User login
Tackling midurethral sling complications
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Breaking down midurethral sling approaches
It has been nearly 20 years since the first minimally invasive midurethral sling was introduced. This development was followed 5 years later with the introduction of the transobturator midurethral sling. The advent of both ambulatory techniques has essentially changed the landscape in the surgical treatment of stress urinary incontinence; midurethral slings are certainly considered the procedure of choice for many women.
The midurethral sling has continued to evolve. Not only does the surgeon have the choice of placing a retropubic midurethral sling (bottom to top or top to bottom) and the transobturator midurethral sling (inside-out or outside-in), but, as of late, single incision midurethral slings (mini-slings or mini-tape) as well.
In the previous Master Class on urinary incontinence, Dr. Eric Sokol discussed issues of sling selection and the evidence in favor of various types of retropubic and transobturator slings. This month, we’ll discuss the technique behind these two approaches. I have elicited the assistance of Dr. Sokol, as well as Dr. Charles Rardin.
Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals.
Dr. Rardin is the director of the robotic surgery program at Women & Infants Hospital of Rhode Island, Providence, a surgeon in the division of urogynecology and reconstructive pelvic surgery, and is the director of the hospital’s fellowship in urogynecology and reconstructive pelvic surgery. He is also an assistant professor at Brown University, also in Providence. He has published numerous articles in peer-reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
It has been nearly 20 years since the first minimally invasive midurethral sling was introduced. This development was followed 5 years later with the introduction of the transobturator midurethral sling. The advent of both ambulatory techniques has essentially changed the landscape in the surgical treatment of stress urinary incontinence; midurethral slings are certainly considered the procedure of choice for many women.
The midurethral sling has continued to evolve. Not only does the surgeon have the choice of placing a retropubic midurethral sling (bottom to top or top to bottom) and the transobturator midurethral sling (inside-out or outside-in), but, as of late, single incision midurethral slings (mini-slings or mini-tape) as well.
In the previous Master Class on urinary incontinence, Dr. Eric Sokol discussed issues of sling selection and the evidence in favor of various types of retropubic and transobturator slings. This month, we’ll discuss the technique behind these two approaches. I have elicited the assistance of Dr. Sokol, as well as Dr. Charles Rardin.
Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals.
Dr. Rardin is the director of the robotic surgery program at Women & Infants Hospital of Rhode Island, Providence, a surgeon in the division of urogynecology and reconstructive pelvic surgery, and is the director of the hospital’s fellowship in urogynecology and reconstructive pelvic surgery. He is also an assistant professor at Brown University, also in Providence. He has published numerous articles in peer-reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
It has been nearly 20 years since the first minimally invasive midurethral sling was introduced. This development was followed 5 years later with the introduction of the transobturator midurethral sling. The advent of both ambulatory techniques has essentially changed the landscape in the surgical treatment of stress urinary incontinence; midurethral slings are certainly considered the procedure of choice for many women.
The midurethral sling has continued to evolve. Not only does the surgeon have the choice of placing a retropubic midurethral sling (bottom to top or top to bottom) and the transobturator midurethral sling (inside-out or outside-in), but, as of late, single incision midurethral slings (mini-slings or mini-tape) as well.
In the previous Master Class on urinary incontinence, Dr. Eric Sokol discussed issues of sling selection and the evidence in favor of various types of retropubic and transobturator slings. This month, we’ll discuss the technique behind these two approaches. I have elicited the assistance of Dr. Sokol, as well as Dr. Charles Rardin.
Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals.
Dr. Rardin is the director of the robotic surgery program at Women & Infants Hospital of Rhode Island, Providence, a surgeon in the division of urogynecology and reconstructive pelvic surgery, and is the director of the hospital’s fellowship in urogynecology and reconstructive pelvic surgery. He is also an assistant professor at Brown University, also in Providence. He has published numerous articles in peer-reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Treatment of stress urinary incontinence
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
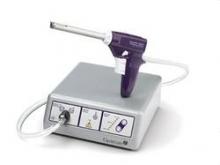
Hysteroscopic electromechanical power morcellation
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
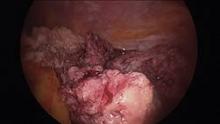
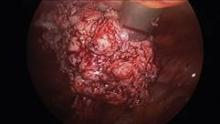
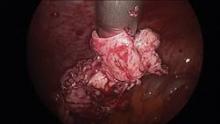
Electromechanical power morcellation confined to a bag
In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.
In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.
In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.
Putting morcellation into perspective – ‘Just the facts, Ma’am, nothing but the facts’
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
The case for robotic-assisted hysterectomy
During my address as president of the Board of Trustees of the AAGL in 2008, I noted that essentially 95% of all cholecystectomies, 95% of all bariatric surgery, and 70% of all appendectomies in the United States were performed laparoscopically. Unfortunately, less than 20% of hysterectomies were performed via a minimally invasive route.
Subsequently, at the time of my 2012 presidential address for the International Society for Gynecologic Endoscopy (ISGE), I noted that the percentage of minimally invasive hysterectomies performed in the United States now reached 50%, while the percentage of laparoscopic and vaginal hysterectomies was still mired at 18% and 14%, respectively. The increase in a minimally invasive approach to hysterectomy appeared to be due to the newest method of hysterectomy; that is, robotic-assisted hysterectomy.
On March 14, 2013, Dr. James T. Breeden, president of the American College of Obstetricians and Gynecologists, released a statement regarding robotic surgery. In that, he noted, "While there may be some advantages to the use of robotics in complex hysterectomies ... studies have shown that adding this expensive technology for routine surgical care does not improve patient outcomes. Consequently, there is no good data proving that robotic hysterectomy is even as good as – let alone better than – existing, and far less costly, minimally invasive alternatives."
Dr. Breeden then went on to refer to a recent article in the Journal of the American Medical Association (JAMA 2013;309:689-98) to make the point that, in a study of 264,758 patients undergoing hysterectomy in 441 hospitals in the Premier hospital group, robotics added an average of $2,000/procedure without any demonstrable benefit.
Interestingly, however, the authors of the JAMA article acknowledge that while uptake of laparoscopic hysterectomy has been slow since its inception in the early 1990s, accounting for only 14% of hysterectomies in 2005, within 3 years of the introduction of the adoption of robotics for hysterectomy, nearly 10% of all cases were completed by this enabling technology. Furthermore, the authors comment that, "The introduction of robotic gynecologic surgery was associated with a decrease in the rate of abdominal hysterectomy and an increase in the use of minimally invasive surgery as a whole, including both laparoscopic and robotic hysterectomy." The authors acknowledge that robotic surgery may be easier to learn and that robotic assistance may allow for the completion of more technically demanding cases. In addition, they note that the increase in numbers of laparoscopic hysterectomy may have occurred because of competitive pressures or an increased awareness and appreciation of minimally invasive surgical options.
In comparison, the authors found that in hospitals at which robotic surgery was not performed as of the first quarter of 2010, nearly 50% of all hysterectomies were performed via an open abdominal route, while less than 40% of hysterectomies were performed with a laparotomy incision when robotic hysterectomy was performed at the hospital. With the future adoption of the robotics in gynecologic surgery, I am sure there will be a continued reduction in open abdominal hysterectomy. Benefit ... a resounding yes!
Another fascinating finding of the JAMA study was the fact that overall complication rates were similar for robotic-assisted and laparoscopic hysterectomy (5.5% vs. 5.3%; relative risk, 1.03; 95% confidence interval, 0.86-1.24). Moreover, patients who underwent a robotic-assisted hysterectomy were less likely to have a length of stay longer than 2 days (19.6% vs. 24.9%; RR, 0.78; 95% CI, 0.67-0.92).
