User login
Tooth Decay Is the Most Prevalent Disease
There are 2 leading types of dental diseases: tooth decay (dental caries or cavities) and gum disease (periodontal disease). Tooth decay is by far the more prevalent of the 2, causing a greater, needless loss in the quality of life.
Prevalence of Tooth Decay
Dental caries is a major public health problem. Even though in the past 50 years there has been a significant decline in dental caries, it is the most common ailment in the U.S., and large segments of the population experience various barriers to care. Among children and adolescents, dental caries is 4 to 5 times more common than asthma.1,2 Data from the National Health and Nutrition Examination Survey, 2011-2012, show that among children aged 2 to 8 years, 37% had dental caries in their primary teeth. Among adolescents aged 12 to 19 years, the prevalence of dental caries in the permanent teeth was 58%. About 90% of adults aged ≥ 20 years had dental caries.3,4
Unmet Dental Needs
The real disease burden of dental caries is the amount of unmet needs or untreated decay. In recent years, there has been a shift in the peak prevalence of untreated tooth decay from children to adults, possibly attributable to socioecologic factors.3,5 The prevalence of untreated decay in children and adolescents was about 15%. However, the prevalence of untreated decay among adults aged 20 to 64 years was higher: 27% of the adults had decayed teeth that were left untreated.3,4
The rates of untreated decay among these adults were higher among Hispanics (36%) and non-Hispanic blacks (42%) than among non-Hispanic whites (22%) and non-Hispanic Asians (17%).3,4 Despite the decline in dental caries, disparities persist among different racial, ethnic, educational, and income groups; it remains a modern curse for large segments of the population.
Basics of Tooth Decay
Tooth decay is caused by Streptococcus mutans (S mutans) bacterial infection. The cariogenic bacteria are transmissible from mother or caregiver to children.6 After tooth eruption, the mouth is quickly colonized by S mutans. Even though the bacteria are ubiquitous, the disease burden is more concentrated in some populations.
What makes some people more susceptible to tooth decay? Contrary to popular belief, it is not caused by childbirth or low dietary intake of calcium.7,8 Tooth decay typically starts on the chewing surfaces or proximal contacts of teeth.
S mutans is introduced into the mouth principally from another person. The bacteria colonize the mouth and with the formation of plaques, adhere to the teeth. Plaque is the soft, sticky film formed on teeth from food degradation. The biofilm is conducive to bacterial proliferation and teeming with bacteria.
The S mutans bacteria break down sugars and produce lactic acids, which cause tooth decay—a process of demineralization, or loss of calcium phosphate, from the tooth structure.2,9 As a result, the tooth “softens” and eventually collapses on itself, forming a cavity. Tooth decay most commonly occurs at the occlusal (chewing) surfaces and the proximal contacts of teeth. The occlusal aspects of teeth have a natural pit-and-fissure morphology that facilitates bacterial adherence and colonization. The proximal contacts of teeth also facilitate adherence of plaque and bacteria.
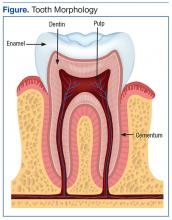
A tooth is made up of 3 layers. The outermost layer of the tooth crown is the enamel, which is the hardest or most mineralized part of the tooth—it is harder than bone. The next layer is the dentin, which has a higher percentage of organic material (collagen) and water than the enamel has and is softer. In the center of the tooth is the pulp (consisting of nerves and blood vessels), which keeps the tooth alive and provides sensation to the tooth. The outermost layer of the tooth root is cementum (instead of enamel), followed by dentin, which encases the pulp tissues contained within the root canals (Figure).
Process of Tooth Loss
At the early stage of tooth decay, when the decay is confined to the enamel, the tooth is asymptomatic, and the damage is reversible. When the decay extends into the dentin, restorations are a consideration. The further the decay extends toward the pulp, the greater the risk of tooth sensitivity and pain. Decay naturally progresses faster in the (less mineralized) dentin than when the decay is still confined to the enamel. If left unchecked, the decay may progress, and the bacteria eventually invade the pulp. At that point, there is risk of not only pain, but also swelling and tooth loss.
At the earlier stage of decay, a tooth may be treated with restorations, which are typically made of dental amalgam, resin (composite), glass ionomer, porcelain, or gold. When the bacteria have invaded the pulp, a dental restoration will not treat the pain and infection. Root canal therapy is needed to remove the infected tissues in the pulp chamber and root canals. Further, if the tooth is too compromised with extensive destruction from the decay or if there are financial or other barriers to root canal therapy, extraction is likely the only option.
Key to Oral Health
Personal daily oral hygiene (brushing and flossing) helps remove plaque from accumulating on tooth surfaces—especially the occlusal surfaces and proximal contacts, which are most prone to decay. Brushing teeth is of limited benefit without the use of fluoride toothpaste.8 The most important time for brushing is before bedtime, because less salivation occurs during sleep. Saliva helps clear fermented bacterial products, buffers the drop in pH, prevents demineralization, and enhances remineralizaton.10
Fluoride benefits children and adults of all ages, and comes in the form of fluoridated tap water, toothpaste, mouth rinse, and professionally applied gel and varnish. Fluoride occurs naturally in the environment, too. Its effect is mainly topical—fluoride gets incorporated into the tooth as fluorapatite, replacing the hydroxyapatite. Fluorapatite is harder than the hydroxyapatite; hence, the tooth is better able to resist demineralization by bacterial acid attacks.11 Professionally applied fluoride is generally recommended for high-risk patients, typically patients with high caries burden, ie, many decayed teeth. The best predictor of future caries is the past caries experience.
Sealants are thin, plastic coatings that are painted on to tooth occlusal surfaces, obliterating the pits and fissures normally found on posterior teeth. The smooth sealants effectively inhibit the colonization of S mutans on the occlusal aspects of teeth.12 Sealants are applied in dental offices (by the dentists or dental hygienists) or in school-based programs.
1. U.S. Department of Health and Human Services. Oral health in America: a report of the Surgeon General. http://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/Report/ExecutiveSummary.htm. Updated March 7, 2014. Accessed August 22, 2016.
2. Centers for Disease Control and Prevention. Hygiene-related diseases. http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html. Updated December 16, 2014. Accessed August 22, 2016.
3. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db191.pdf. Published March 2015. NCHS Data Brief. Accessed August 22, 2016.
4. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db197.pdf. NCHS Data Brief. Published May 2015. Accessed August 22, 2016.
5. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658.
6. Smith RE, Badner VM, Morse DE, Freeman K. Maternal risk indicators for childhood caries in an inner city population. Community Dent Oral Epidemiol. 2002;30(3):176-181.
7. Boggess KA, Urlaub DM, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. J Am Dent Assoc. 2011;142(11):1275-1282.
8. Roberts-Thomson KF, Spencer AJ. Public knowledge of the prevention of dental decay and gum diseases. Aust Dent J. 1999;44(4):253-258.
9. Cochrane NJ, Cai F, Hug NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187-1197.
10. Prabhakar AR, Dodawad R, Os R. Evaluation of flow Rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an in vivo study. Int J Clin Pediatr Dent. 2009;2(1):9-12.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remuneration (part 3). J Clin Pediatr Dent. 2004;28(3):203-214.
12. Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74(2):171-177.
There are 2 leading types of dental diseases: tooth decay (dental caries or cavities) and gum disease (periodontal disease). Tooth decay is by far the more prevalent of the 2, causing a greater, needless loss in the quality of life.
