User login
Erythrasma
THE COMPARISON
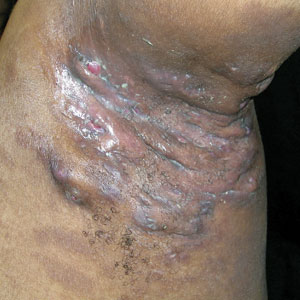
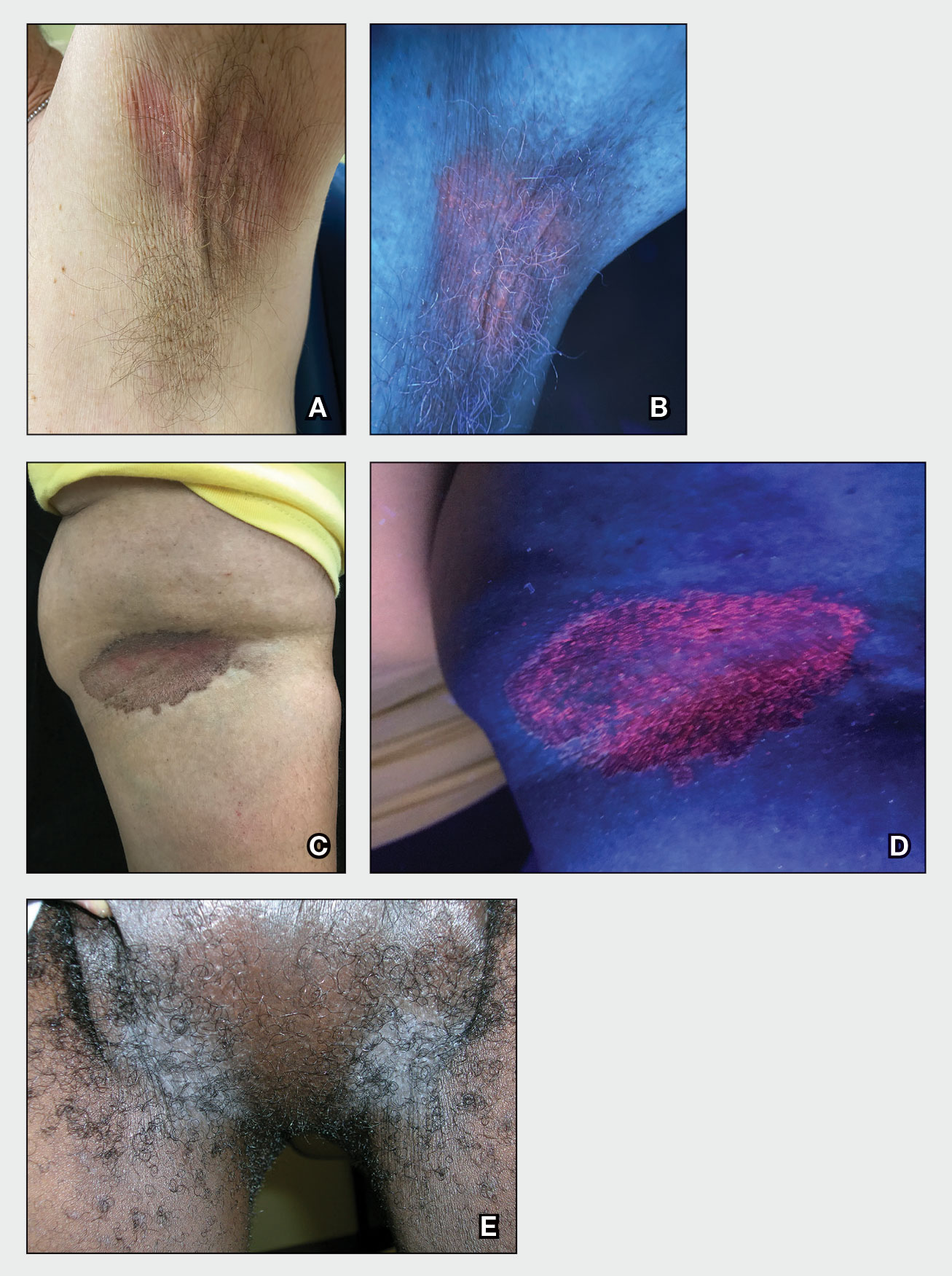
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
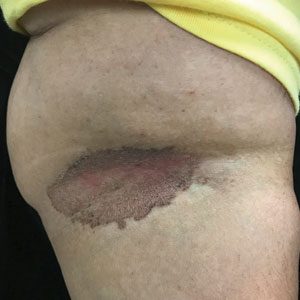