Despite no differences in complications in the JAMA study, given the fact that robotics is an emerging technology, one can easily extrapolate that the percentage of cases performed in the study by relatively inexperienced robotic surgeons, as compared with laparoscopic surgeons, was higher. Therefore, with increased surgeon experience, as with any new technology, the rate of complications would be expected to be further decreased. To this end, one must remember that early in its inception, the New York Assembly voiced concerns with laparoscopic cholecystectomy secondary to complications. Now, virtually 95% of all cholecystectomies in the United States are performed via a laparoscopic route. Currently, what is the latest focus in cholecystectomy ... robotic assisted single site cholecystectomy that is being rapidly adopted throughout the country.
While one must acknowledge that, at present, robotic-assisted surgery would appear to be more expensive to perform than laparoscopic surgery is, it is difficult to ascertain what that cost differential is truly. Furthermore, one would anticipate with increased experience and efficiency that cost would, indeed, decrease. While in 1996, Dr. James H. Dorsey published an article on the higher costs associated with laparoscopic surgery (N. Engl. J. Med. 1996;335:476-82), more recent studies by Warren L., et al. (J. Minim. Invasive Gynecol. 2009;16:581-8), and Jonsdottir G.M., et al. (Obstet. Gynecol. 2011;117:1142-9) actually show that the laparoscopic route can be more cost effective.
In this era of cost containment, it is imperative that surgical innovation thrive. Where would all specialties involved in minimally invasive surgery be if surgical pioneer and visionary Professor Kurt Semm were not allowed to perform early operative laparoscopic cases in Kiel, Germany? As chronicled by his associate, Professor Lisolette Mettler, in the July-September 2003 NewsScope of the AAGL, "Kurt endured much resistance, including a request for him to undergo a brain scan to rule out brain damage when attempting to introduce operative laparoscopy; the laughter of general surgeons when he recommended laparoscopic cholecystectomy in the late 1970s; a call for suspension by the president of the German Surgical Society after a 1981 lecture on laparoscopic appendectomy; and rejection of a paper on laparoscopic appendectomy to the American Journal of Obstetrics & Gynecology as unethical." Where would the cholecystectomy market be if general surgeons headed the randomized controlled trial of open vs. laparoscopic cholecystectomy published in Lancet in 1996 (Lancet 1996;347:989-94)? The study concluded that the open procedure was superior because there was no difference in hospital stay or recovery, compared with the laparoscopic route. Where would minimally invasive gynecologic surgery be if our specialty fell in line behind Dr. Roy Pitkin, then president of ACOG, who in 1992 entitled an editorial in Obstetrics & Gynecology "Operative Laparoscopy: Surgical Advance or Technical Gimmick?" (Obstet. Gynecol. 1992;79:441-2). In this editorial he questioned operative laparoscopy on the following:
• How does one separate technical feasibility from therapeutic appropriateness?
• What is the nature of "quality assurance"?
• How can appropriate credentialing criteria be established for procedures not taught in residency and for which no present member of the medical staff can claim experience?
• To what extent are these procedures "experimental," requiring review by an institutional body charged with protection of human subjects, and how should truly informed consent be obtained?
• What about fees? When the procedure is not part of established clinical care, is it ethical to charge for professional services?
Dr. Pitkin concluded by commenting, "Our approach to evaluation of these newer surgical techniques is not something of which we can be proud." Many of these same concerns are currently being voiced by those who do not see the brilliant potential of robotics in gynecologic surgery.
Eighteen years later, in a subsequent editorial (Obstet. Gynecol. 2010;115:890-1), Dr. Pitkin acknowledged that "A substantial body of evidence has accumulated in the recent years to support the laparoscopic approach to various gynecologic operations. ... From this extensive literature, it is now clear that many, if not most gynecologic operations traditionally done by laparotomy are amenable to a laparoscopic approach. Further, the studies are consistent in indicating that operative laparoscopy confers unequivocal advantages over older surgical approaches."
Dr. Pitkin and his coauthor, Dr. William Parker, then go on to discuss the issue of cost, "All health care financial studies are complicated by inconsistencies and uncertainties regarding the meaning of cost. ... Increase in operating time with laparoscopic surgery and disposable instruments are offset, by decreased charges reflecting shortened postoperative hospital stays. If a societal cost that included financial results from early return to work or full home activity were calculated, the advantage of endoscopic surgery would be even greater."
Just as it is imperative that our surgical specialty must remain innovative, we must remember, as can be learned with Dr. Pitkin’s two editorials, that scientific evidence behind the innovation takes time. The fact that, in its infancy, robotic assisted surgery has enabled more gynecologic surgeons to perform minimally invasive surgery for more patients cannot be denied. As seen by the JAMA article, even early on, it can be performed safely and effectively. Data collected in the final decade of the 20th century and the first decade of the 21st have enabled operative laparoscopy to enter mainstream surgical care. One can foresee, with the accumulation of knowledge and experience, that robotics will have a similar – if not even greater – role within our specialty. We must learn from William Shakespeare, who provided Marc Anthony the words, "I have come to bury Caesar, not to praise him." We must not come here to bury robotic assisted surgery, but to praise it!
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column. Dr. Miller has received grants from Intuitive Surgical Inc. He also has served as a consultant for and served on the speakers bureau for Intuitive.
During my address as president of the Board of Trustees of the AAGL in 2008, I noted that essentially 95% of all cholecystectomies, 95% of all bariatric surgery, and 70% of all appendectomies in the United States were performed laparoscopically. Unfortunately, less than 20% of hysterectomies were performed via a minimally invasive route.
Subsequently, at the time of my 2012 presidential address for the International Society for Gynecologic Endoscopy (ISGE), I noted that the percentage of minimally invasive hysterectomies performed in the United States now reached 50%, while the percentage of laparoscopic and vaginal hysterectomies was still mired at 18% and 14%, respectively. The increase in a minimally invasive approach to hysterectomy appeared to be due to the newest method of hysterectomy; that is, robotic-assisted hysterectomy.
On March 14, 2013, Dr. James T. Breeden, president of the American College of Obstetricians and Gynecologists, released a statement regarding robotic surgery. In that, he noted, "While there may be some advantages to the use of robotics in complex hysterectomies ... studies have shown that adding this expensive technology for routine surgical care does not improve patient outcomes. Consequently, there is no good data proving that robotic hysterectomy is even as good as – let alone better than – existing, and far less costly, minimally invasive alternatives."
Dr. Breeden then went on to refer to a recent article in the Journal of the American Medical Association (JAMA 2013;309:689-98) to make the point that, in a study of 264,758 patients undergoing hysterectomy in 441 hospitals in the Premier hospital group, robotics added an average of $2,000/procedure without any demonstrable benefit.
Interestingly, however, the authors of the JAMA article acknowledge that while uptake of laparoscopic hysterectomy has been slow since its inception in the early 1990s, accounting for only 14% of hysterectomies in 2005, within 3 years of the introduction of the adoption of robotics for hysterectomy, nearly 10% of all cases were completed by this enabling technology. Furthermore, the authors comment that, "The introduction of robotic gynecologic surgery was associated with a decrease in the rate of abdominal hysterectomy and an increase in the use of minimally invasive surgery as a whole, including both laparoscopic and robotic hysterectomy." The authors acknowledge that robotic surgery may be easier to learn and that robotic assistance may allow for the completion of more technically demanding cases. In addition, they note that the increase in numbers of laparoscopic hysterectomy may have occurred because of competitive pressures or an increased awareness and appreciation of minimally invasive surgical options.
In comparison, the authors found that in hospitals at which robotic surgery was not performed as of the first quarter of 2010, nearly 50% of all hysterectomies were performed via an open abdominal route, while less than 40% of hysterectomies were performed with a laparotomy incision when robotic hysterectomy was performed at the hospital. With the future adoption of the robotics in gynecologic surgery, I am sure there will be a continued reduction in open abdominal hysterectomy. Benefit ... a resounding yes!
Another fascinating finding of the JAMA study was the fact that overall complication rates were similar for robotic-assisted and laparoscopic hysterectomy (5.5% vs. 5.3%; relative risk, 1.03; 95% confidence interval, 0.86-1.24). Moreover, patients who underwent a robotic-assisted hysterectomy were less likely to have a length of stay longer than 2 days (19.6% vs. 24.9%; RR, 0.78; 95% CI, 0.67-0.92).