Prevalence of Tooth Decay
Dental caries is a major public health problem. Even though in the past 50 years there has been a significant decline in dental caries, it is the most common ailment in the U.S., and large segments of the population experience various barriers to care. Among children and adolescents, dental caries is 4 to 5 times more common than asthma.1,2 Data from the National Health and Nutrition Examination Survey, 2011-2012, show that among children aged 2 to 8 years, 37% had dental caries in their primary teeth. Among adolescents aged 12 to 19 years, the prevalence of dental caries in the permanent teeth was 58%. About 90% of adults aged ≥ 20 years had dental caries.3,4
Unmet Dental Needs
The real disease burden of dental caries is the amount of unmet needs or untreated decay. In recent years, there has been a shift in the peak prevalence of untreated tooth decay from children to adults, possibly attributable to socioecologic factors.3,5 The prevalence of untreated decay in children and adolescents was about 15%. However, the prevalence of untreated decay among adults aged 20 to 64 years was higher: 27% of the adults had decayed teeth that were left untreated.3,4
The rates of untreated decay among these adults were higher among Hispanics (36%) and non-Hispanic blacks (42%) than among non-Hispanic whites (22%) and non-Hispanic Asians (17%).3,4 Despite the decline in dental caries, disparities persist among different racial, ethnic, educational, and income groups; it remains a modern curse for large segments of the population.
Basics of Tooth Decay
Tooth decay is caused by Streptococcus mutans (S mutans) bacterial infection. The cariogenic bacteria are transmissible from mother or caregiver to children.6 After tooth eruption, the mouth is quickly colonized by S mutans. Even though the bacteria are ubiquitous, the disease burden is more concentrated in some populations.
What makes some people more susceptible to tooth decay? Contrary to popular belief, it is not caused by childbirth or low dietary intake of calcium.7,8 Tooth decay typically starts on the chewing surfaces or proximal contacts of teeth.
S mutans is introduced into the mouth principally from another person. The bacteria colonize the mouth and with the formation of plaques, adhere to the teeth. Plaque is the soft, sticky film formed on teeth from food degradation. The biofilm is conducive to bacterial proliferation and teeming with bacteria.
The S mutans bacteria break down sugars and produce lactic acids, which cause tooth decay—a process of demineralization, or loss of calcium phosphate, from the tooth structure.2,9 As a result, the tooth “softens” and eventually collapses on itself, forming a cavity. Tooth decay most commonly occurs at the occlusal (chewing) surfaces and the proximal contacts of teeth. The occlusal aspects of teeth have a natural pit-and-fissure morphology that facilitates bacterial adherence and colonization. The proximal contacts of teeth also facilitate adherence of plaque and bacteria.
A tooth is made up of 3 layers. The outermost layer of the tooth crown is the enamel, which is the hardest or most mineralized part of the tooth—it is harder than bone. The next layer is the dentin, which has a higher percentage of organic material (collagen) and water than the enamel has and is softer. In the center of the tooth is the pulp (consisting of nerves and blood vessels), which keeps the tooth alive and provides sensation to the tooth. The outermost layer of the tooth root is cementum (instead of enamel), followed by dentin, which encases the pulp tissues contained within the root canals (Figure).
Process of Tooth Loss
At the early stage of tooth decay, when the decay is confined to the enamel, the tooth is asymptomatic, and the damage is reversible. When the decay extends into the dentin, restorations are a consideration. The further the decay extends toward the pulp, the greater the risk of tooth sensitivity and pain. Decay naturally progresses faster in the (less mineralized) dentin than when the decay is still confined to the enamel. If left unchecked, the decay may progress, and the bacteria eventually invade the pulp. At that point, there is risk of not only pain, but also swelling and tooth loss.
At the earlier stage of decay, a tooth may be treated with restorations, which are typically made of dental amalgam, resin (composite), glass ionomer, porcelain, or gold. When the bacteria have invaded the pulp, a dental restoration will not treat the pain and infection. Root canal therapy is needed to remove the infected tissues in the pulp chamber and root canals. Further, if the tooth is too compromised with extensive destruction from the decay or if there are financial or other barriers to root canal therapy, extraction is likely the only option.
Key to Oral Health
Personal daily oral hygiene (brushing and flossing) helps remove plaque from accumulating on tooth surfaces—especially the occlusal surfaces and proximal contacts, which are most prone to decay. Brushing teeth is of limited benefit without the use of fluoride toothpaste.8 The most important time for brushing is before bedtime, because less salivation occurs during sleep. Saliva helps clear fermented bacterial products, buffers the drop in pH, prevents demineralization, and enhances remineralizaton.10
Fluoride benefits children and adults of all ages, and comes in the form of fluoridated tap water, toothpaste, mouth rinse, and professionally applied gel and varnish. Fluoride occurs naturally in the environment, too. Its effect is mainly topical—fluoride gets incorporated into the tooth as fluorapatite, replacing the hydroxyapatite. Fluorapatite is harder than the hydroxyapatite; hence, the tooth is better able to resist demineralization by bacterial acid attacks.11 Professionally applied fluoride is generally recommended for high-risk patients, typically patients with high caries burden, ie, many decayed teeth. The best predictor of future caries is the past caries experience.
Sealants are thin, plastic coatings that are painted on to tooth occlusal surfaces, obliterating the pits and fissures normally found on posterior teeth. The smooth sealants effectively inhibit the colonization of S mutans on the occlusal aspects of teeth.12 Sealants are applied in dental offices (by the dentists or dental hygienists) or in school-based programs.
There are 2 leading types of dental diseases: tooth decay (dental caries or cavities) and gum disease (periodontal disease). Tooth decay is by far the more prevalent of the 2, causing a greater, needless loss in the quality of life.
Prevalence of Tooth Decay
Dental caries is a major public health problem. Even though in the past 50 years there has been a significant decline in dental caries, it is the most common ailment in the U.S., and large segments of the population experience various barriers to care. Among children and adolescents, dental caries is 4 to 5 times more common than asthma.1,2 Data from the National Health and Nutrition Examination Survey, 2011-2012, show that among children aged 2 to 8 years, 37% had dental caries in their primary teeth. Among adolescents aged 12 to 19 years, the prevalence of dental caries in the permanent teeth was 58%. About 90% of adults aged ≥ 20 years had dental caries.3,4
Unmet Dental Needs
The real disease burden of dental caries is the amount of unmet needs or untreated decay. In recent years, there has been a shift in the peak prevalence of untreated tooth decay from children to adults, possibly attributable to socioecologic factors.3,5 The prevalence of untreated decay in children and adolescents was about 15%. However, the prevalence of untreated decay among adults aged 20 to 64 years was higher: 27% of the adults had decayed teeth that were left untreated.3,4
The rates of untreated decay among these adults were higher among Hispanics (36%) and non-Hispanic blacks (42%) than among non-Hispanic whites (22%) and non-Hispanic Asians (17%).3,4 Despite the decline in dental caries, disparities persist among different racial, ethnic, educational, and income groups; it remains a modern curse for large segments of the population.
Basics of Tooth Decay
Tooth decay is caused by Streptococcus mutans (S mutans) bacterial infection. The cariogenic bacteria are transmissible from mother or caregiver to children.6 After tooth eruption, the mouth is quickly colonized by S mutans. Even though the bacteria are ubiquitous, the disease burden is more concentrated in some populations.
What makes some people more susceptible to tooth decay? Contrary to popular belief, it is not caused by childbirth or low dietary intake of calcium.7,8 Tooth decay typically starts on the chewing surfaces or proximal contacts of teeth.
S mutans is introduced into the mouth principally from another person. The bacteria colonize the mouth and with the formation of plaques, adhere to the teeth. Plaque is the soft, sticky film formed on teeth from food degradation. The biofilm is conducive to bacterial proliferation and teeming with bacteria.
The S mutans bacteria break down sugars and produce lactic acids, which cause tooth decay—a process of demineralization, or loss of calcium phosphate, from the tooth structure.2,9 As a result, the tooth “softens” and eventually collapses on itself, forming a cavity. Tooth decay most commonly occurs at the occlusal (chewing) surfaces and the proximal contacts of teeth. The occlusal aspects of teeth have a natural pit-and-fissure morphology that facilitates bacterial adherence and colonization. The proximal contacts of teeth also facilitate adherence of plaque and bacteria.
