Article
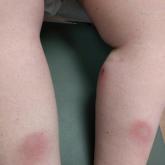
Pembrolizumab-Induced Lobular Panniculitis in the Setting of Metastatic Melanoma
- Author:
- Caitlin M. Peterman, MD
- Leslie Robinson-Bostom, MD
- So Yeon Paek, MD
Pembrolizumab may cause lobular panniculitis years after treatment initiation. The authors report a case of pembrolizumab-induced, self-limited...