Despite no differences in complications in the JAMA study, given the fact that robotics is an emerging technology, one can easily extrapolate that the percentage of cases performed in the study by relatively inexperienced robotic surgeons, as compared with laparoscopic surgeons, was higher. Therefore, with increased surgeon experience, as with any new technology, the rate of complications would be expected to be further decreased. To this end, one must remember that early in its inception, the New York Assembly voiced concerns with laparoscopic cholecystectomy secondary to complications. Now, virtually 95% of all cholecystectomies in the United States are performed via a laparoscopic route. Currently, what is the latest focus in cholecystectomy ... robotic assisted single site cholecystectomy that is being rapidly adopted throughout the country.
While one must acknowledge that, at present, robotic-assisted surgery would appear to be more expensive to perform than laparoscopic surgery is, it is difficult to ascertain what that cost differential is truly. Furthermore, one would anticipate with increased experience and efficiency that cost would, indeed, decrease. While in 1996, Dr. James H. Dorsey published an article on the higher costs associated with laparoscopic surgery (N. Engl. J. Med. 1996;335:476-82), more recent studies by Warren L., et al. (J. Minim. Invasive Gynecol. 2009;16:581-8), and Jonsdottir G.M., et al. (Obstet. Gynecol. 2011;117:1142-9) actually show that the laparoscopic route can be more cost effective.
In this era of cost containment, it is imperative that surgical innovation thrive. Where would all specialties involved in minimally invasive surgery be if surgical pioneer and visionary Professor Kurt Semm were not allowed to perform early operative laparoscopic cases in Kiel, Germany? As chronicled by his associate, Professor Lisolette Mettler, in the July-September 2003 NewsScope of the AAGL, "Kurt endured much resistance, including a request for him to undergo a brain scan to rule out brain damage when attempting to introduce operative laparoscopy; the laughter of general surgeons when he recommended laparoscopic cholecystectomy in the late 1970s; a call for suspension by the president of the German Surgical Society after a 1981 lecture on laparoscopic appendectomy; and rejection of a paper on laparoscopic appendectomy to the American Journal of Obstetrics & Gynecology as unethical." Where would the cholecystectomy market be if general surgeons headed the randomized controlled trial of open vs. laparoscopic cholecystectomy published in Lancet in 1996 (Lancet 1996;347:989-94)? The study concluded that the open procedure was superior because there was no difference in hospital stay or recovery, compared with the laparoscopic route. Where would minimally invasive gynecologic surgery be if our specialty fell in line behind Dr. Roy Pitkin, then president of ACOG, who in 1992 entitled an editorial in Obstetrics & Gynecology "Operative Laparoscopy: Surgical Advance or Technical Gimmick?" (Obstet. Gynecol. 1992;79:441-2). In this editorial he questioned operative laparoscopy on the following:
• How does one separate technical feasibility from therapeutic appropriateness?
• What is the nature of "quality assurance"?
• How can appropriate credentialing criteria be established for procedures not taught in residency and for which no present member of the medical staff can claim experience?
• To what extent are these procedures "experimental," requiring review by an institutional body charged with protection of human subjects, and how should truly informed consent be obtained?
• What about fees? When the procedure is not part of established clinical care, is it ethical to charge for professional services?
Dr. Pitkin concluded by commenting, "Our approach to evaluation of these newer surgical techniques is not something of which we can be proud." Many of these same concerns are currently being voiced by those who do not see the brilliant potential of robotics in gynecologic surgery.
Eighteen years later, in a subsequent editorial (Obstet. Gynecol. 2010;115:890-1), Dr. Pitkin acknowledged that "A substantial body of evidence has accumulated in the recent years to support the laparoscopic approach to various gynecologic operations. ... From this extensive literature, it is now clear that many, if not most gynecologic operations traditionally done by laparotomy are amenable to a laparoscopic approach. Further, the studies are consistent in indicating that operative laparoscopy confers unequivocal advantages over older surgical approaches."
Dr. Pitkin and his coauthor, Dr. William Parker, then go on to discuss the issue of cost, "All health care financial studies are complicated by inconsistencies and uncertainties regarding the meaning of cost. ... Increase in operating time with laparoscopic surgery and disposable instruments are offset, by decreased charges reflecting shortened postoperative hospital stays. If a societal cost that included financial results from early return to work or full home activity were calculated, the advantage of endoscopic surgery would be even greater."
Just as it is imperative that our surgical specialty must remain innovative, we must remember, as can be learned with Dr. Pitkin’s two editorials, that scientific evidence behind the innovation takes time. The fact that, in its infancy, robotic assisted surgery has enabled more gynecologic surgeons to perform minimally invasive surgery for more patients cannot be denied. As seen by the JAMA article, even early on, it can be performed safely and effectively. Data collected in the final decade of the 20th century and the first decade of the 21st have enabled operative laparoscopy to enter mainstream surgical care. One can foresee, with the accumulation of knowledge and experience, that robotics will have a similar – if not even greater – role within our specialty. We must learn from William Shakespeare, who provided Marc Anthony the words, "I have come to bury Caesar, not to praise him." We must not come here to bury robotic assisted surgery, but to praise it!
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column. Dr. Miller has received grants from Intuitive Surgical Inc. He also has served as a consultant for and served on the speakers bureau for Intuitive.
During my address as president of the Board of Trustees of the AAGL in 2008, I noted that essentially 95% of all cholecystectomies, 95% of all bariatric surgery, and 70% of all appendectomies in the United States were performed laparoscopically. Unfortunately, less than 20% of hysterectomies were performed via a minimally invasive route.
Subsequently, at the time of my 2012 presidential address for the International Society for Gynecologic Endoscopy (ISGE), I noted that the percentage of minimally invasive hysterectomies performed in the United States now reached 50%, while the percentage of laparoscopic and vaginal hysterectomies was still mired at 18% and 14%, respectively. The increase in a minimally invasive approach to hysterectomy appeared to be due to the newest method of hysterectomy; that is, robotic-assisted hysterectomy.
On March 14, 2013, Dr. James T. Breeden, president of the American College of Obstetricians and Gynecologists, released a statement regarding robotic surgery. In that, he noted, "While there may be some advantages to the use of robotics in complex hysterectomies ... studies have shown that adding this expensive technology for routine surgical care does not improve patient outcomes. Consequently, there is no good data proving that robotic hysterectomy is even as good as – let alone better than – existing, and far less costly, minimally invasive alternatives."
Dr. Breeden then went on to refer to a recent article in the Journal of the American Medical Association (JAMA 2013;309:689-98) to make the point that, in a study of 264,758 patients undergoing hysterectomy in 441 hospitals in the Premier hospital group, robotics added an average of $2,000/procedure without any demonstrable benefit.
Interestingly, however, the authors of the JAMA article acknowledge that while uptake of laparoscopic hysterectomy has been slow since its inception in the early 1990s, accounting for only 14% of hysterectomies in 2005, within 3 years of the introduction of the adoption of robotics for hysterectomy, nearly 10% of all cases were completed by this enabling technology. Furthermore, the authors comment that, "The introduction of robotic gynecologic surgery was associated with a decrease in the rate of abdominal hysterectomy and an increase in the use of minimally invasive surgery as a whole, including both laparoscopic and robotic hysterectomy." The authors acknowledge that robotic surgery may be easier to learn and that robotic assistance may allow for the completion of more technically demanding cases. In addition, they note that the increase in numbers of laparoscopic hysterectomy may have occurred because of competitive pressures or an increased awareness and appreciation of minimally invasive surgical options.
In comparison, the authors found that in hospitals at which robotic surgery was not performed as of the first quarter of 2010, nearly 50% of all hysterectomies were performed via an open abdominal route, while less than 40% of hysterectomies were performed with a laparotomy incision when robotic hysterectomy was performed at the hospital. With the future adoption of the robotics in gynecologic surgery, I am sure there will be a continued reduction in open abdominal hysterectomy. Benefit ... a resounding yes!