A tooth is made up of 3 layers. The outermost layer of the tooth crown is the enamel, which is the hardest or most mineralized part of the tooth—it is harder than bone. The next layer is the dentin, which has a higher percentage of organic material (collagen) and water than the enamel has and is softer. In the center of the tooth is the pulp (consisting of nerves and blood vessels), which keeps the tooth alive and provides sensation to the tooth. The outermost layer of the tooth root is cementum (instead of enamel), followed by dentin, which encases the pulp tissues contained within the root canals (Figure).
Process of Tooth Loss
At the early stage of tooth decay, when the decay is confined to the enamel, the tooth is asymptomatic, and the damage is reversible. When the decay extends into the dentin, restorations are a consideration. The further the decay extends toward the pulp, the greater the risk of tooth sensitivity and pain. Decay naturally progresses faster in the (less mineralized) dentin than when the decay is still confined to the enamel. If left unchecked, the decay may progress, and the bacteria eventually invade the pulp. At that point, there is risk of not only pain, but also swelling and tooth loss.
At the earlier stage of decay, a tooth may be treated with restorations, which are typically made of dental amalgam, resin (composite), glass ionomer, porcelain, or gold. When the bacteria have invaded the pulp, a dental restoration will not treat the pain and infection. Root canal therapy is needed to remove the infected tissues in the pulp chamber and root canals. Further, if the tooth is too compromised with extensive destruction from the decay or if there are financial or other barriers to root canal therapy, extraction is likely the only option.
Key to Oral Health
Personal daily oral hygiene (brushing and flossing) helps remove plaque from accumulating on tooth surfaces—especially the occlusal surfaces and proximal contacts, which are most prone to decay. Brushing teeth is of limited benefit without the use of fluoride toothpaste.8 The most important time for brushing is before bedtime, because less salivation occurs during sleep. Saliva helps clear fermented bacterial products, buffers the drop in pH, prevents demineralization, and enhances remineralizaton.10
Fluoride benefits children and adults of all ages, and comes in the form of fluoridated tap water, toothpaste, mouth rinse, and professionally applied gel and varnish. Fluoride occurs naturally in the environment, too. Its effect is mainly topical—fluoride gets incorporated into the tooth as fluorapatite, replacing the hydroxyapatite. Fluorapatite is harder than the hydroxyapatite; hence, the tooth is better able to resist demineralization by bacterial acid attacks.11 Professionally applied fluoride is generally recommended for high-risk patients, typically patients with high caries burden, ie, many decayed teeth. The best predictor of future caries is the past caries experience.
Sealants are thin, plastic coatings that are painted on to tooth occlusal surfaces, obliterating the pits and fissures normally found on posterior teeth. The smooth sealants effectively inhibit the colonization of S mutans on the occlusal aspects of teeth.12 Sealants are applied in dental offices (by the dentists or dental hygienists) or in school-based programs.
1. U.S. Department of Health and Human Services. Oral health in America: a report of the Surgeon General. http://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/Report/ExecutiveSummary.htm. Updated March 7, 2014. Accessed August 22, 2016.
2. Centers for Disease Control and Prevention. Hygiene-related diseases. http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html. Updated December 16, 2014. Accessed August 22, 2016.
3. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db191.pdf. Published March 2015. NCHS Data Brief. Accessed August 22, 2016.
4. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db197.pdf. NCHS Data Brief. Published May 2015. Accessed August 22, 2016.
5. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658.
6. Smith RE, Badner VM, Morse DE, Freeman K. Maternal risk indicators for childhood caries in an inner city population. Community Dent Oral Epidemiol. 2002;30(3):176-181.
7. Boggess KA, Urlaub DM, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. J Am Dent Assoc. 2011;142(11):1275-1282.
8. Roberts-Thomson KF, Spencer AJ. Public knowledge of the prevention of dental decay and gum diseases. Aust Dent J. 1999;44(4):253-258.
9. Cochrane NJ, Cai F, Hug NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187-1197.
10. Prabhakar AR, Dodawad R, Os R. Evaluation of flow Rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an in vivo study. Int J Clin Pediatr Dent. 2009;2(1):9-12.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remuneration (part 3). J Clin Pediatr Dent. 2004;28(3):203-214.
12. Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74(2):171-177.
1. U.S. Department of Health and Human Services. Oral health in America: a report of the Surgeon General. http://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/Report/ExecutiveSummary.htm. Updated March 7, 2014. Accessed August 22, 2016.
2. Centers for Disease Control and Prevention. Hygiene-related diseases. http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html. Updated December 16, 2014. Accessed August 22, 2016.
3. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db191.pdf. Published March 2015. NCHS Data Brief. Accessed August 22, 2016.
4. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db197.pdf. NCHS Data Brief. Published May 2015. Accessed August 22, 2016.
5. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658.
6. Smith RE, Badner VM, Morse DE, Freeman K. Maternal risk indicators for childhood caries in an inner city population. Community Dent Oral Epidemiol. 2002;30(3):176-181.
7. Boggess KA, Urlaub DM, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. J Am Dent Assoc. 2011;142(11):1275-1282.
8. Roberts-Thomson KF, Spencer AJ. Public knowledge of the prevention of dental decay and gum diseases. Aust Dent J. 1999;44(4):253-258.
9. Cochrane NJ, Cai F, Hug NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187-1197.
10. Prabhakar AR, Dodawad R, Os R. Evaluation of flow Rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an in vivo study. Int J Clin Pediatr Dent. 2009;2(1):9-12.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remuneration (part 3). J Clin Pediatr Dent. 2004;28(3):203-214.
12. Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74(2):171-177.
Changing Treatment Landscape of Hepatitis C Virus Infection Among Penitentiary Inmates
The incidence of hepatitis C virus (HCV) infection increased markedly in the 1970s and 1980s. These increases were mainly attributable to blood transfusions and injection drug use.1,2 The blood supply was not screened for HCV before 1992 (now, HCV infection by blood transfusion is rare).2,3 Surveillance of the period 1992-2003 showed a substantial decrease in the incidence of acute hepatitis C cases, and the rate remained steady through 2010.2,3 Over the past 5 years, HCV has returned to national attention with a rising infection rate (2.5-fold increase during 2010-2013) and a rapid succession of FDA approvals of direct-acting antiviral agents (DAAs).4 Currently, the most prevalent mode of infection is injection drug use, accounting for > 50% of all cases of HCV infection and 84% of acute HCV infections.5
Baby boomers (people born between 1945 and 1965) account for three-fourths of the population currently living with chronic HCV infection resulting from past infection.6 Historically, rates of acute and chronic infection have been far higher for blacks than for whites and Hispanics.2,4,7,8 In 2004, that trend started to reverse, with the incidence in whites surpassing that in blacks.4 Recent reports have identified a new cohort of HCV-infected injection drug users (IDUs) who are young (aged ≤ 24 years) and white nonurban dwellers.5
HCV Infection Among High Risk Individuals
In the U.S., unlike in other parts of the world, HCV infection is more prevalent than hepatitis B virus (HBV) infection.4,9,10 According to the National Health and Nutrition Examination Survey (NHANES), about 2.7 million Americans have chronic HCV infection. However, NHANES surveys do not sample certain populations, including the incarcerated and the homeless, in whom infection rates are thought to be higher.11 The incarcerated, the largest institutionalized group, have the highest incidence: One in 3 is infected with HCV.12 This rate is attributable to high rates of injection drug use and other high-risk behaviors. Drug-related offenses account for 50% of federal prison incarceration.13 For IDUs, the HCV infection rate is as high as 70% to 90%. Despite widespread implementation of needle-exchange programs after the discovery of HIV in the 1980s, recent surveys have indicated that about one-third of 18- to 30-year-old active IDUs are infected with HCV.14
Penitentiary Inmates Infected With HCV
A 2015 search of the Federal Bureau of Prisons (BOP) electronic medical records at the U.S. Penitentiary Canaan (USP Canaan) found that out of a population of about 1,600 inmates, 182 (11%) had tested positive for HCV antibodies (anti-HCV). This percentage likely is an underestimation, because HCV testing is not mandatory, and many (45%-85%) of the infected are unaware of their HCV infection status.2 Most of the infected remain chronically infected and are not being treated.
