User login
Bilateral thigh and knee pain • leg weakness • no history of trauma • Dx?
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
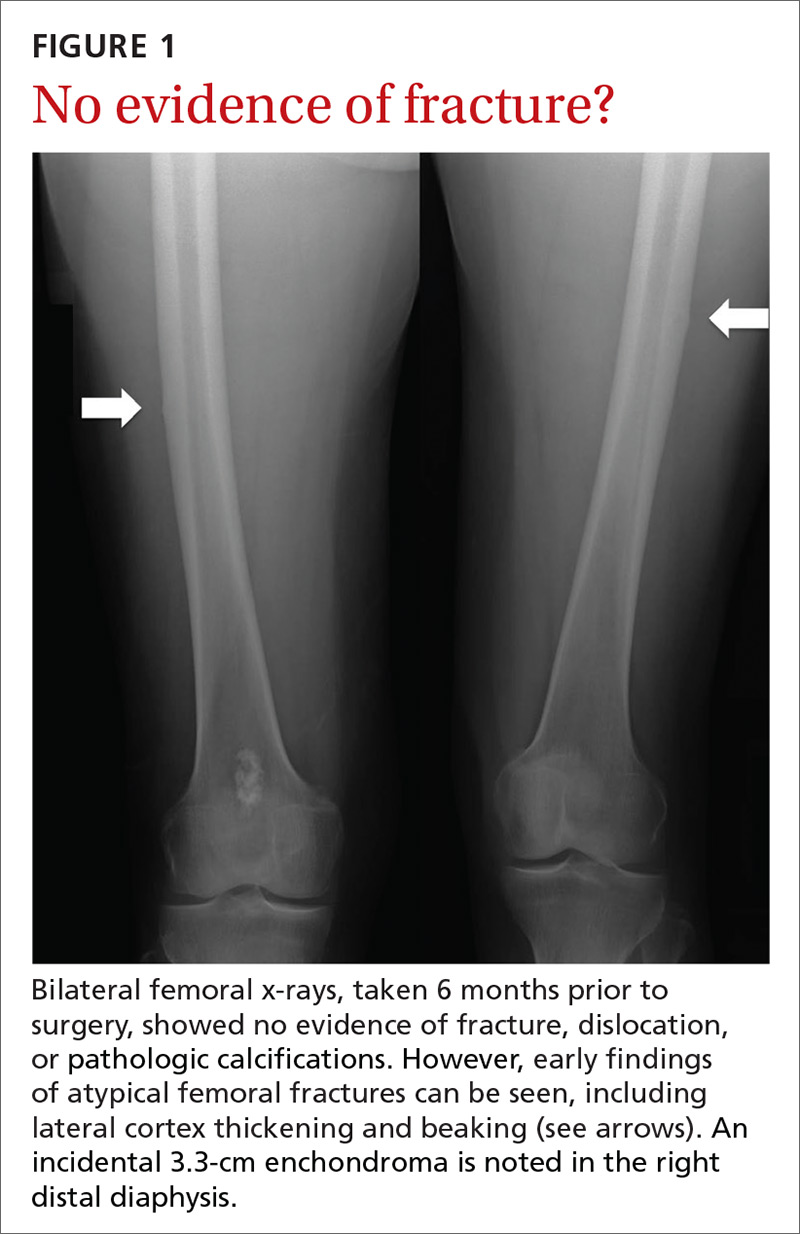
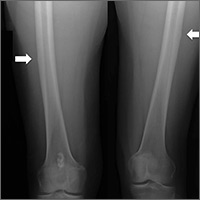
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
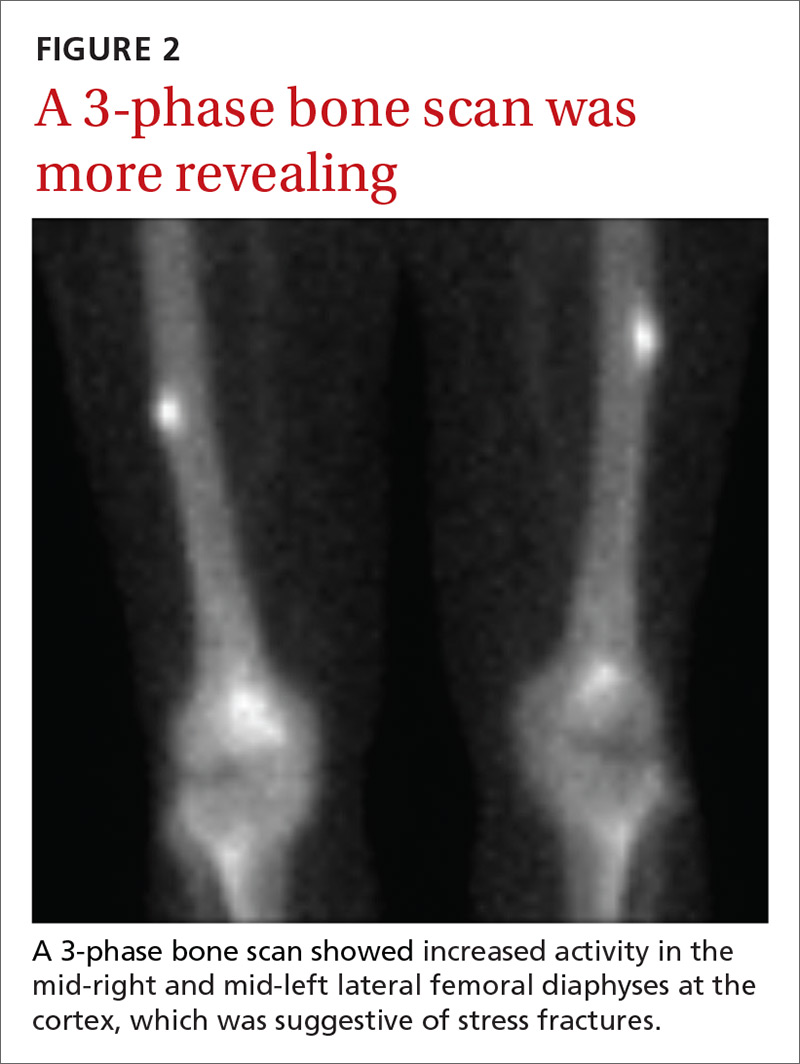
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