Another fascinating finding of the JAMA study was the fact that overall complication rates were similar for robotic-assisted and laparoscopic hysterectomy (5.5% vs. 5.3%; relative risk, 1.03; 95% confidence interval, 0.86-1.24). Moreover, patients who underwent a robotic-assisted hysterectomy were less likely to have a length of stay longer than 2 days (19.6% vs. 24.9%; RR, 0.78; 95% CI, 0.67-0.92).
Despite no differences in complications in the JAMA study, given the fact that robotics is an emerging technology, one can easily extrapolate that the percentage of cases performed in the study by relatively inexperienced robotic surgeons, as compared with laparoscopic surgeons, was higher. Therefore, with increased surgeon experience, as with any new technology, the rate of complications would be expected to be further decreased. To this end, one must remember that early in its inception, the New York Assembly voiced concerns with laparoscopic cholecystectomy secondary to complications. Now, virtually 95% of all cholecystectomies in the United States are performed via a laparoscopic route. Currently, what is the latest focus in cholecystectomy ... robotic assisted single site cholecystectomy that is being rapidly adopted throughout the country.
While one must acknowledge that, at present, robotic-assisted surgery would appear to be more expensive to perform than laparoscopic surgery is, it is difficult to ascertain what that cost differential is truly. Furthermore, one would anticipate with increased experience and efficiency that cost would, indeed, decrease. While in 1996, Dr. James H. Dorsey published an article on the higher costs associated with laparoscopic surgery (N. Engl. J. Med. 1996;335:476-82), more recent studies by Warren L., et al. (J. Minim. Invasive Gynecol. 2009;16:581-8), and Jonsdottir G.M., et al. (Obstet. Gynecol. 2011;117:1142-9) actually show that the laparoscopic route can be more cost effective.
In this era of cost containment, it is imperative that surgical innovation thrive. Where would all specialties involved in minimally invasive surgery be if surgical pioneer and visionary Professor Kurt Semm were not allowed to perform early operative laparoscopic cases in Kiel, Germany? As chronicled by his associate, Professor Lisolette Mettler, in the July-September 2003 NewsScope of the AAGL, "Kurt endured much resistance, including a request for him to undergo a brain scan to rule out brain damage when attempting to introduce operative laparoscopy; the laughter of general surgeons when he recommended laparoscopic cholecystectomy in the late 1970s; a call for suspension by the president of the German Surgical Society after a 1981 lecture on laparoscopic appendectomy; and rejection of a paper on laparoscopic appendectomy to the American Journal of Obstetrics & Gynecology as unethical." Where would the cholecystectomy market be if general surgeons headed the randomized controlled trial of open vs. laparoscopic cholecystectomy published in Lancet in 1996 (Lancet 1996;347:989-94)? The study concluded that the open procedure was superior because there was no difference in hospital stay or recovery, compared with the laparoscopic route. Where would minimally invasive gynecologic surgery be if our specialty fell in line behind Dr. Roy Pitkin, then president of ACOG, who in 1992 entitled an editorial in Obstetrics & Gynecology "Operative Laparoscopy: Surgical Advance or Technical Gimmick?" (Obstet. Gynecol. 1992;79:441-2). In this editorial he questioned operative laparoscopy on the following:
• How does one separate technical feasibility from therapeutic appropriateness?
• What is the nature of "quality assurance"?
• How can appropriate credentialing criteria be established for procedures not taught in residency and for which no present member of the medical staff can claim experience?
• To what extent are these procedures "experimental," requiring review by an institutional body charged with protection of human subjects, and how should truly informed consent be obtained?
• What about fees? When the procedure is not part of established clinical care, is it ethical to charge for professional services?
Dr. Pitkin concluded by commenting, "Our approach to evaluation of these newer surgical techniques is not something of which we can be proud." Many of these same concerns are currently being voiced by those who do not see the brilliant potential of robotics in gynecologic surgery.
Eighteen years later, in a subsequent editorial (Obstet. Gynecol. 2010;115:890-1), Dr. Pitkin acknowledged that "A substantial body of evidence has accumulated in the recent years to support the laparoscopic approach to various gynecologic operations. ... From this extensive literature, it is now clear that many, if not most gynecologic operations traditionally done by laparotomy are amenable to a laparoscopic approach. Further, the studies are consistent in indicating that operative laparoscopy confers unequivocal advantages over older surgical approaches."
Dr. Pitkin and his coauthor, Dr. William Parker, then go on to discuss the issue of cost, "All health care financial studies are complicated by inconsistencies and uncertainties regarding the meaning of cost. ... Increase in operating time with laparoscopic surgery and disposable instruments are offset, by decreased charges reflecting shortened postoperative hospital stays. If a societal cost that included financial results from early return to work or full home activity were calculated, the advantage of endoscopic surgery would be even greater."
Just as it is imperative that our surgical specialty must remain innovative, we must remember, as can be learned with Dr. Pitkin’s two editorials, that scientific evidence behind the innovation takes time. The fact that, in its infancy, robotic assisted surgery has enabled more gynecologic surgeons to perform minimally invasive surgery for more patients cannot be denied. As seen by the JAMA article, even early on, it can be performed safely and effectively. Data collected in the final decade of the 20th century and the first decade of the 21st have enabled operative laparoscopy to enter mainstream surgical care. One can foresee, with the accumulation of knowledge and experience, that robotics will have a similar – if not even greater – role within our specialty. We must learn from William Shakespeare, who provided Marc Anthony the words, "I have come to bury Caesar, not to praise him." We must not come here to bury robotic assisted surgery, but to praise it!
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column. Dr. Miller has received grants from Intuitive Surgical Inc. He also has served as a consultant for and served on the speakers bureau for Intuitive.
Does minimally invasive hysterectomy cost more than the abdominal approach?
The evolution of the laparoscope from diagnostic tool to operative instrument came as quite a shake-up to some. For example, when Professor Kurt Semm pioneered efforts in operative laparoscopy in Germany in the late 1960s and early 1970s, one of his peers suggested he undergo a scan to rule out brain damage!1 (Until then, the laparoscope had been used only to perform tubal sterilization.)
Fast forward to 1988, when Harry Reich, MD, performed the first laparoscopic hysterectomy.2 Within 5 years of that milestone, the literature was replete with information on laparoscopic-assisted vaginal hysterectomy and total laparoscopic hysterectomy. Surgeons such as Tom Lyons, MD, and Harry Hasson, MD, described the advantages, relative ease, and safety profile of laparoscopic supracervical hysterectomy. However, despite accumulating evidence of the benefits of minimally invasive hysterectomy, including speedier recovery and fewer complications, the president of ACOG felt compelled to question, in a 1992 editorial in Obstetrics & Gynecology, whether operative laparoscopy was a “surgical advance” or a “technical gimmick.”3
By 2007, a Cochrane review of hysterectomy for benign gynecologic disease had concluded that the laparoscopic approach is associated with less intraoperative blood loss, a smaller decline in hemoglobin levels, faster recovery, and fewer wound and abdominal infections and febrile episodes than the abdominal approach is.
Moreover, the laparoscopic approach to hysterectomy is more cost-effective than the abdominal route. In a 2009 study, unadjusted expenditures for laparoscopic hysterectomy averaged $10,868 per case, compared with $12,086 for abdominal hysterectomy and $9,544 for vaginal hysterectomy. Adjusted expenditures for outpatient minimally invasive hysterectomy (laparoscopic or vaginal) were markedly lower than for inpatient abdominal hysterectomy.4
Nevertheless, as I noted in my 2008 presidential address to the 37th Global Congress of Minimally Invasive Gynecology of the AAGL, we have not yet achieved widespread adoption of laparoscopic hysterectomy in the United States. Although more than 95% of cholecystectomy cases are performed laparoscopically—as well as 70% of hernia repairs and 80% of bariatric procedures—only 15% of hysterectomies are laparoscopic, and nearly two thirds are completed using an open abdominal technique.