Prevalence of HCV infection commonly refers to chronic HCV infection. The diagnosis of chronic HCV infection is established by presence of HCV RNA on polymerase chain reaction assays. Of the 182 inmates who tested positive for anti-HCV, 45 had their cases resolved (undetectable HCV RNA), 34 spontaneously, and the other 11 after treatment, primarily with peginterferon and ribavirin (pegINF/RBV) dual therapies. The remaining 137 who tested positive remained chronically infected. This chronically infected group represented 9% of the population of 1,600 inmates. Although the infection rate is significantly higher than that in the general population (1% incidence), the inmates’ true rate of infection in all probability is much higher.11
Earlier NHANES data showed HCV more prevalent in minorities, particularly blacks, compared with whites.2,7,8 At USP Canaan, however, the incidence of chronic HCV infection was 21% in whites (mean age, 42 years), 4% in blacks (mean age, 51 years), and 7% in Hispanics (mean age, 39 years). The lower rates in blacks and Hispanics could have resulted from a lack of awareness about getting tested or from having fewer opportunities to obtain medical care in the community before incarceration (the infection can remain asymptomatic for several decades).
HCV genotype 1 is the most common HCV genotype in the U.S.5,15 At USP Canaan, genotype 1 accounted for 56% of the cases of chronic HCV infection in whites, 90% in blacks, and 79% in Hispanics. The majority genotype was subtype 1a.
Of the 137 inmates with HCV co-infections, 8 (6%) had HIV/HCV co-infection, and 4 (3%) had HBV/HCV co-infection. Also, 7 (5%) were diabetic. According to the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidelines, patients with comorbidities are a high priority for treatment, as there is a high risk for complications, with liver fibrosis progressing more rapidly.16
Changing Landscape of HCV Treatment
Treatments for chronic HCV infection have never been more promising. There is the prospect of a cure with the new DAAs. These new medications are active against HCV and interfere with viral enzymes and other proteins essential for viral replication. Until recently, the mainstay of treatment for chronic HCV infection was pegINF/RBV. However, INF-based dual therapies were associated with high rates of adverse effects (AEs) and treatment discontinuation. In 2011, release of the protease inhibitors (PIs) boceprevir and telaprevir improved sustained virologic response (SVR) rates for treatment-naïve patients with genotype 1 from about 50% (pegINF/RBV dual therapies) to 70% to 75% (PI in combination with pegINF/RBV triple therapies). However, first-generation DAAs were INF-based, and their dosing was cumbersome.15,17-19
Starting with the 2013 approval of simeprevir (second-wave PI) and sofosbuvir (polymerase inhibitor), most patients’ SVR rates improved to 75% to 90%.20,21 Sustained virologic response rates were boosted to > 95% after the 2014 approval of Harvoni, coformulated ledispasvir (replication complex inhibitor) and sofosbuvir, and Viekira Pak, a combination of ombitasvir (replication complex inhibitor), paritaprevir (PI), and ritonavir (inhibitor of CYP3A4 enzyme) co-packaged with dasabuvir (polymerase inhibitor).22-24 In 2015, daclatasvir (replication complex inhibitor) was approved, followed in 2016 by Zepatier, coformulated elbasvir (replication complex inhibitor) and grazoprevir (PI). Harvoni has simplified the treatment regimen to 1 pill a day and shortened the duration of treatment to as few as 8 weeks for some
patients.25
The option of an all-oral, INF-free treatment regimen and the prospect of freedom from the HCV could not come at a more opportune time, given the aging of baby boomers with chronic HCV infection and the high rates of HCV and HIV infections contracted in the 1970s and 1980s. An estimated one-third of those infected is expected to develop cirrhosis within 20 years.26
Cost of HCV Treatment
The U.S. has the highest health care costs in the world—consuming 17% of the nation’s gross domestic product.27,28 Health care costs also have been steadily increasing in U.S. prisons because of the expanding and aging incarcerated population. The Eighth Amendment provides inmates with the constitutional right to health care. The BOP’s overall expense of pharmaceuticals for HCV treatment has soared in recent years. It was kept below $2 million in fiscal years 2010 and 2011 but more than doubled the next 2 years, to more than $4 million in 2012 and 2013, and increased in 2014 to about $6 million. Hepititis C treatment accounted for 3% of the BOP pharmaceutical budget in 2011 and 7% in 2014.29 Increased HCV pharmaceutical expenses were attributable to the introduction of DAAs. Even so, the majority of inmates with chronic HCV infection remained untreated.
Compared with DAA PIs, sofosbuvir is a game changer. Its all-oral, INF-free regimen makes treatment of chronic HCV infection more effective and safer. However, its cost is prohibitive, even in rich countries: A 12-week course costs $84,000, and Harvoni (ledispasvir/ sofosbuvir) costs $94,000.30,31 A generic version of sofosbuvir is not expected until 2025.32 Many studies have been conducted on the cost-effectiveness of these newer DAAs, but the picture is unclear, as the results were sensitive to drug price, drug efficacy (SVR rates vary with genotype and patient profile), quality of life after successful treatment, and the willingness-to-pay threshold.30 Ironically, treatment cost could be the primary barrier to HCV eradication.
At USP Canaan, 137 inmates with chronic HCV infection potentially could have benefited from treatment. A majority (91 inmates) were infected with HCV genotype 1; of the other 46 inmates, 12 had genotype 2, 18 had genotype 3, 2 had genotype 4, and 14 lacked genotyping.
The all-oral, INF-free regimen obviates the need for weekly injection, and treatment duration is shorter. The AASLD/IDSA treatment guidelines recommend all-oral, INF-free treatment regimens for all patients with genotype 1. Typically, treatment lasts 12 or 24 weeks, depending on presence or absence of liver cirrhosis, among other considerations.16
Because of the high cost of treating all inmates with chronic HCV infection, the large number of inmates who are asymptomatic, and the potential decrease in medication costs after the introduction of generic versions, inmates are being prioritized for treatment based primarily on staging (presence or absence of liver disease). The rationale for using staging for prioritization is that patients with chronic HCV infection and no or minimal fibrosis at presentation seem to progress slowly, and treatment possibly can be delayed or withheld; whereas patients with significant fibrosis (septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period, so antiviral treatment becomes urgent.33
APRI: Biomarker for Liver Fibrosis
A liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although generally safe, it is costly. It is also subject to sampling error and examiner discrepancy, which lead to incorrect staging of fibrosis in 20% of patients.5,33 Alternatively, various biologic markers can be used to diagnose liver disease. The aspartate aminotransferase (AST) platelet ratio index (APRI) is a simple and useful index based on readily available laboratory results: AST level and platelet count. APRI correlated significantly with fibrosis stage in patients with chronic HCV infection.33
At USP Canaan, 16 (12%) of the 137 inmates with chronic HCV infection had an APRI higher than 1, and 5 of the 16 had an APRI higher than 2.
Conclusion
In coming years, treatment of chronic HCV infection will consume a more significant portion of the health care budget. As treatment becomes more efficacious and safer, the paradigm may shift from a stage-based strategy to a treat-all strategy. Possibly, more inmates will ask for treatment as the treatment burden lessens due to fewer treatment-associated AEs. However, despite the efficacy of HCV treatment, there is no reduction in the overall lifetime medical costs, because the offset in downstream direct medical costs (from successful treatment) is less than the cost of a cure.30
In the BOP, many challenges remain: HCV infection rates are expected to be high, treatment costs astronomical, resources limited, and treated patients may become reinfected if high-risk behavior continues. Patient education is, therefore, an important component of effective prevention and treatment strategies. The U.S. Preventive Services Task Force recommends HCV screening for all high-risk persons and a onetime screening for all baby boomers.34 Federal prisons offer HCV testing to all inmates with risk factors, when clinically indicated, or on
request.