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
Tinea capitis
THE COMPARISON
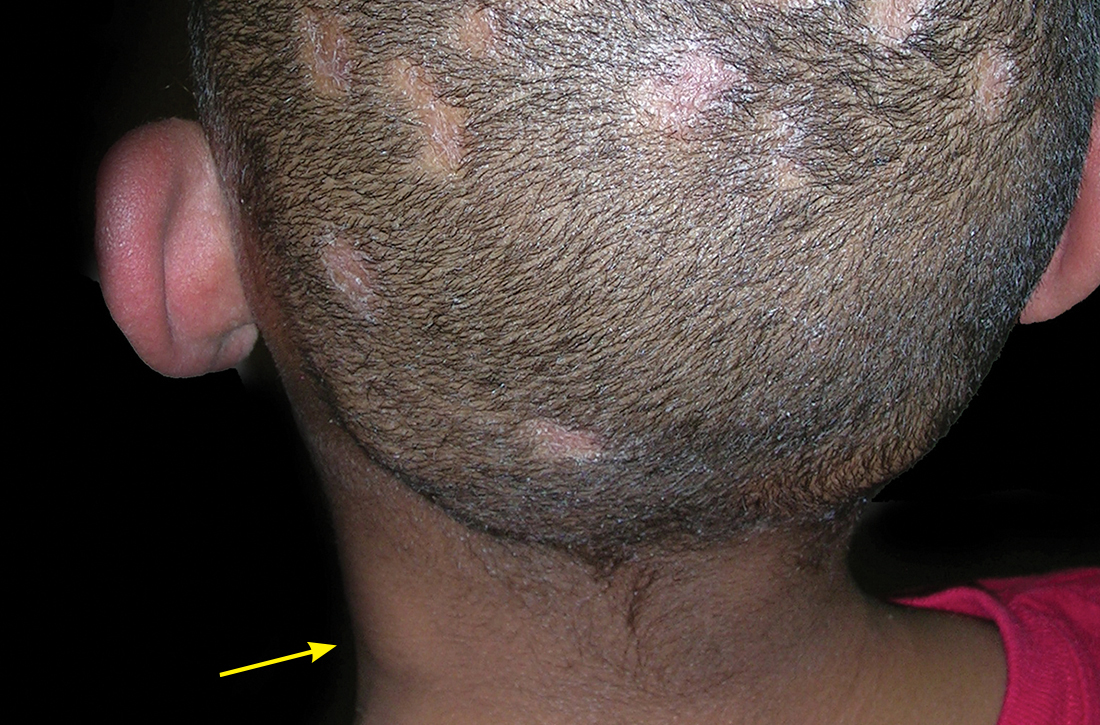
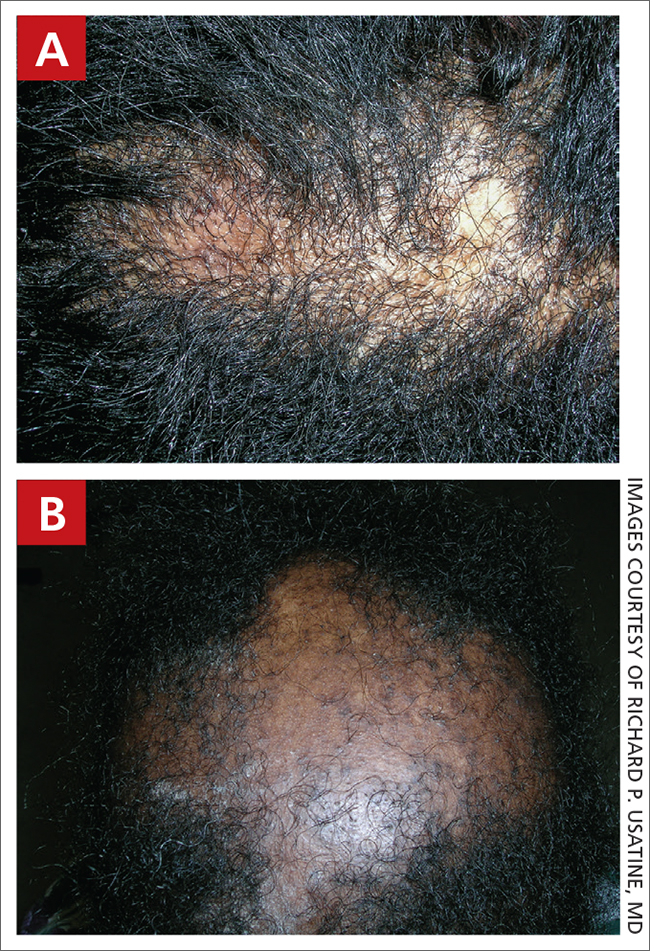
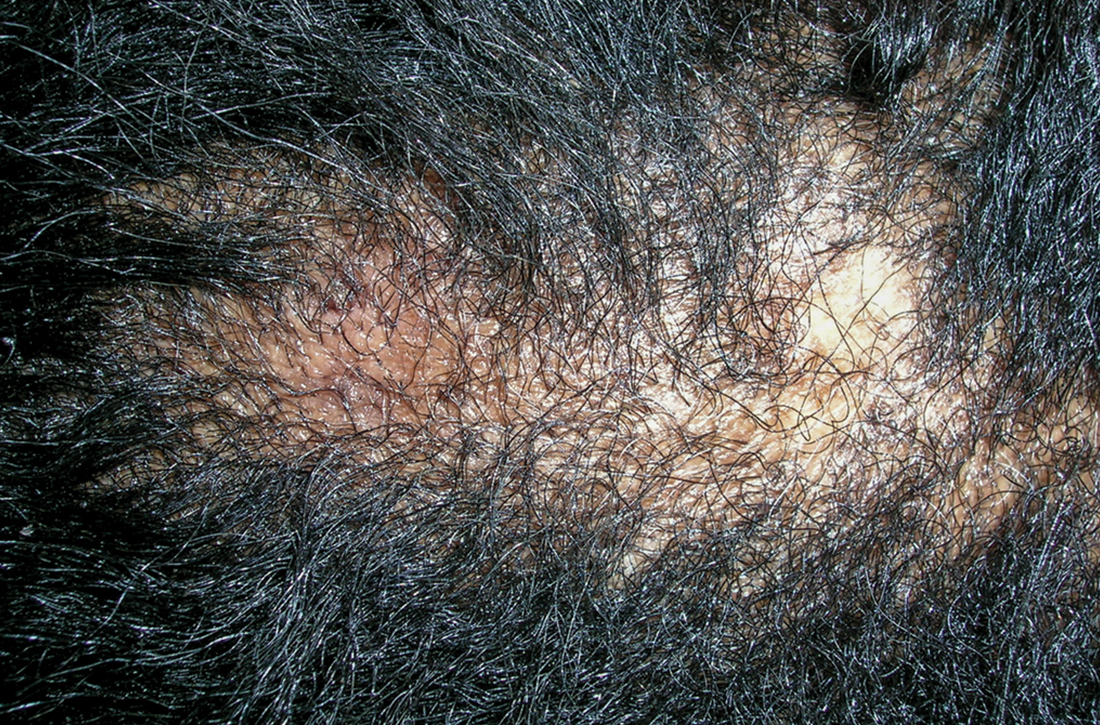