Jonsdottir et al: Details of the study
Enter the current study: a retrospective analysis from the Division of Minimally Invasive Gynecologic Surgery at Brigham and Women’s Hospital in Boston. It may finally deliver the much-needed death knell for open abdominal hysterectomy. The study of 2,133 women who underwent hysterectomy (1,054 in 2006 and 1,079 in 2009) found that the total number of hysterectomies remained stable while the percentages of abdominal and laparoscopic cases changed markedly. The percentage of hysterectomies that were performed abdominally declined from 64.7% in 2006 to 35.8% in 2009, and the percentage of laparoscopic procedures rose from 17.7% to 46% over the same period.
Along with this change, the overall rate of intraoperative complications decreased significantly—from 7.2% to 4%—and so did the mean percentage of postoperative complications—from 18% to 5.7%. Although operative costs did increase significantly, there was no change in the mean total cost.
Jonsdottir and colleagues cite various reasons for the shift toward minimally invasive hysterectomy:
- a nationwide change in practice patterns
- increasing awareness among patients of the benefits of minimally invasive procedures
- establishment of a minimally invasive gynecologic surgery program at Brigham and Women’s Hospital in 2006
- a preponderance of hysterectomies performed by the gynecologic oncology service or the minimally invasive gynecologic surgery group, both of which are adept in minimally invasive hysterectomy.
Use of the robot increased dramatically
Although robotic hysterectomy was the least common approach identified in this study, the rate nearly tripled over 3 years. This trend toward increasing use of robotic assistance in hysterectomy is seen nationally; according to industry estimates, 26% of all hysterectomy procedures are performed via the robot, as opposed to 18% performed via laparoscopy, 15% performed vaginally, and 38% performed via the open abdominal approach. Although a study by Pasic and colleagues noted increased hospital costs per case with use of the robot for hysterectomy, Jonsdottir and coworkers found the calculated cost to society to be lowest with robotic hysterectomy.5
These findings in context
In another retrospective study that evaluated the use of minimally invasive hysterectomy over a 20-year period at my institution—a tertiary-care, community teaching hospital—Moen and colleagues noted a decrease in the percentage of abdominal hysterectomy from 77% to 35%, whereas minimally invasive hysterectomy (vaginal and laparoscopic approaches) increased from 23% to 64.8%.6 By 2009, 13.6% of hysterectomies at my institution were completed with robotic assistance.
Interestingly, the majority of abdominal, laparoscopic supracervical, and robotic-assisted hysterectomies were performed by generalists, whereas the majority of vaginal and total laparoscopic hysterectomies were performed by fellowship-trained specialists.6
I agree with a recent position statement from the AAGL, which concludes that most hysterectomies for benign indications should utilize the vaginal or laparoscopic approach.7 AAGL also recommends that efforts to facilitate these approaches continue. Surgeons who lack the requisite skills and training should seek assistance from expert colleagues or refer the patient to a surgeon with such expertise.—CHARLES E. MILLER, MD
1. Miller CE. In tribute to Professor Kurt Semm: a true innovator—1927–2003. NewsScope: Newsletter of American Association of Gynecologic Laparoscopists. 2003;17(3):7.-
2. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
3. Pitkin RM. Operative laparoscopy: surgical advance or technical gimmick. Obstet Gynecol. 1992;79(3):441-442.
4. Warren L, Ladapo JA, Borah BJ, Gunnarsson CL. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16(5):581-588.
5. Pasic RP, Rizzo JA, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17(6):730-738.
6. Moen MD, Noone M, Pesch D, Vassallo B, Miller C. Minimally invasive surgery for hysterectomy: evolving trends over 20 years at a tertiary care community teaching hospital. Female Pelvic Med Reconstr Surg. 2011;17(2):S9.-
7. AAGL Position Statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
The evolution of the laparoscope from diagnostic tool to operative instrument came as quite a shake-up to some. For example, when Professor Kurt Semm pioneered efforts in operative laparoscopy in Germany in the late 1960s and early 1970s, one of his peers suggested he undergo a scan to rule out brain damage!1 (Until then, the laparoscope had been used only to perform tubal sterilization.)
Fast forward to 1988, when Harry Reich, MD, performed the first laparoscopic hysterectomy.2 Within 5 years of that milestone, the literature was replete with information on laparoscopic-assisted vaginal hysterectomy and total laparoscopic hysterectomy. Surgeons such as Tom Lyons, MD, and Harry Hasson, MD, described the advantages, relative ease, and safety profile of laparoscopic supracervical hysterectomy. However, despite accumulating evidence of the benefits of minimally invasive hysterectomy, including speedier recovery and fewer complications, the president of ACOG felt compelled to question, in a 1992 editorial in Obstetrics & Gynecology, whether operative laparoscopy was a “surgical advance” or a “technical gimmick.”3
By 2007, a Cochrane review of hysterectomy for benign gynecologic disease had concluded that the laparoscopic approach is associated with less intraoperative blood loss, a smaller decline in hemoglobin levels, faster recovery, and fewer wound and abdominal infections and febrile episodes than the abdominal approach is.
Moreover, the laparoscopic approach to hysterectomy is more cost-effective than the abdominal route. In a 2009 study, unadjusted expenditures for laparoscopic hysterectomy averaged $10,868 per case, compared with $12,086 for abdominal hysterectomy and $9,544 for vaginal hysterectomy. Adjusted expenditures for outpatient minimally invasive hysterectomy (laparoscopic or vaginal) were markedly lower than for inpatient abdominal hysterectomy.4
Nevertheless, as I noted in my 2008 presidential address to the 37th Global Congress of Minimally Invasive Gynecology of the AAGL, we have not yet achieved widespread adoption of laparoscopic hysterectomy in the United States. Although more than 95% of cholecystectomy cases are performed laparoscopically—as well as 70% of hernia repairs and 80% of bariatric procedures—only 15% of hysterectomies are laparoscopic, and nearly two thirds are completed using an open abdominal technique.
Jonsdottir et al: Details of the study
Enter the current study: a retrospective analysis from the Division of Minimally Invasive Gynecologic Surgery at Brigham and Women’s Hospital in Boston. It may finally deliver the much-needed death knell for open abdominal hysterectomy. The study of 2,133 women who underwent hysterectomy (1,054 in 2006 and 1,079 in 2009) found that the total number of hysterectomies remained stable while the percentages of abdominal and laparoscopic cases changed markedly. The percentage of hysterectomies that were performed abdominally declined from 64.7% in 2006 to 35.8% in 2009, and the percentage of laparoscopic procedures rose from 17.7% to 46% over the same period.
Along with this change, the overall rate of intraoperative complications decreased significantly—from 7.2% to 4%—and so did the mean percentage of postoperative complications—from 18% to 5.7%. Although operative costs did increase significantly, there was no change in the mean total cost.
Jonsdottir and colleagues cite various reasons for the shift toward minimally invasive hysterectomy:
- a nationwide change in practice patterns
- increasing awareness among patients of the benefits of minimally invasive procedures
- establishment of a minimally invasive gynecologic surgery program at Brigham and Women’s Hospital in 2006
- a preponderance of hysterectomies performed by the gynecologic oncology service or the minimally invasive gynecologic surgery group, both of which are adept in minimally invasive hysterectomy.
Use of the robot increased dramatically
Although robotic hysterectomy was the least common approach identified in this study, the rate nearly tripled over 3 years. This trend toward increasing use of robotic assistance in hysterectomy is seen nationally; according to industry estimates, 26% of all hysterectomy procedures are performed via the robot, as opposed to 18% performed via laparoscopy, 15% performed vaginally, and 38% performed via the open abdominal approach. Although a study by Pasic and colleagues noted increased hospital costs per case with use of the robot for hysterectomy, Jonsdottir and coworkers found the calculated cost to society to be lowest with robotic hysterectomy.5
These findings in context
In another retrospective study that evaluated the use of minimally invasive hysterectomy over a 20-year period at my institution—a tertiary-care, community teaching hospital—Moen and colleagues noted a decrease in the percentage of abdominal hysterectomy from 77% to 35%, whereas minimally invasive hysterectomy (vaginal and laparoscopic approaches) increased from 23% to 64.8%.6 By 2009, 13.6% of hysterectomies at my institution were completed with robotic assistance.