All inmates with chronic HCV infection were being monitored for treatment prioritization, as some were at higher risk for complications or disease progression and required more urgent treatment.35 Ideally, all inmates should be treated, as incarcerated persons are at elevated risk for HCV transmission, and successful treatment would benefit public health by reducing infection rates in the community.16 However, resource constraints are a reality in health care, particularly among underserved populations, and this situation provides the rationale for screening, monitoring, and treatment prioritization. This step-by-step approach, which rests on sound clinical judgment, helps control the budget.
Click here for the digital edition.
1. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27.
4. Centers for Disease Control and Prevention, Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis Surveillance—United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Updated April 24, 2015. Accessed May 20, 2015.
5. Hepatitis C Online. Hepatitis C Online Website. http://www.hepatitisc.uw.edu. Accessed March 3, 2016.
6. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
7. Alter KJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
8. Liu G, Holmberg SD, Kamili S, Xu F. Racial disparities in the proportion of current, unresolved hepatitis C virus infections in the United States, 2003-2010. Dig Dis Sci. 2014;59(8):1950-1957.
9. World Health Organization. Hepatitis B [fact sheet 204]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs204/en. Updated July 2015. Accessed March 3, 2016.
10. World Health Organization. Hepatitis C [fact sheet 164]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs164/en. Updated July 2015. Accessed March 3, 2016.
11. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
12. Centers for Disease Control and Prevention. Hepatitis C and Incarceration. Publication No. 21-1306. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/PDFs/HepCIncarcerationFactSheet.pdf. October 2013. Accessed March 3, 2016.
13. Federal Bureau of Prisons. Inmate statistics: offenses. Federal Bureau of Prisons Website. http://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. Updated January 30, 2016. Accessed March 3, 2016.
14. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Updated January 8, 2016. Accessed March 4, 2016.
15. Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675.
16. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Website. http://hcvguidelines.org. Updated February 2016. Accessed March 4, 2016.
17. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
18. Kwo PY. Boceprevir: a novel nonstructural 3 (NS3) protease inhibitor for the treatment of chronic hepatitis C infection. Therap Adv Gastroenterol. 2012;5(3):179-188.
19. Stahmeyer JT, Rossol S, Krauth C. Outcomes, costs and cost-effectiveness of treating
hepatitis C with direct acting antivirals. J Comp Eff Res. 2015;4(3):267-277.
20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
21. Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
22. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
23. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
24. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
25. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and
sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
26. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530-537.
27. The Economist Don’t kill Obamacare. The Economist Website. http://www.economist.com/news/leaders/21645730-supreme-court-considers-whether-gut-obamacare-evidence-mounting-law. Published May 7, 2015. Accessed March 4, 2016.
28. The World Bank. Health expenditure, total (% of GDP). The World Bank Website. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Published 2015. Accessed March 4, 2016.
29. Federal Bureau of Prisons, Health Services Division. 2015 BOP National P&T Minutes. Federal Bureau of Prisons intranet website. http://sallyport.bop.gov/co/hsd/pharmacy/docs/BOP_National_P&T_Minutes/2015%20BOP%20National%20P&T%20Minutes_Final.pdf. Published August 13, 2014. Accessed November 9, 2015.
30. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406.
31. Sachs J. The drug that is bankrupting America. Huffington Post Website. http://www.huffingtonpost.com/jeffrey-sachs/the-drug-that-is-bankrupt_b_6692340
.html. Published February 16, 2015. Updated April 18, 2015. Accessed March 4, 2016.
32. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928-936.
33. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
34. U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force Website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening?ds=1&s=hepatitis c. Updated July 2015. Accessed March 4, 2016.
35. Federal Bureau of Prisons. Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection [clinical practice guidelines]. Federal Bureau of Prisons Website. http://www.bop.gov/resources/pdfs/hepatitis_c.pdf. Published July 2015. Accessed March 4, 2016.
The incidence of hepatitis C virus (HCV) infection increased markedly in the 1970s and 1980s. These increases were mainly attributable to blood transfusions and injection drug use.1,2 The blood supply was not screened for HCV before 1992 (now, HCV infection by blood transfusion is rare).2,3 Surveillance of the period 1992-2003 showed a substantial decrease in the incidence of acute hepatitis C cases, and the rate remained steady through 2010.2,3 Over the past 5 years, HCV has returned to national attention with a rising infection rate (2.5-fold increase during 2010-2013) and a rapid succession of FDA approvals of direct-acting antiviral agents (DAAs).4 Currently, the most prevalent mode of infection is injection drug use, accounting for > 50% of all cases of HCV infection and 84% of acute HCV infections.5
Baby boomers (people born between 1945 and 1965) account for three-fourths of the population currently living with chronic HCV infection resulting from past infection.6 Historically, rates of acute and chronic infection have been far higher for blacks than for whites and Hispanics.2,4,7,8 In 2004, that trend started to reverse, with the incidence in whites surpassing that in blacks.4 Recent reports have identified a new cohort of HCV-infected injection drug users (IDUs) who are young (aged ≤ 24 years) and white nonurban dwellers.5
HCV Infection Among High Risk Individuals
In the U.S., unlike in other parts of the world, HCV infection is more prevalent than hepatitis B virus (HBV) infection.4,9,10 According to the National Health and Nutrition Examination Survey (NHANES), about 2.7 million Americans have chronic HCV infection. However, NHANES surveys do not sample certain populations, including the incarcerated and the homeless, in whom infection rates are thought to be higher.11 The incarcerated, the largest institutionalized group, have the highest incidence: One in 3 is infected with HCV.12 This rate is attributable to high rates of injection drug use and other high-risk behaviors. Drug-related offenses account for 50% of federal prison incarceration.13 For IDUs, the HCV infection rate is as high as 70% to 90%. Despite widespread implementation of needle-exchange programs after the discovery of HIV in the 1980s, recent surveys have indicated that about one-third of 18- to 30-year-old active IDUs are infected with HCV.14
Penitentiary Inmates Infected With HCV
A 2015 search of the Federal Bureau of Prisons (BOP) electronic medical records at the U.S. Penitentiary Canaan (USP Canaan) found that out of a population of about 1,600 inmates, 182 (11%) had tested positive for HCV antibodies (anti-HCV). This percentage likely is an underestimation, because HCV testing is not mandatory, and many (45%-85%) of the infected are unaware of their HCV infection status.2 Most of the infected remain chronically infected and are not being treated.
Prevalence of HCV infection commonly refers to chronic HCV infection. The diagnosis of chronic HCV infection is established by presence of HCV RNA on polymerase chain reaction assays. Of the 182 inmates who tested positive for anti-HCV, 45 had their cases resolved (undetectable HCV RNA), 34 spontaneously, and the other 11 after treatment, primarily with peginterferon and ribavirin (pegINF/RBV) dual therapies. The remaining 137 who tested positive remained chronically infected. This chronically infected group represented 9% of the population of 1,600 inmates. Although the infection rate is significantly higher than that in the general population (1% incidence), the inmates’ true rate of infection in all probability is much higher.11
Earlier NHANES data showed HCV more prevalent in minorities, particularly blacks, compared with whites.2,7,8 At USP Canaan, however, the incidence of chronic HCV infection was 21% in whites (mean age, 42 years), 4% in blacks (mean age, 51 years), and 7% in Hispanics (mean age, 39 years). The lower rates in blacks and Hispanics could have resulted from a lack of awareness about getting tested or from having fewer opportunities to obtain medical care in the community before incarceration (the infection can remain asymptomatic for several decades).
HCV genotype 1 is the most common HCV genotype in the U.S.5,15 At USP Canaan, genotype 1 accounted for 56% of the cases of chronic HCV infection in whites, 90% in blacks, and 79% in Hispanics. The majority genotype was subtype 1a.
