User login
Improved Coordination of Care for Patients with Abnormalities on Chest Imaging: The Rapid Access Chest and Lung Assessment Program
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
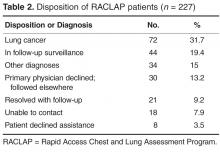
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
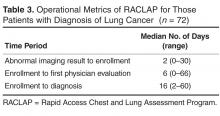
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, Meisenberg@AAHS.org.
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, Meisenberg@AAHS.org.
Financial disclosures: None.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, Meisenberg@AAHS.org.
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.