Interestingly, the majority of abdominal, laparoscopic supracervical, and robotic-assisted hysterectomies were performed by generalists, whereas the majority of vaginal and total laparoscopic hysterectomies were performed by fellowship-trained specialists.6
I agree with a recent position statement from the AAGL, which concludes that most hysterectomies for benign indications should utilize the vaginal or laparoscopic approach.7 AAGL also recommends that efforts to facilitate these approaches continue. Surgeons who lack the requisite skills and training should seek assistance from expert colleagues or refer the patient to a surgeon with such expertise.—CHARLES E. MILLER, MD
The evolution of the laparoscope from diagnostic tool to operative instrument came as quite a shake-up to some. For example, when Professor Kurt Semm pioneered efforts in operative laparoscopy in Germany in the late 1960s and early 1970s, one of his peers suggested he undergo a scan to rule out brain damage!1 (Until then, the laparoscope had been used only to perform tubal sterilization.)
Fast forward to 1988, when Harry Reich, MD, performed the first laparoscopic hysterectomy.2 Within 5 years of that milestone, the literature was replete with information on laparoscopic-assisted vaginal hysterectomy and total laparoscopic hysterectomy. Surgeons such as Tom Lyons, MD, and Harry Hasson, MD, described the advantages, relative ease, and safety profile of laparoscopic supracervical hysterectomy. However, despite accumulating evidence of the benefits of minimally invasive hysterectomy, including speedier recovery and fewer complications, the president of ACOG felt compelled to question, in a 1992 editorial in Obstetrics & Gynecology, whether operative laparoscopy was a “surgical advance” or a “technical gimmick.”3
By 2007, a Cochrane review of hysterectomy for benign gynecologic disease had concluded that the laparoscopic approach is associated with less intraoperative blood loss, a smaller decline in hemoglobin levels, faster recovery, and fewer wound and abdominal infections and febrile episodes than the abdominal approach is.
Moreover, the laparoscopic approach to hysterectomy is more cost-effective than the abdominal route. In a 2009 study, unadjusted expenditures for laparoscopic hysterectomy averaged $10,868 per case, compared with $12,086 for abdominal hysterectomy and $9,544 for vaginal hysterectomy. Adjusted expenditures for outpatient minimally invasive hysterectomy (laparoscopic or vaginal) were markedly lower than for inpatient abdominal hysterectomy.4
Nevertheless, as I noted in my 2008 presidential address to the 37th Global Congress of Minimally Invasive Gynecology of the AAGL, we have not yet achieved widespread adoption of laparoscopic hysterectomy in the United States. Although more than 95% of cholecystectomy cases are performed laparoscopically—as well as 70% of hernia repairs and 80% of bariatric procedures—only 15% of hysterectomies are laparoscopic, and nearly two thirds are completed using an open abdominal technique.
Jonsdottir et al: Details of the study
Enter the current study: a retrospective analysis from the Division of Minimally Invasive Gynecologic Surgery at Brigham and Women’s Hospital in Boston. It may finally deliver the much-needed death knell for open abdominal hysterectomy. The study of 2,133 women who underwent hysterectomy (1,054 in 2006 and 1,079 in 2009) found that the total number of hysterectomies remained stable while the percentages of abdominal and laparoscopic cases changed markedly. The percentage of hysterectomies that were performed abdominally declined from 64.7% in 2006 to 35.8% in 2009, and the percentage of laparoscopic procedures rose from 17.7% to 46% over the same period.
Along with this change, the overall rate of intraoperative complications decreased significantly—from 7.2% to 4%—and so did the mean percentage of postoperative complications—from 18% to 5.7%. Although operative costs did increase significantly, there was no change in the mean total cost.
Jonsdottir and colleagues cite various reasons for the shift toward minimally invasive hysterectomy:
- a nationwide change in practice patterns
- increasing awareness among patients of the benefits of minimally invasive procedures
- establishment of a minimally invasive gynecologic surgery program at Brigham and Women’s Hospital in 2006
- a preponderance of hysterectomies performed by the gynecologic oncology service or the minimally invasive gynecologic surgery group, both of which are adept in minimally invasive hysterectomy.
Use of the robot increased dramatically
Although robotic hysterectomy was the least common approach identified in this study, the rate nearly tripled over 3 years. This trend toward increasing use of robotic assistance in hysterectomy is seen nationally; according to industry estimates, 26% of all hysterectomy procedures are performed via the robot, as opposed to 18% performed via laparoscopy, 15% performed vaginally, and 38% performed via the open abdominal approach. Although a study by Pasic and colleagues noted increased hospital costs per case with use of the robot for hysterectomy, Jonsdottir and coworkers found the calculated cost to society to be lowest with robotic hysterectomy.5
These findings in context
In another retrospective study that evaluated the use of minimally invasive hysterectomy over a 20-year period at my institution—a tertiary-care, community teaching hospital—Moen and colleagues noted a decrease in the percentage of abdominal hysterectomy from 77% to 35%, whereas minimally invasive hysterectomy (vaginal and laparoscopic approaches) increased from 23% to 64.8%.6 By 2009, 13.6% of hysterectomies at my institution were completed with robotic assistance.
Interestingly, the majority of abdominal, laparoscopic supracervical, and robotic-assisted hysterectomies were performed by generalists, whereas the majority of vaginal and total laparoscopic hysterectomies were performed by fellowship-trained specialists.6
I agree with a recent position statement from the AAGL, which concludes that most hysterectomies for benign indications should utilize the vaginal or laparoscopic approach.7 AAGL also recommends that efforts to facilitate these approaches continue. Surgeons who lack the requisite skills and training should seek assistance from expert colleagues or refer the patient to a surgeon with such expertise.—CHARLES E. MILLER, MD
1. Miller CE. In tribute to Professor Kurt Semm: a true innovator—1927–2003. NewsScope: Newsletter of American Association of Gynecologic Laparoscopists. 2003;17(3):7.-
2. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
3. Pitkin RM. Operative laparoscopy: surgical advance or technical gimmick. Obstet Gynecol. 1992;79(3):441-442.
4. Warren L, Ladapo JA, Borah BJ, Gunnarsson CL. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16(5):581-588.
5. Pasic RP, Rizzo JA, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17(6):730-738.
6. Moen MD, Noone M, Pesch D, Vassallo B, Miller C. Minimally invasive surgery for hysterectomy: evolving trends over 20 years at a tertiary care community teaching hospital. Female Pelvic Med Reconstr Surg. 2011;17(2):S9.-
7. AAGL Position Statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
1. Miller CE. In tribute to Professor Kurt Semm: a true innovator—1927–2003. NewsScope: Newsletter of American Association of Gynecologic Laparoscopists. 2003;17(3):7.-
2. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
3. Pitkin RM. Operative laparoscopy: surgical advance or technical gimmick. Obstet Gynecol. 1992;79(3):441-442.
4. Warren L, Ladapo JA, Borah BJ, Gunnarsson CL. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16(5):581-588.
5. Pasic RP, Rizzo JA, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17(6):730-738.
6. Moen MD, Noone M, Pesch D, Vassallo B, Miller C. Minimally invasive surgery for hysterectomy: evolving trends over 20 years at a tertiary care community teaching hospital. Female Pelvic Med Reconstr Surg. 2011;17(2):S9.-
7. AAGL Position Statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
“You say you want a revolution. Well….”
You say you want a revolution
Well, you know
We all want to change the world
—John Lennon
Gynecology was once a revolutionary specialty. Innovative and enterprising, its members were pioneers in operative laparoscopy, and they educated other surgical services on the merits and techniques of endoscopy.