Of the 137 inmates with HCV co-infections, 8 (6%) had HIV/HCV co-infection, and 4 (3%) had HBV/HCV co-infection. Also, 7 (5%) were diabetic. According to the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidelines, patients with comorbidities are a high priority for treatment, as there is a high risk for complications, with liver fibrosis progressing more rapidly.16
Changing Landscape of HCV Treatment
Treatments for chronic HCV infection have never been more promising. There is the prospect of a cure with the new DAAs. These new medications are active against HCV and interfere with viral enzymes and other proteins essential for viral replication. Until recently, the mainstay of treatment for chronic HCV infection was pegINF/RBV. However, INF-based dual therapies were associated with high rates of adverse effects (AEs) and treatment discontinuation. In 2011, release of the protease inhibitors (PIs) boceprevir and telaprevir improved sustained virologic response (SVR) rates for treatment-naïve patients with genotype 1 from about 50% (pegINF/RBV dual therapies) to 70% to 75% (PI in combination with pegINF/RBV triple therapies). However, first-generation DAAs were INF-based, and their dosing was cumbersome.15,17-19
Starting with the 2013 approval of simeprevir (second-wave PI) and sofosbuvir (polymerase inhibitor), most patients’ SVR rates improved to 75% to 90%.20,21 Sustained virologic response rates were boosted to > 95% after the 2014 approval of Harvoni, coformulated ledispasvir (replication complex inhibitor) and sofosbuvir, and Viekira Pak, a combination of ombitasvir (replication complex inhibitor), paritaprevir (PI), and ritonavir (inhibitor of CYP3A4 enzyme) co-packaged with dasabuvir (polymerase inhibitor).22-24 In 2015, daclatasvir (replication complex inhibitor) was approved, followed in 2016 by Zepatier, coformulated elbasvir (replication complex inhibitor) and grazoprevir (PI). Harvoni has simplified the treatment regimen to 1 pill a day and shortened the duration of treatment to as few as 8 weeks for some
patients.25
The option of an all-oral, INF-free treatment regimen and the prospect of freedom from the HCV could not come at a more opportune time, given the aging of baby boomers with chronic HCV infection and the high rates of HCV and HIV infections contracted in the 1970s and 1980s. An estimated one-third of those infected is expected to develop cirrhosis within 20 years.26
Cost of HCV Treatment
The U.S. has the highest health care costs in the world—consuming 17% of the nation’s gross domestic product.27,28 Health care costs also have been steadily increasing in U.S. prisons because of the expanding and aging incarcerated population. The Eighth Amendment provides inmates with the constitutional right to health care. The BOP’s overall expense of pharmaceuticals for HCV treatment has soared in recent years. It was kept below $2 million in fiscal years 2010 and 2011 but more than doubled the next 2 years, to more than $4 million in 2012 and 2013, and increased in 2014 to about $6 million. Hepititis C treatment accounted for 3% of the BOP pharmaceutical budget in 2011 and 7% in 2014.29 Increased HCV pharmaceutical expenses were attributable to the introduction of DAAs. Even so, the majority of inmates with chronic HCV infection remained untreated.
Compared with DAA PIs, sofosbuvir is a game changer. Its all-oral, INF-free regimen makes treatment of chronic HCV infection more effective and safer. However, its cost is prohibitive, even in rich countries: A 12-week course costs $84,000, and Harvoni (ledispasvir/ sofosbuvir) costs $94,000.30,31 A generic version of sofosbuvir is not expected until 2025.32 Many studies have been conducted on the cost-effectiveness of these newer DAAs, but the picture is unclear, as the results were sensitive to drug price, drug efficacy (SVR rates vary with genotype and patient profile), quality of life after successful treatment, and the willingness-to-pay threshold.30 Ironically, treatment cost could be the primary barrier to HCV eradication.
At USP Canaan, 137 inmates with chronic HCV infection potentially could have benefited from treatment. A majority (91 inmates) were infected with HCV genotype 1; of the other 46 inmates, 12 had genotype 2, 18 had genotype 3, 2 had genotype 4, and 14 lacked genotyping.
The all-oral, INF-free regimen obviates the need for weekly injection, and treatment duration is shorter. The AASLD/IDSA treatment guidelines recommend all-oral, INF-free treatment regimens for all patients with genotype 1. Typically, treatment lasts 12 or 24 weeks, depending on presence or absence of liver cirrhosis, among other considerations.16
Because of the high cost of treating all inmates with chronic HCV infection, the large number of inmates who are asymptomatic, and the potential decrease in medication costs after the introduction of generic versions, inmates are being prioritized for treatment based primarily on staging (presence or absence of liver disease). The rationale for using staging for prioritization is that patients with chronic HCV infection and no or minimal fibrosis at presentation seem to progress slowly, and treatment possibly can be delayed or withheld; whereas patients with significant fibrosis (septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period, so antiviral treatment becomes urgent.33
APRI: Biomarker for Liver Fibrosis
A liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although generally safe, it is costly. It is also subject to sampling error and examiner discrepancy, which lead to incorrect staging of fibrosis in 20% of patients.5,33 Alternatively, various biologic markers can be used to diagnose liver disease. The aspartate aminotransferase (AST) platelet ratio index (APRI) is a simple and useful index based on readily available laboratory results: AST level and platelet count. APRI correlated significantly with fibrosis stage in patients with chronic HCV infection.33
At USP Canaan, 16 (12%) of the 137 inmates with chronic HCV infection had an APRI higher than 1, and 5 of the 16 had an APRI higher than 2.
Conclusion
In coming years, treatment of chronic HCV infection will consume a more significant portion of the health care budget. As treatment becomes more efficacious and safer, the paradigm may shift from a stage-based strategy to a treat-all strategy. Possibly, more inmates will ask for treatment as the treatment burden lessens due to fewer treatment-associated AEs. However, despite the efficacy of HCV treatment, there is no reduction in the overall lifetime medical costs, because the offset in downstream direct medical costs (from successful treatment) is less than the cost of a cure.30
In the BOP, many challenges remain: HCV infection rates are expected to be high, treatment costs astronomical, resources limited, and treated patients may become reinfected if high-risk behavior continues. Patient education is, therefore, an important component of effective prevention and treatment strategies. The U.S. Preventive Services Task Force recommends HCV screening for all high-risk persons and a onetime screening for all baby boomers.34 Federal prisons offer HCV testing to all inmates with risk factors, when clinically indicated, or on
request.
All inmates with chronic HCV infection were being monitored for treatment prioritization, as some were at higher risk for complications or disease progression and required more urgent treatment.35 Ideally, all inmates should be treated, as incarcerated persons are at elevated risk for HCV transmission, and successful treatment would benefit public health by reducing infection rates in the community.16 However, resource constraints are a reality in health care, particularly among underserved populations, and this situation provides the rationale for screening, monitoring, and treatment prioritization. This step-by-step approach, which rests on sound clinical judgment, helps control the budget.
Click here for the digital edition.
The incidence of hepatitis C virus (HCV) infection increased markedly in the 1970s and 1980s. These increases were mainly attributable to blood transfusions and injection drug use.1,2 The blood supply was not screened for HCV before 1992 (now, HCV infection by blood transfusion is rare).2,3 Surveillance of the period 1992-2003 showed a substantial decrease in the incidence of acute hepatitis C cases, and the rate remained steady through 2010.2,3 Over the past 5 years, HCV has returned to national attention with a rising infection rate (2.5-fold increase during 2010-2013) and a rapid succession of FDA approvals of direct-acting antiviral agents (DAAs).4 Currently, the most prevalent mode of infection is injection drug use, accounting for > 50% of all cases of HCV infection and 84% of acute HCV infections.5
Baby boomers (people born between 1945 and 1965) account for three-fourths of the population currently living with chronic HCV infection resulting from past infection.6 Historically, rates of acute and chronic infection have been far higher for blacks than for whites and Hispanics.2,4,7,8 In 2004, that trend started to reverse, with the incidence in whites surpassing that in blacks.4 Recent reports have identified a new cohort of HCV-infected injection drug users (IDUs) who are young (aged ≤ 24 years) and white nonurban dwellers.5
HCV Infection Among High Risk Individuals
In the U.S., unlike in other parts of the world, HCV infection is more prevalent than hepatitis B virus (HBV) infection.4,9,10 According to the National Health and Nutrition Examination Survey (NHANES), about 2.7 million Americans have chronic HCV infection. However, NHANES surveys do not sample certain populations, including the incarcerated and the homeless, in whom infection rates are thought to be higher.11 The incarcerated, the largest institutionalized group, have the highest incidence: One in 3 is infected with HCV.12 This rate is attributable to high rates of injection drug use and other high-risk behaviors. Drug-related offenses account for 50% of federal prison incarceration.13 For IDUs, the HCV infection rate is as high as 70% to 90%. Despite widespread implementation of needle-exchange programs after the discovery of HIV in the 1980s, recent surveys have indicated that about one-third of 18- to 30-year-old active IDUs are infected with HCV.14
Penitentiary Inmates Infected With HCV
A 2015 search of the Federal Bureau of Prisons (BOP) electronic medical records at the U.S. Penitentiary Canaan (USP Canaan) found that out of a population of about 1,600 inmates, 182 (11%) had tested positive for HCV antibodies (anti-HCV). This percentage likely is an underestimation, because HCV testing is not mandatory, and many (45%-85%) of the infected are unaware of their HCV infection status.2 Most of the infected remain chronically infected and are not being treated.
