Article
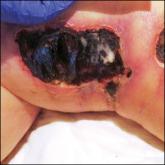
Nonuremic Calciphylaxis Triggered by Rapid Weight Loss and Hypotension
- Author:
- Logan J. Kolb, DO
- Carolyn Ellis, DO
- Ann Lafond, MD
Calciphylaxis most commonly is seen in patients with renal disease requiring dialysis, but it also may be triggered by nonuremic causes in...
Article
Angioimmunoblastic T-Cell Lymphoma Mimicking Diffuse Large B-Cell Lymphoma
- Author:
- Carolyn Ellis, DO
- James Ramirez, MD
- Ann Ammond LaFond, MD
Angioimmunoblastic T-cell lymphoma (AITL) is an aggressive form of peripheral T-cell lymphoma that is characterized by...