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
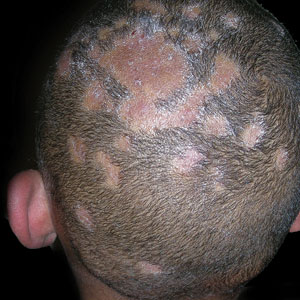
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
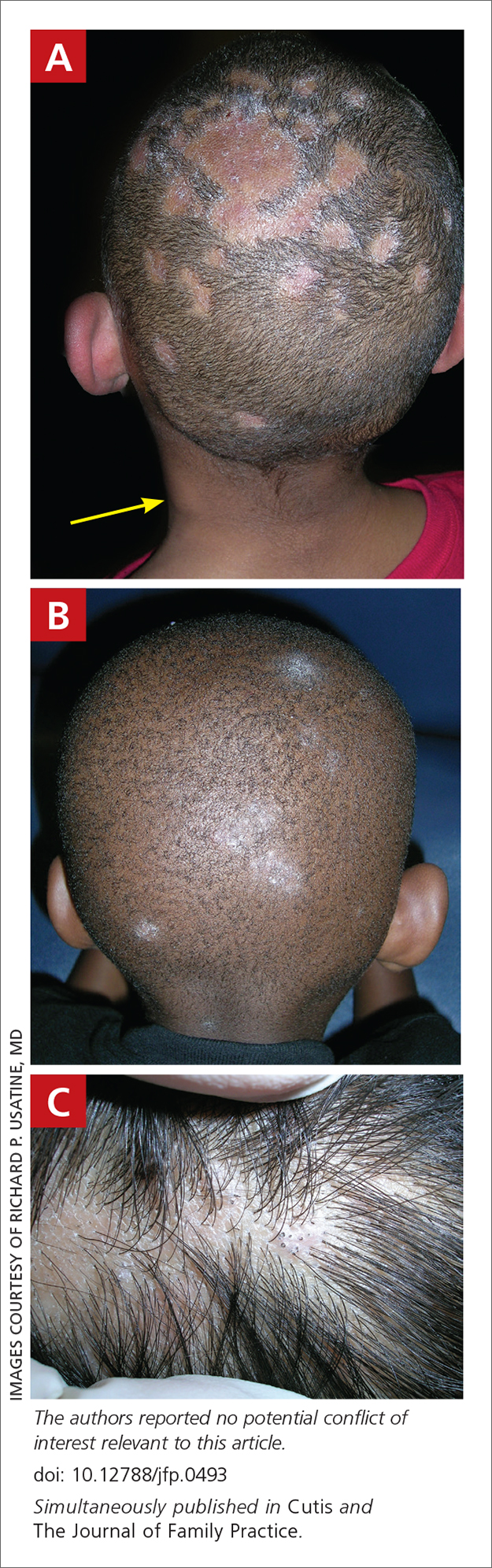