Prevalence of HCV infection commonly refers to chronic HCV infection. The diagnosis of chronic HCV infection is established by presence of HCV RNA on polymerase chain reaction assays. Of the 182 inmates who tested positive for anti-HCV, 45 had their cases resolved (undetectable HCV RNA), 34 spontaneously, and the other 11 after treatment, primarily with peginterferon and ribavirin (pegINF/RBV) dual therapies. The remaining 137 who tested positive remained chronically infected. This chronically infected group represented 9% of the population of 1,600 inmates. Although the infection rate is significantly higher than that in the general population (1% incidence), the inmates’ true rate of infection in all probability is much higher.11
Earlier NHANES data showed HCV more prevalent in minorities, particularly blacks, compared with whites.2,7,8 At USP Canaan, however, the incidence of chronic HCV infection was 21% in whites (mean age, 42 years), 4% in blacks (mean age, 51 years), and 7% in Hispanics (mean age, 39 years). The lower rates in blacks and Hispanics could have resulted from a lack of awareness about getting tested or from having fewer opportunities to obtain medical care in the community before incarceration (the infection can remain asymptomatic for several decades).
HCV genotype 1 is the most common HCV genotype in the U.S.5,15 At USP Canaan, genotype 1 accounted for 56% of the cases of chronic HCV infection in whites, 90% in blacks, and 79% in Hispanics. The majority genotype was subtype 1a.
Of the 137 inmates with HCV co-infections, 8 (6%) had HIV/HCV co-infection, and 4 (3%) had HBV/HCV co-infection. Also, 7 (5%) were diabetic. According to the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidelines, patients with comorbidities are a high priority for treatment, as there is a high risk for complications, with liver fibrosis progressing more rapidly.16
Changing Landscape of HCV Treatment
Treatments for chronic HCV infection have never been more promising. There is the prospect of a cure with the new DAAs. These new medications are active against HCV and interfere with viral enzymes and other proteins essential for viral replication. Until recently, the mainstay of treatment for chronic HCV infection was pegINF/RBV. However, INF-based dual therapies were associated with high rates of adverse effects (AEs) and treatment discontinuation. In 2011, release of the protease inhibitors (PIs) boceprevir and telaprevir improved sustained virologic response (SVR) rates for treatment-naïve patients with genotype 1 from about 50% (pegINF/RBV dual therapies) to 70% to 75% (PI in combination with pegINF/RBV triple therapies). However, first-generation DAAs were INF-based, and their dosing was cumbersome.15,17-19
Starting with the 2013 approval of simeprevir (second-wave PI) and sofosbuvir (polymerase inhibitor), most patients’ SVR rates improved to 75% to 90%.20,21 Sustained virologic response rates were boosted to > 95% after the 2014 approval of Harvoni, coformulated ledispasvir (replication complex inhibitor) and sofosbuvir, and Viekira Pak, a combination of ombitasvir (replication complex inhibitor), paritaprevir (PI), and ritonavir (inhibitor of CYP3A4 enzyme) co-packaged with dasabuvir (polymerase inhibitor).22-24 In 2015, daclatasvir (replication complex inhibitor) was approved, followed in 2016 by Zepatier, coformulated elbasvir (replication complex inhibitor) and grazoprevir (PI). Harvoni has simplified the treatment regimen to 1 pill a day and shortened the duration of treatment to as few as 8 weeks for some
patients.25
The option of an all-oral, INF-free treatment regimen and the prospect of freedom from the HCV could not come at a more opportune time, given the aging of baby boomers with chronic HCV infection and the high rates of HCV and HIV infections contracted in the 1970s and 1980s. An estimated one-third of those infected is expected to develop cirrhosis within 20 years.26
Cost of HCV Treatment
The U.S. has the highest health care costs in the world—consuming 17% of the nation’s gross domestic product.27,28 Health care costs also have been steadily increasing in U.S. prisons because of the expanding and aging incarcerated population. The Eighth Amendment provides inmates with the constitutional right to health care. The BOP’s overall expense of pharmaceuticals for HCV treatment has soared in recent years. It was kept below $2 million in fiscal years 2010 and 2011 but more than doubled the next 2 years, to more than $4 million in 2012 and 2013, and increased in 2014 to about $6 million. Hepititis C treatment accounted for 3% of the BOP pharmaceutical budget in 2011 and 7% in 2014.29 Increased HCV pharmaceutical expenses were attributable to the introduction of DAAs. Even so, the majority of inmates with chronic HCV infection remained untreated.
Compared with DAA PIs, sofosbuvir is a game changer. Its all-oral, INF-free regimen makes treatment of chronic HCV infection more effective and safer. However, its cost is prohibitive, even in rich countries: A 12-week course costs $84,000, and Harvoni (ledispasvir/ sofosbuvir) costs $94,000.30,31 A generic version of sofosbuvir is not expected until 2025.32 Many studies have been conducted on the cost-effectiveness of these newer DAAs, but the picture is unclear, as the results were sensitive to drug price, drug efficacy (SVR rates vary with genotype and patient profile), quality of life after successful treatment, and the willingness-to-pay threshold.30 Ironically, treatment cost could be the primary barrier to HCV eradication.
At USP Canaan, 137 inmates with chronic HCV infection potentially could have benefited from treatment. A majority (91 inmates) were infected with HCV genotype 1; of the other 46 inmates, 12 had genotype 2, 18 had genotype 3, 2 had genotype 4, and 14 lacked genotyping.
The all-oral, INF-free regimen obviates the need for weekly injection, and treatment duration is shorter. The AASLD/IDSA treatment guidelines recommend all-oral, INF-free treatment regimens for all patients with genotype 1. Typically, treatment lasts 12 or 24 weeks, depending on presence or absence of liver cirrhosis, among other considerations.16
Because of the high cost of treating all inmates with chronic HCV infection, the large number of inmates who are asymptomatic, and the potential decrease in medication costs after the introduction of generic versions, inmates are being prioritized for treatment based primarily on staging (presence or absence of liver disease). The rationale for using staging for prioritization is that patients with chronic HCV infection and no or minimal fibrosis at presentation seem to progress slowly, and treatment possibly can be delayed or withheld; whereas patients with significant fibrosis (septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period, so antiviral treatment becomes urgent.33
APRI: Biomarker for Liver Fibrosis
A liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although generally safe, it is costly. It is also subject to sampling error and examiner discrepancy, which lead to incorrect staging of fibrosis in 20% of patients.5,33 Alternatively, various biologic markers can be used to diagnose liver disease. The aspartate aminotransferase (AST) platelet ratio index (APRI) is a simple and useful index based on readily available laboratory results: AST level and platelet count. APRI correlated significantly with fibrosis stage in patients with chronic HCV infection.33
At USP Canaan, 16 (12%) of the 137 inmates with chronic HCV infection had an APRI higher than 1, and 5 of the 16 had an APRI higher than 2.