Today the specialty lags behind others in both innovation and adoption of minimally invasive surgical techniques (FIGURE). By 2007, 95% of cholecystectomies were performed laparoscopically, as well as 90% of bariatric procedures and 70% of appendectomies. In contrast, only 20% of hysterectomies were performed using the laparoscopic approach. One reason for this discrepancy may be the extraordinary confidence that patients have in their gynecologist.
Consider the two sides of a coin that were revealed by the findings of an Internet survey of 526 women conducted by Russell Research and commissioned by the Patient Awareness Program of the AAGL. On one hand, investigators found that survey participants shared a steadfast opinion that their gynecologist would describe all available treatment choices, including the least traumatic and safest surgical procedures. Ninety-eight percent expected their physician to describe minimally invasive treatment options even if he or she was not proficient in them at the time—and to mention options that entail the least amount of pain. In addition, 94% of respondents expected their gynecologist to promote options with the lowest impact on lifestyle.1 Yet, on the other hand, although 517 of 528 (98%) respondents who were 18 years or older had experienced stress urinary incontinence, fibroids, or uterine prolapse, fewer than 40% were aware that a number of minimally invasive techniques could greatly reduce the need for hysterectomy. Fewer than 50% of respondents who suffered from menorrhagia were aware of endometrial ablation as a treatment, and only 21% realized that it could be performed in an office. Only 45% of women who had leiomyomata had heard of myomectomy. And one of every three women who had stress urinary incontinence was unaware of sling procedures. Fewer than 20% knew that sterilization could be performed in an office.1 Although these women expected to be thoroughly informed by their physician, their lack of awareness suggests the opposite.
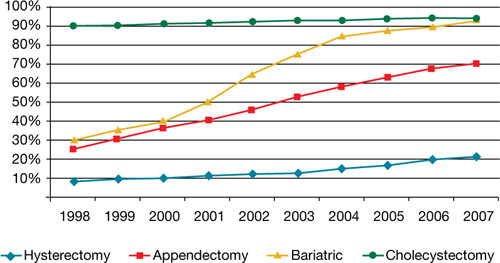
Use of minimally invasive options for 4 common surgeries
Resident physicians need more hands-on experience
A barrier to adoption of minimally invasive surgical approaches is the limited access many residents have to training and experience. This barrier could become especially problematic as third-party payers gain awareness of the advantages of minimally invasive surgery and shift reimbursement accordingly. In association with the American Society of Reproductive Medicine (ASRM), AAGL is actively engaged in enhancing surgical education through its Fellowship in Minimally Invasive Gynecology and is designing programs to supplement resident education. It is also establishing centers of excellence through its professional interest partner, the Council on Gynecologic Endoscopy (CGE).
Although the solution to our problem seems simple—increase the number of gynecologists who perform minimally invasive gynecologic surgery—that is easier said than done. According to data from the Accreditation Council for Graduate Medical Education ( ACGME), one of every three graduating residents has limited experience in minimally invasive procedures, and 30% of residents perform an average of only 12 laparoscopic operations a year as the primary surgeon. The average is even lower for hysteroscopic procedures!2

In an article published earlier this year in the Journal of Minimally Invasive Gynecology, Jon I. Einarsson, MD, MPH, and colleagues explore attitudes toward hysterectomy among gynecologists in the United States.3 Although nearly three quarters of hysterectomies are performed using an open abdominal approach, only 8% of respondents said they would choose the abdominal approach for themselves or their spouse. Among respondents who reported the highest surgical volumes, the percentage likely to choose a laparoscopic approach was significantly higher. The main barriers to laparoscopic hysterectomy? Limited opportunity for training during residency, technical difficulty, personal surgical experience, and operating time.3
We need an advanced curriculum
We have made tremendous progress in postgraduate education, thanks to the efforts of AAGL and ASRM. Nevertheless, we lack a specific, unified curriculum to train and ultimately credential gynecology residents and fellows in minimally invasive surgery.
Once again, our specialty lags behind general surgery. As of July 2009, all residents performing general surgery are required to complete and pass a course, “Fundamentals of laparoscopic surgery” (FLS). This joint undertaking of the American College of Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons has established minimum standards for basic cognitive and technical skills required for laparoscopic surgery.
Using the FLS course as a model, AAGL is designing a structured core curriculum to educate not only residents and fellows, but practicing gynecologists as well. Like the FLS course, the AAGL curriculum will emphasize both didactic teaching and lab skills, but it will be even more extensive because it will include hysteroscopy.
What we are doing now
After analyzing the needs of patients and providers around the world and acknowledging the lack of national standards to improve outcomes, AAGL and CGE have launched two other ambitious programs:
- a registry of physicians that includes operative experience and complication rate, as reviewed by their peers. This system lists physicians by level of practice, according to complexity of procedures, and by proactive experience and specialization
- a center of excellence in minimally invasive gynecology. The CGE recognizes that the achievement of best-in-class surgical outcomes requires not only an expert minimally invasive gynecologic surgeon but also an integrated, multidisciplinary surgical facility with systems and procedures to maximize quality, cost-effectiveness, and safety. For example, it is the vision of AAGL President C. Y. Liu that complex endometriosis surgery will one day be performed only by competent and experienced laparoscopic surgeons in endometriosis surgery centers.
Another initiative, launched in 2009, is the American Institute of Minimally Invasive Surgery (AIMIS). This not-for-profit organization offers member surgeons and hospitals not only accreditation and recognition, but also a number of useful products and programs, including:
- education
- guidance on technology
- business development
- insurance
- marketing strategies
- financial services
- consultation in practice management.
AIMIS is not a medical society but a national institute of which surgeons and hospitals become affiliated members.
Minimally invasive approach reduces costs, too
Rising health care costs have focused attention on ways to improve quality of care and contain costs at the same time, and minimally invasive surgery has emerged as a means of doing both. A recent study of 15,404 patients compared total abdominal hysterectomy with the minimally invasive alternatives (vaginal and laparoscopic hysterectomy) and found that the latter shortened hospitalization, reduced infection, and decreased the cost by $4,000 for the vaginal approach and $2,000 for laparoscopy.4 A study of more than 11,000 patients demonstrated a 52% reduction in the rate of hospital-acquired infection when a minimally invasive approach was utilized rather than open abdominal surgery.4
Some initiatives focus on the patient as a way of increasing utilization of minimally invasive approaches. For example, in Colorado, a 6,000-member self-funded medical plan launched a value-based program for patients undergoing colectomy, cholecystectomy, hysterectomy, appendectomy, and bariatric surgery. The program educated patients about minimally invasive options and encouraged them to seek consultation with a minimally invasive surgeon. Members who chose a minimally invasive procedure saw their copayment go down significantly. In addition, any surgeon who decided to perform one of these five procedures using an open approach was required to obtain preauthorization. Referral physicians were notified of the initiative, and minimally invasive surgeons were identified on the plan’s Web site.4
After 2 years, the plan saved nearly $1 million in direct hospital and surgeon claim costs. (Indirect savings from reduced need for prescription drugs and fewer complications were not included in this estimate. Nor were the economic advantages gained from the patient’s faster return to work and increased productivity.) After only 1 year, the utilization of minimally invasive hysterectomy rose from 28% to 80%!4
In New England, a 167-store grocery chain with 9,000 employees enrolled in the company’s self-funded health plan was able to reduce costs by identifying minimally invasive surgeons and steering members to them. The company covered 80% of surgical costs if minimally invasive surgery was performed, versus 70% for open surgery. Ultimately, the company’s per capita cost was 40% lower than all available benchmarks.4
We’re at a tipping point
The time has come for gynecologic surgeons to rejoin the revolution. Although we face many challenges, from limited experience and restricted access to training opportunities to lack of patient awareness of the benefits of minimally invasive surgery, it is imperative that we utilize minimally invasive approaches as often as possible. Educational opportunities are available, and third-party payers are beginning to demand it.
I believe it is only a matter of time before minimally invasive gynecologic procedures are the norm, not the exception.