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
Tinea Capitis
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
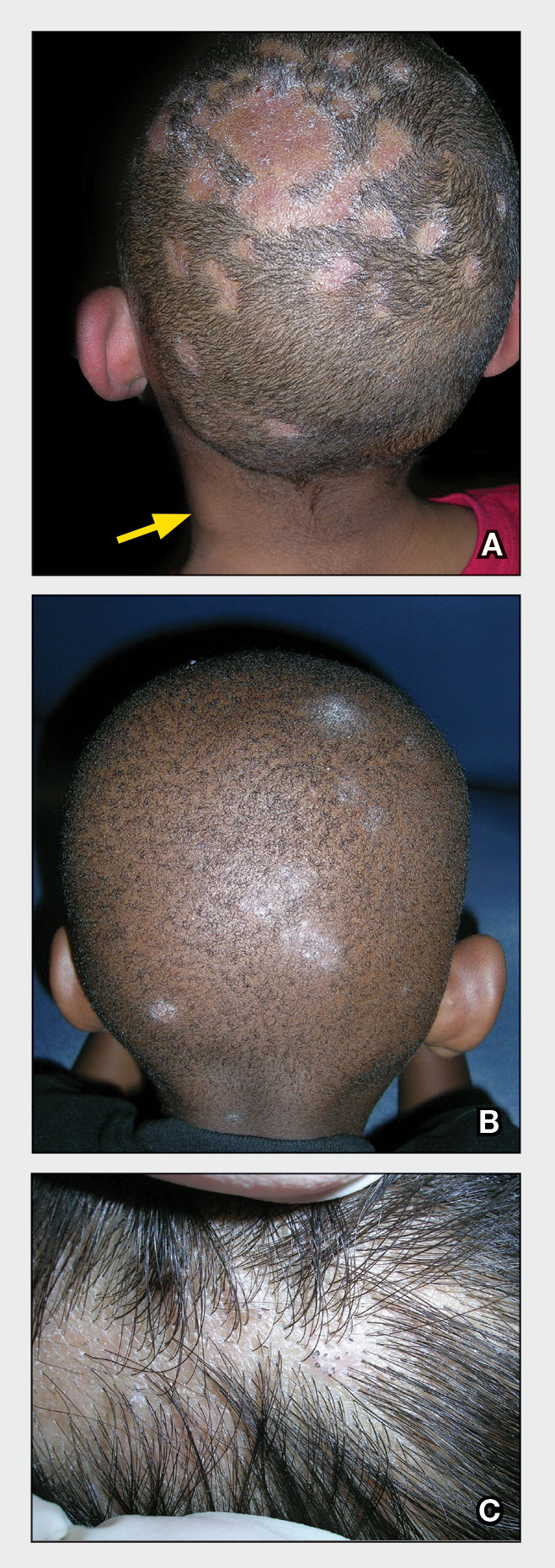
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
Hidradenitis suppurativa