Conclusion
In coming years, treatment of chronic HCV infection will consume a more significant portion of the health care budget. As treatment becomes more efficacious and safer, the paradigm may shift from a stage-based strategy to a treat-all strategy. Possibly, more inmates will ask for treatment as the treatment burden lessens due to fewer treatment-associated AEs. However, despite the efficacy of HCV treatment, there is no reduction in the overall lifetime medical costs, because the offset in downstream direct medical costs (from successful treatment) is less than the cost of a cure.30
In the BOP, many challenges remain: HCV infection rates are expected to be high, treatment costs astronomical, resources limited, and treated patients may become reinfected if high-risk behavior continues. Patient education is, therefore, an important component of effective prevention and treatment strategies. The U.S. Preventive Services Task Force recommends HCV screening for all high-risk persons and a onetime screening for all baby boomers.34 Federal prisons offer HCV testing to all inmates with risk factors, when clinically indicated, or on
request.
All inmates with chronic HCV infection were being monitored for treatment prioritization, as some were at higher risk for complications or disease progression and required more urgent treatment.35 Ideally, all inmates should be treated, as incarcerated persons are at elevated risk for HCV transmission, and successful treatment would benefit public health by reducing infection rates in the community.16 However, resource constraints are a reality in health care, particularly among underserved populations, and this situation provides the rationale for screening, monitoring, and treatment prioritization. This step-by-step approach, which rests on sound clinical judgment, helps control the budget.
Click here for the digital edition.
1. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27.
4. Centers for Disease Control and Prevention, Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis Surveillance—United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Updated April 24, 2015. Accessed May 20, 2015.
5. Hepatitis C Online. Hepatitis C Online Website. http://www.hepatitisc.uw.edu. Accessed March 3, 2016.
6. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
7. Alter KJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
8. Liu G, Holmberg SD, Kamili S, Xu F. Racial disparities in the proportion of current, unresolved hepatitis C virus infections in the United States, 2003-2010. Dig Dis Sci. 2014;59(8):1950-1957.
9. World Health Organization. Hepatitis B [fact sheet 204]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs204/en. Updated July 2015. Accessed March 3, 2016.
10. World Health Organization. Hepatitis C [fact sheet 164]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs164/en. Updated July 2015. Accessed March 3, 2016.
11. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
12. Centers for Disease Control and Prevention. Hepatitis C and Incarceration. Publication No. 21-1306. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/PDFs/HepCIncarcerationFactSheet.pdf. October 2013. Accessed March 3, 2016.
13. Federal Bureau of Prisons. Inmate statistics: offenses. Federal Bureau of Prisons Website. http://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. Updated January 30, 2016. Accessed March 3, 2016.
14. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Updated January 8, 2016. Accessed March 4, 2016.
15. Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675.
16. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Website. http://hcvguidelines.org. Updated February 2016. Accessed March 4, 2016.
17. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
18. Kwo PY. Boceprevir: a novel nonstructural 3 (NS3) protease inhibitor for the treatment of chronic hepatitis C infection. Therap Adv Gastroenterol. 2012;5(3):179-188.
19. Stahmeyer JT, Rossol S, Krauth C. Outcomes, costs and cost-effectiveness of treating
hepatitis C with direct acting antivirals. J Comp Eff Res. 2015;4(3):267-277.
20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
21. Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
22. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
23. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
24. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
25. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and
sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
26. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530-537.
27. The Economist Don’t kill Obamacare. The Economist Website. http://www.economist.com/news/leaders/21645730-supreme-court-considers-whether-gut-obamacare-evidence-mounting-law. Published May 7, 2015. Accessed March 4, 2016.
28. The World Bank. Health expenditure, total (% of GDP). The World Bank Website. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Published 2015. Accessed March 4, 2016.
29. Federal Bureau of Prisons, Health Services Division. 2015 BOP National P&T Minutes. Federal Bureau of Prisons intranet website. http://sallyport.bop.gov/co/hsd/pharmacy/docs/BOP_National_P&T_Minutes/2015%20BOP%20National%20P&T%20Minutes_Final.pdf. Published August 13, 2014. Accessed November 9, 2015.
30. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406.
31. Sachs J. The drug that is bankrupting America. Huffington Post Website. http://www.huffingtonpost.com/jeffrey-sachs/the-drug-that-is-bankrupt_b_6692340
.html. Published February 16, 2015. Updated April 18, 2015. Accessed March 4, 2016.
32. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928-936.
33. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
34. U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force Website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening?ds=1&s=hepatitis c. Updated July 2015. Accessed March 4, 2016.
35. Federal Bureau of Prisons. Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection [clinical practice guidelines]. Federal Bureau of Prisons Website. http://www.bop.gov/resources/pdfs/hepatitis_c.pdf. Published July 2015. Accessed March 4, 2016.
1. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27.
4. Centers for Disease Control and Prevention, Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis Surveillance—United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Updated April 24, 2015. Accessed May 20, 2015.
5. Hepatitis C Online. Hepatitis C Online Website. http://www.hepatitisc.uw.edu. Accessed March 3, 2016.
6. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
7. Alter KJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
8. Liu G, Holmberg SD, Kamili S, Xu F. Racial disparities in the proportion of current, unresolved hepatitis C virus infections in the United States, 2003-2010. Dig Dis Sci. 2014;59(8):1950-1957.
9. World Health Organization. Hepatitis B [fact sheet 204]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs204/en. Updated July 2015. Accessed March 3, 2016.
10. World Health Organization. Hepatitis C [fact sheet 164]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs164/en. Updated July 2015. Accessed March 3, 2016.
11. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
12. Centers for Disease Control and Prevention. Hepatitis C and Incarceration. Publication No. 21-1306. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/PDFs/HepCIncarcerationFactSheet.pdf. October 2013. Accessed March 3, 2016.
13. Federal Bureau of Prisons. Inmate statistics: offenses. Federal Bureau of Prisons Website. http://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. Updated January 30, 2016. Accessed March 3, 2016.
14. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Updated January 8, 2016. Accessed March 4, 2016.
15. Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675.
16. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Website. http://hcvguidelines.org. Updated February 2016. Accessed March 4, 2016.
17. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
18. Kwo PY. Boceprevir: a novel nonstructural 3 (NS3) protease inhibitor for the treatment of chronic hepatitis C infection. Therap Adv Gastroenterol. 2012;5(3):179-188.
19. Stahmeyer JT, Rossol S, Krauth C. Outcomes, costs and cost-effectiveness of treating
hepatitis C with direct acting antivirals. J Comp Eff Res. 2015;4(3):267-277.
20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
21. Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
22. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
23. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
24. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
25. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and
sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
26. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530-537.
27. The Economist Don’t kill Obamacare. The Economist Website. http://www.economist.com/news/leaders/21645730-supreme-court-considers-whether-gut-obamacare-evidence-mounting-law. Published May 7, 2015. Accessed March 4, 2016.
28. The World Bank. Health expenditure, total (% of GDP). The World Bank Website. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Published 2015. Accessed March 4, 2016.
29. Federal Bureau of Prisons, Health Services Division. 2015 BOP National P&T Minutes. Federal Bureau of Prisons intranet website. http://sallyport.bop.gov/co/hsd/pharmacy/docs/BOP_National_P&T_Minutes/2015%20BOP%20National%20P&T%20Minutes_Final.pdf. Published August 13, 2014. Accessed November 9, 2015.
30. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406.
31. Sachs J. The drug that is bankrupting America. Huffington Post Website. http://www.huffingtonpost.com/jeffrey-sachs/the-drug-that-is-bankrupt_b_6692340
.html. Published February 16, 2015. Updated April 18, 2015. Accessed March 4, 2016.
32. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928-936.
33. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
34. U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force Website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening?ds=1&s=hepatitis c. Updated July 2015. Accessed March 4, 2016.
35. Federal Bureau of Prisons. Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection [clinical practice guidelines]. Federal Bureau of Prisons Website. http://www.bop.gov/resources/pdfs/hepatitis_c.pdf. Published July 2015. Accessed March 4, 2016.