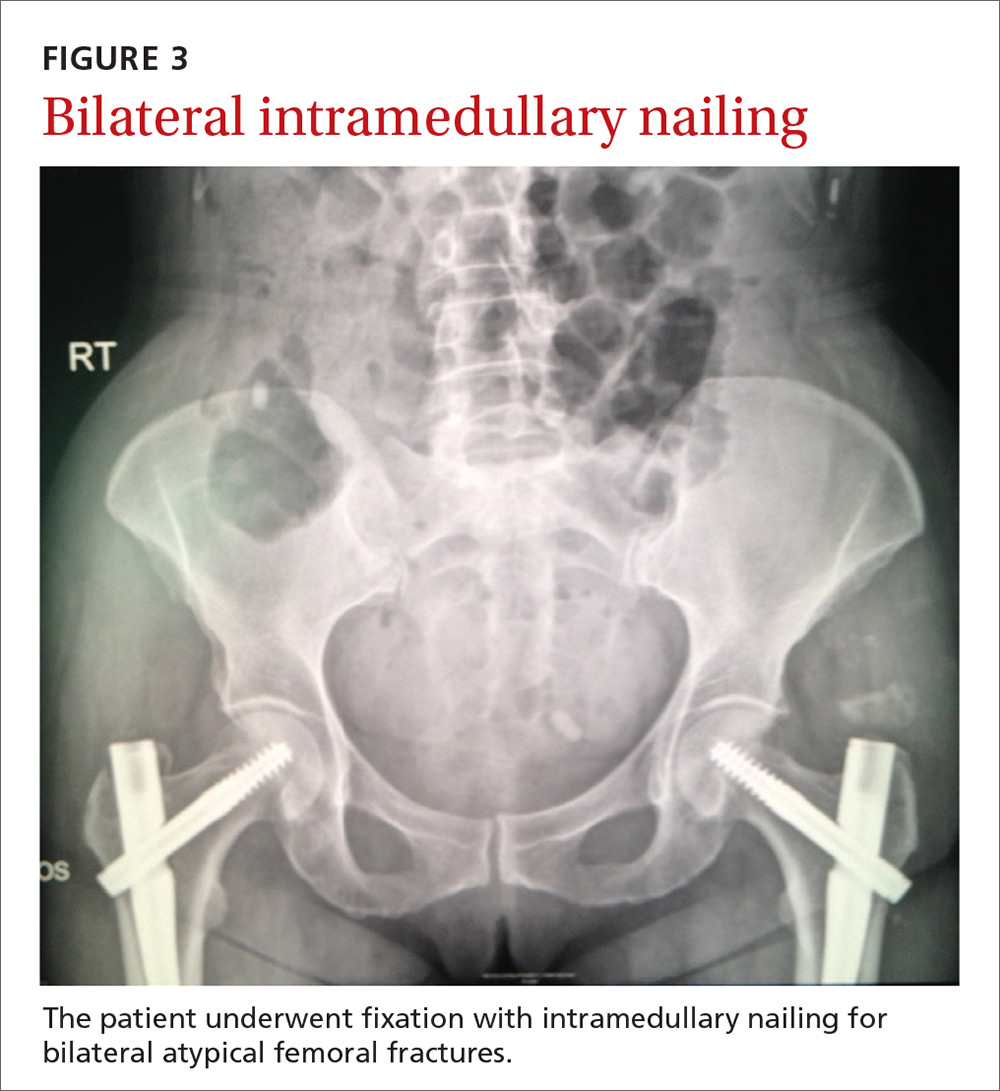
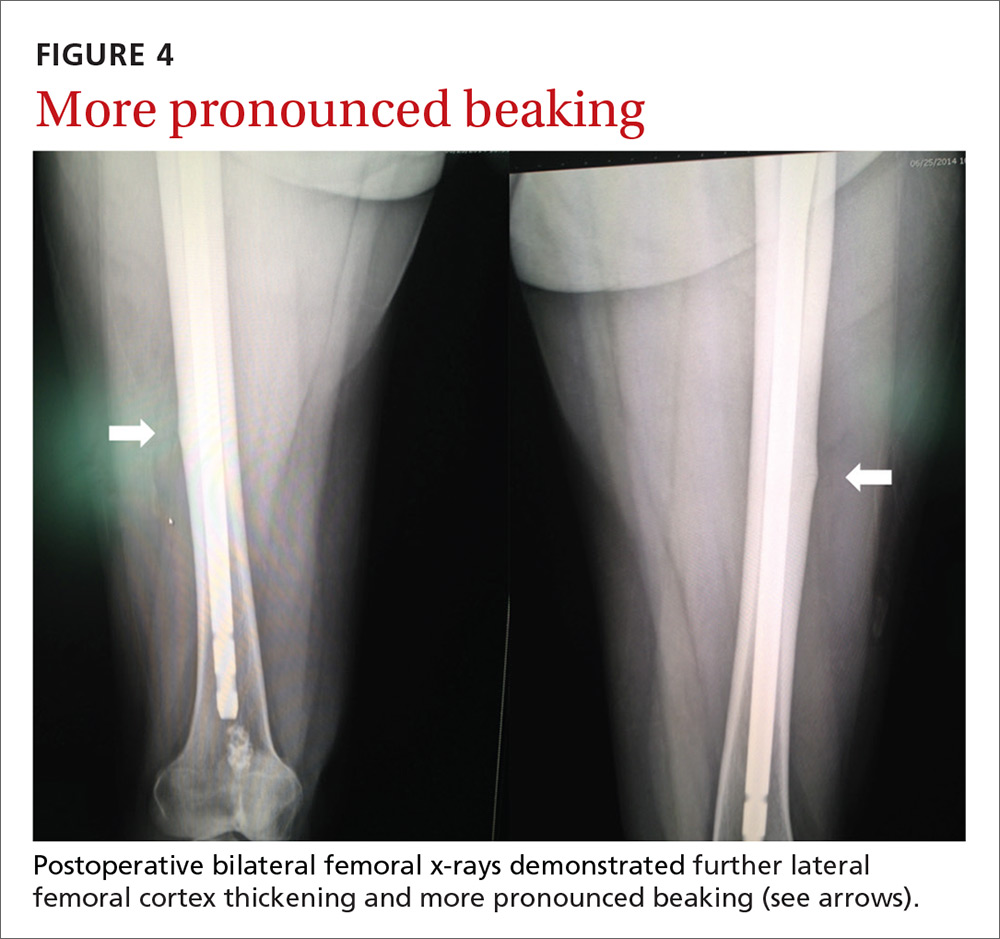
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
Transformation of Benign Giant Cell Tumor of Bone Into Epithelioid Angiosarcoma
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
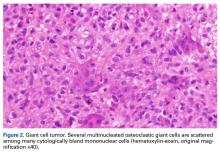
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
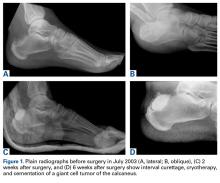
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
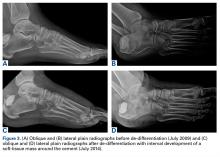
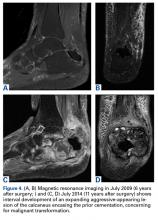
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
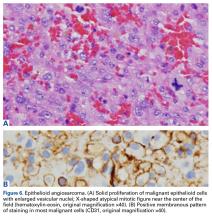
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
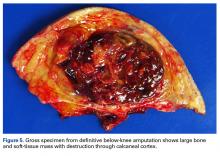
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.