THE COMPARISON
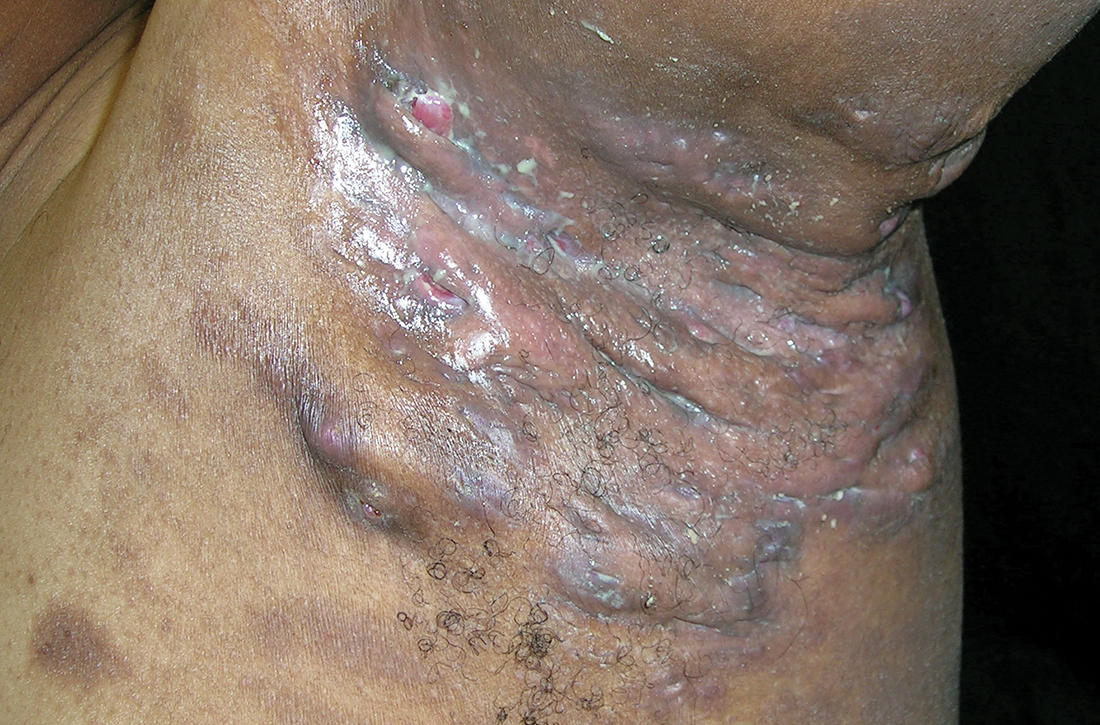
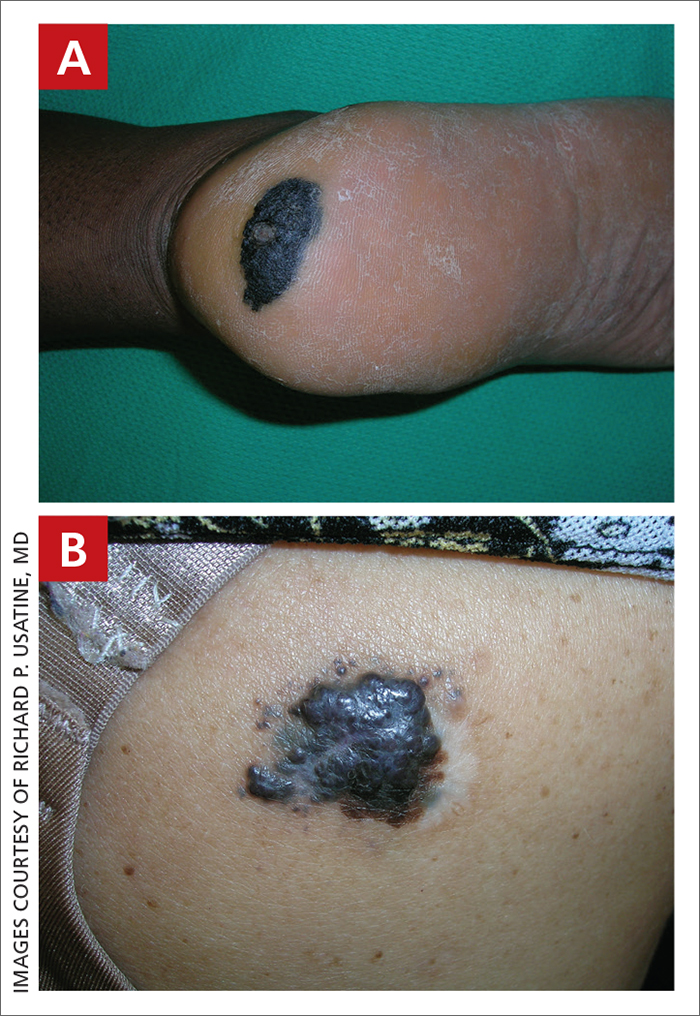
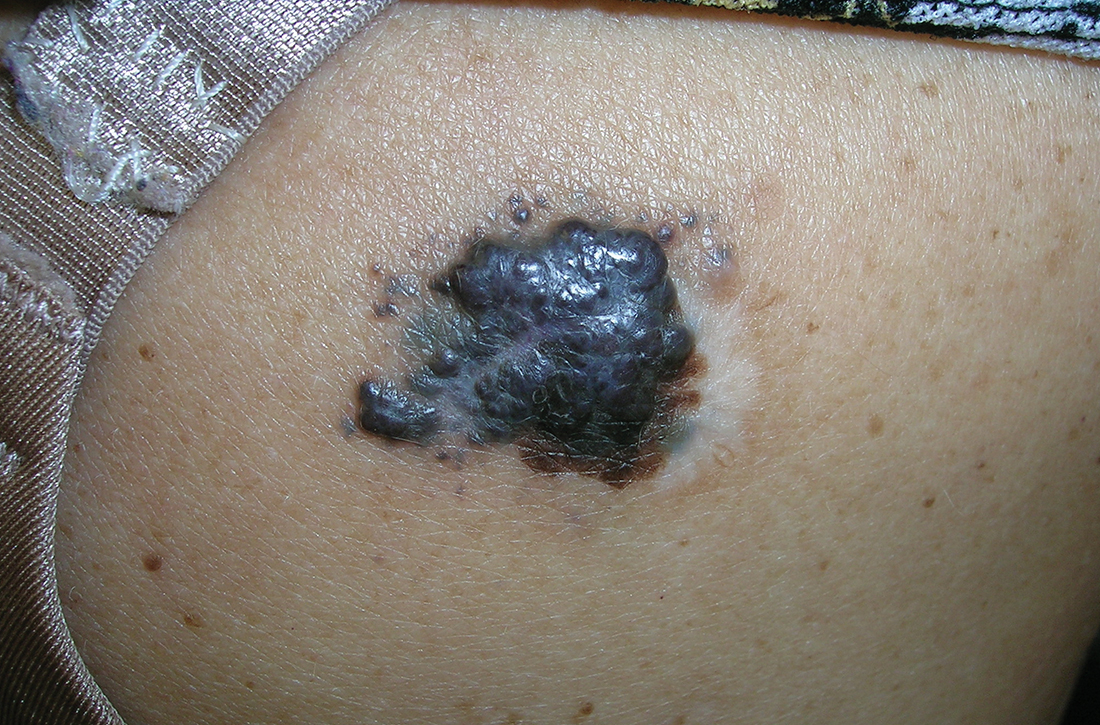
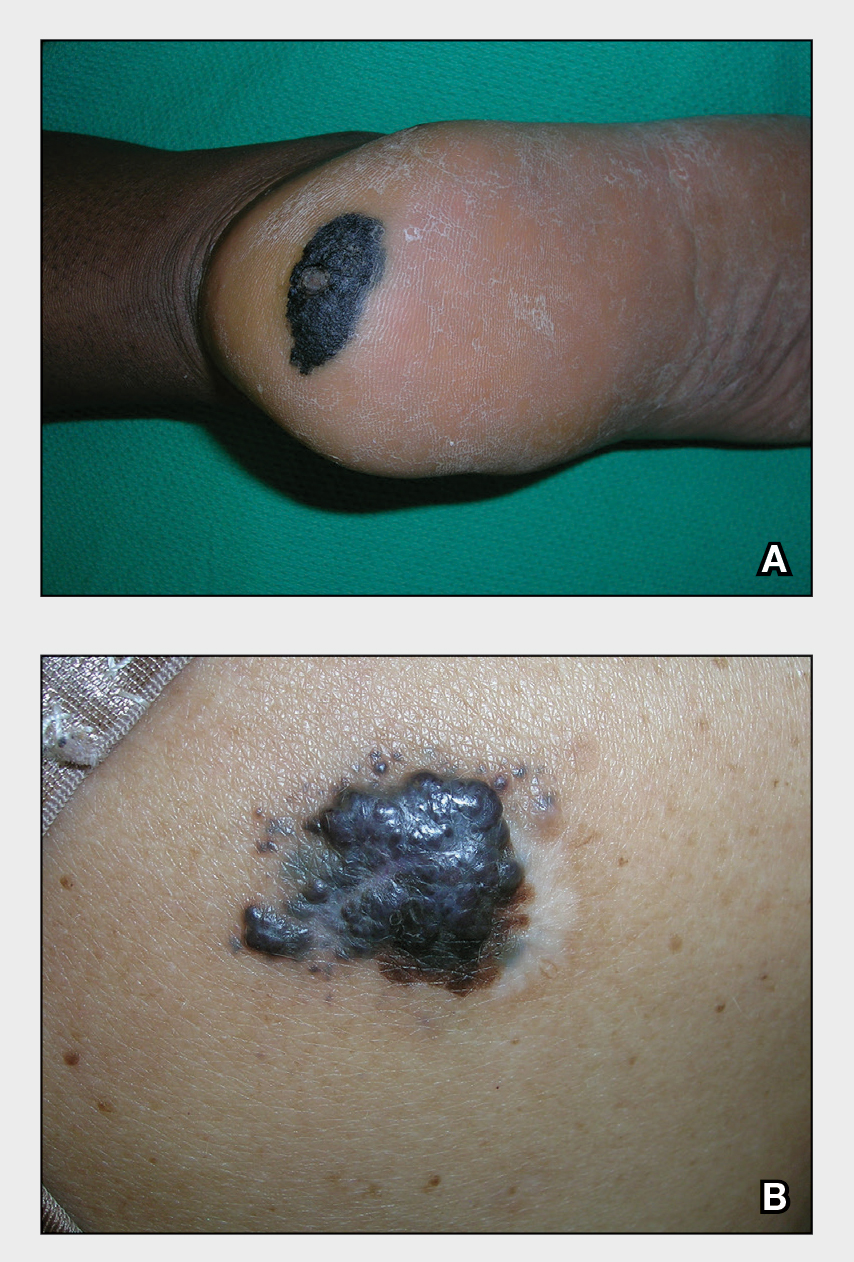
