Article
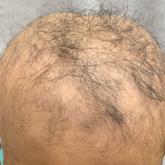
Adjuvant Scalp Rolling for Patients With Refractory Alopecia Areata
- Author:
- Jordan Phillipps, BS
- Bruin Pollard, MD
- Caroline Mann, MD
Dermatologists should be aware of scalp rolling as a safe, affordable, and potentially effective adjuvant to conventional therapy for alopecia...
Article
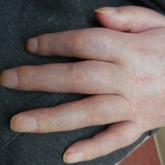
Complex Regional Pain Syndrome Type II After a Brachial Plexus and C6 Nerve Root Injury
- Author:
- Ji Won Ahn, MD
- Caroline Mann, MD
Complex regional pain syndrome (CRPS) is a neuropathic disorder of the extremities characterized by pain, a variety of autonomic and motor...