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.
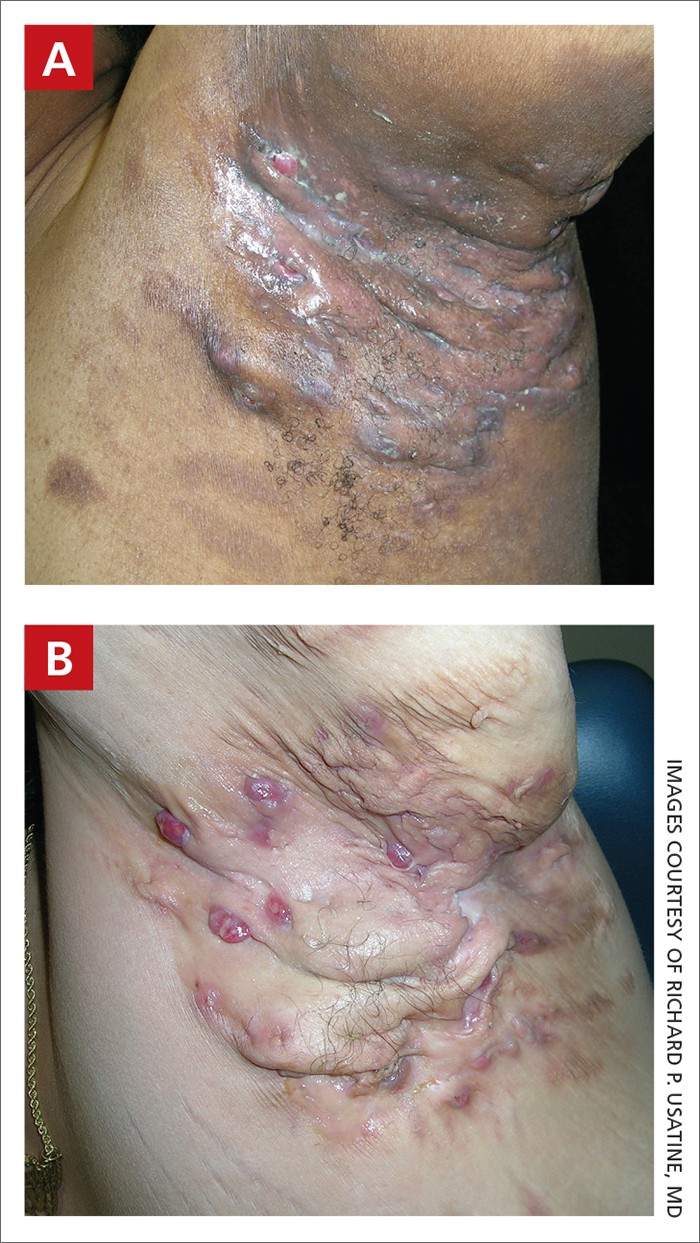
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
Hidradenitis Suppurativa
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).
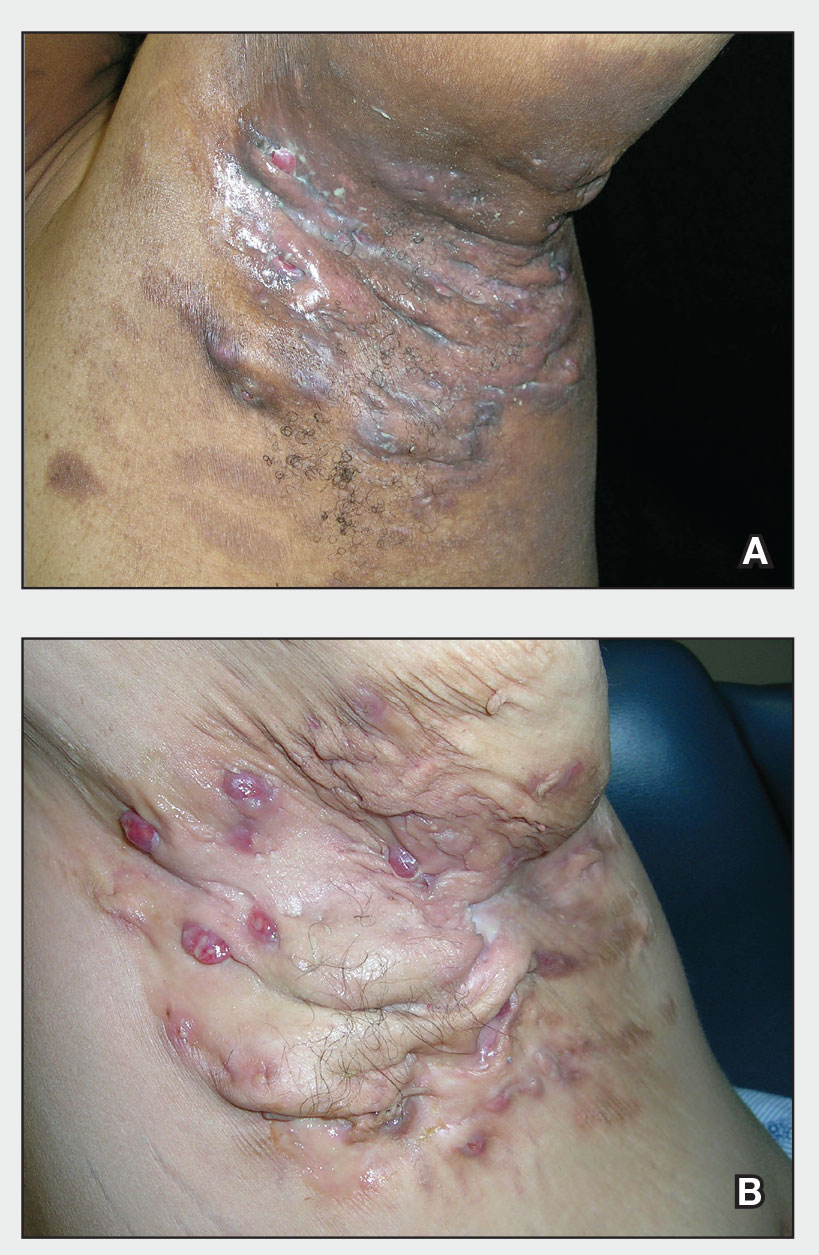
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
Basal cell carcinoma
THE COMPARISON
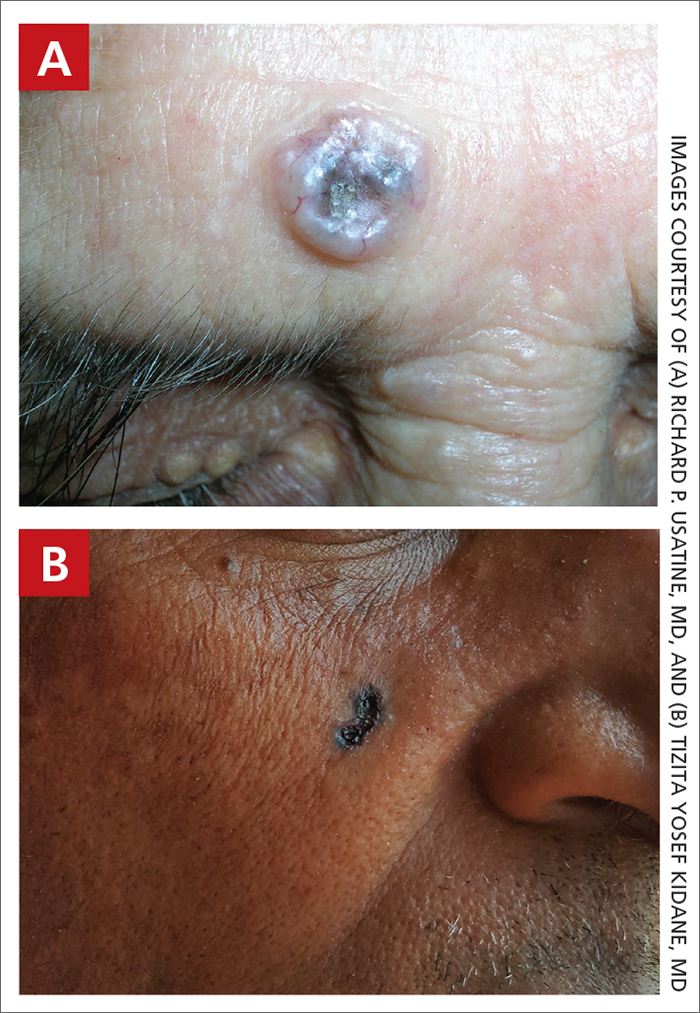
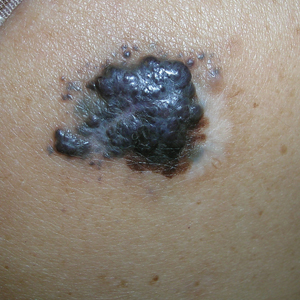
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
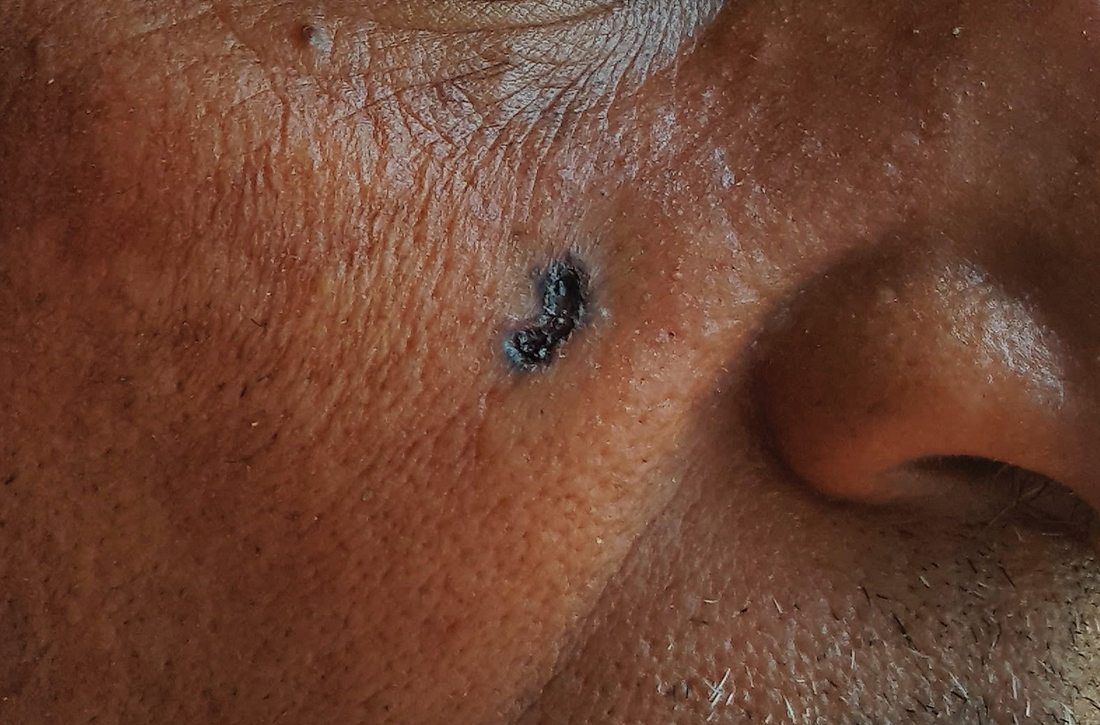
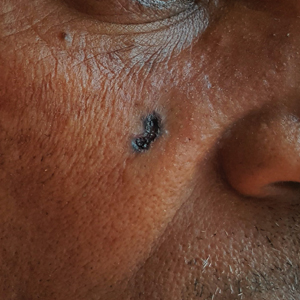
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
Basal Cell Carcinoma
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
Melanoma
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
Melanoma
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
Central centrifugal cicatricial alopecia
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia.1 CCCA (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
CCCA predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. CCCA has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
- CCCA begins in the central scalp (crown area, vertex) and spreads centrifugally.
- Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
- Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
- Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
- CCCA can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Continue to: Due to the inflammatory...
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral antiinflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
1. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/ j.1600-0560.2001.280701.x
2. Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/ archderm.147.12.1453
3. Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111/ 1346-8138.14217
4. Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
5. Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
6. Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
7. Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056/ NEJMe1900042
8. Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
9. Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
10. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/ j.jdcr.2019.12.008.
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia.1 CCCA (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
CCCA predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. CCCA has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
- CCCA begins in the central scalp (crown area, vertex) and spreads centrifugally.
- Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
- Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
- Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
- CCCA can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Continue to: Due to the inflammatory...
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral antiinflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia.1 CCCA (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
CCCA predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. CCCA has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
- CCCA begins in the central scalp (crown area, vertex) and spreads centrifugally.
- Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
- Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
- Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
- CCCA can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Continue to: Due to the inflammatory...
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral antiinflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
1. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/ j.1600-0560.2001.280701.x
2. Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/ archderm.147.12.1453
3. Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111/ 1346-8138.14217
4. Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
5. Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
6. Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
7. Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056/ NEJMe1900042
8. Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
9. Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
10. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/ j.jdcr.2019.12.008.
1. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/ j.1600-0560.2001.280701.x
2. Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/ archderm.147.12.1453
3. Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111/ 1346-8138.14217
4. Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
5. Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
6. Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
7. Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056/ NEJMe1900042
8. Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
9. Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
10. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/ j.jdcr.2019.12.008.