You tell me that it’s evolution…
1. AAGL study finds women still in the dark about minimally invasive treatments for pelvic health disorders [press release]. Cypress, Calif: AAGL; 2008.
2. Accreditation Council for Graduate Medical Education. Obstetrics and gynecology case logs. National data report. 2008–2009. http://www.acgme.org/acWebsite/RRC_220/ObGynNatData0809.pdf. Accessed May 6, 2010.
3. Einarsson JI, Matteson KA, Schulkin J, Chavan NR, Sangi-Haghpeykar H. Minimally invasive hysterectomies—a survey on attitudes and barriers among practicing gynecologists. JMIG. 2010;17(2):167-175
4. Detweiler K, Hayes P, Cardinal A. Targeting surgery to reduce costs for employers. Employee Benefit Adviser. 2009. http://eba.benefitnews.com/news/targeting-surgery-to-reduce-costs-for-employers-2682543-1.html. Accessed May 6, 2010.
This commentary is based on the author’s 2008 presidential address to the 37th World Congress and Annual Meeting of the AAGL, which took place October 30 in Las Vegas, Nevada.
You say you want a revolution
Well, you know
We all want to change the world
—John Lennon
Gynecology was once a revolutionary specialty. Innovative and enterprising, its members were pioneers in operative laparoscopy, and they educated other surgical services on the merits and techniques of endoscopy.
Today the specialty lags behind others in both innovation and adoption of minimally invasive surgical techniques (FIGURE). By 2007, 95% of cholecystectomies were performed laparoscopically, as well as 90% of bariatric procedures and 70% of appendectomies. In contrast, only 20% of hysterectomies were performed using the laparoscopic approach. One reason for this discrepancy may be the extraordinary confidence that patients have in their gynecologist.
Consider the two sides of a coin that were revealed by the findings of an Internet survey of 526 women conducted by Russell Research and commissioned by the Patient Awareness Program of the AAGL. On one hand, investigators found that survey participants shared a steadfast opinion that their gynecologist would describe all available treatment choices, including the least traumatic and safest surgical procedures. Ninety-eight percent expected their physician to describe minimally invasive treatment options even if he or she was not proficient in them at the time—and to mention options that entail the least amount of pain. In addition, 94% of respondents expected their gynecologist to promote options with the lowest impact on lifestyle.1 Yet, on the other hand, although 517 of 528 (98%) respondents who were 18 years or older had experienced stress urinary incontinence, fibroids, or uterine prolapse, fewer than 40% were aware that a number of minimally invasive techniques could greatly reduce the need for hysterectomy. Fewer than 50% of respondents who suffered from menorrhagia were aware of endometrial ablation as a treatment, and only 21% realized that it could be performed in an office. Only 45% of women who had leiomyomata had heard of myomectomy. And one of every three women who had stress urinary incontinence was unaware of sling procedures. Fewer than 20% knew that sterilization could be performed in an office.1 Although these women expected to be thoroughly informed by their physician, their lack of awareness suggests the opposite.

Use of minimally invasive options for 4 common surgeries
Resident physicians need more hands-on experience
A barrier to adoption of minimally invasive surgical approaches is the limited access many residents have to training and experience. This barrier could become especially problematic as third-party payers gain awareness of the advantages of minimally invasive surgery and shift reimbursement accordingly. In association with the American Society of Reproductive Medicine (ASRM), AAGL is actively engaged in enhancing surgical education through its Fellowship in Minimally Invasive Gynecology and is designing programs to supplement resident education. It is also establishing centers of excellence through its professional interest partner, the Council on Gynecologic Endoscopy (CGE).
Although the solution to our problem seems simple—increase the number of gynecologists who perform minimally invasive gynecologic surgery—that is easier said than done. According to data from the Accreditation Council for Graduate Medical Education ( ACGME), one of every three graduating residents has limited experience in minimally invasive procedures, and 30% of residents perform an average of only 12 laparoscopic operations a year as the primary surgeon. The average is even lower for hysteroscopic procedures!2

In an article published earlier this year in the Journal of Minimally Invasive Gynecology, Jon I. Einarsson, MD, MPH, and colleagues explore attitudes toward hysterectomy among gynecologists in the United States.3 Although nearly three quarters of hysterectomies are performed using an open abdominal approach, only 8% of respondents said they would choose the abdominal approach for themselves or their spouse. Among respondents who reported the highest surgical volumes, the percentage likely to choose a laparoscopic approach was significantly higher. The main barriers to laparoscopic hysterectomy? Limited opportunity for training during residency, technical difficulty, personal surgical experience, and operating time.3
We need an advanced curriculum
We have made tremendous progress in postgraduate education, thanks to the efforts of AAGL and ASRM. Nevertheless, we lack a specific, unified curriculum to train and ultimately credential gynecology residents and fellows in minimally invasive surgery.
Once again, our specialty lags behind general surgery. As of July 2009, all residents performing general surgery are required to complete and pass a course, “Fundamentals of laparoscopic surgery” (FLS). This joint undertaking of the American College of Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons has established minimum standards for basic cognitive and technical skills required for laparoscopic surgery.
Using the FLS course as a model, AAGL is designing a structured core curriculum to educate not only residents and fellows, but practicing gynecologists as well. Like the FLS course, the AAGL curriculum will emphasize both didactic teaching and lab skills, but it will be even more extensive because it will include hysteroscopy.
What we are doing now
After analyzing the needs of patients and providers around the world and acknowledging the lack of national standards to improve outcomes, AAGL and CGE have launched two other ambitious programs:
- a registry of physicians that includes operative experience and complication rate, as reviewed by their peers. This system lists physicians by level of practice, according to complexity of procedures, and by proactive experience and specialization
- a center of excellence in minimally invasive gynecology. The CGE recognizes that the achievement of best-in-class surgical outcomes requires not only an expert minimally invasive gynecologic surgeon but also an integrated, multidisciplinary surgical facility with systems and procedures to maximize quality, cost-effectiveness, and safety. For example, it is the vision of AAGL President C. Y. Liu that complex endometriosis surgery will one day be performed only by competent and experienced laparoscopic surgeons in endometriosis surgery centers.
Another initiative, launched in 2009, is the American Institute of Minimally Invasive Surgery (AIMIS). This not-for-profit organization offers member surgeons and hospitals not only accreditation and recognition, but also a number of useful products and programs, including:
- education
- guidance on technology
- business development
- insurance
- marketing strategies
- financial services
- consultation in practice management.
AIMIS is not a medical society but a national institute of which surgeons and hospitals become affiliated members.
Minimally invasive approach reduces costs, too
Rising health care costs have focused attention on ways to improve quality of care and contain costs at the same time, and minimally invasive surgery has emerged as a means of doing both. A recent study of 15,404 patients compared total abdominal hysterectomy with the minimally invasive alternatives (vaginal and laparoscopic hysterectomy) and found that the latter shortened hospitalization, reduced infection, and decreased the cost by $4,000 for the vaginal approach and $2,000 for laparoscopy.4 A study of more than 11,000 patients demonstrated a 52% reduction in the rate of hospital-acquired infection when a minimally invasive approach was utilized rather than open abdominal surgery.4
Some initiatives focus on the patient as a way of increasing utilization of minimally invasive approaches. For example, in Colorado, a 6,000-member self-funded medical plan launched a value-based program for patients undergoing colectomy, cholecystectomy, hysterectomy, appendectomy, and bariatric surgery. The program educated patients about minimally invasive options and encouraged them to seek consultation with a minimally invasive surgeon. Members who chose a minimally invasive procedure saw their copayment go down significantly. In addition, any surgeon who decided to perform one of these five procedures using an open approach was required to obtain preauthorization. Referral physicians were notified of the initiative, and minimally invasive surgeons were identified on the plan’s Web site.4
After 2 years, the plan saved nearly $1 million in direct hospital and surgeon claim costs. (Indirect savings from reduced need for prescription drugs and fewer complications were not included in this estimate. Nor were the economic advantages gained from the patient’s faster return to work and increased productivity.) After only 1 year, the utilization of minimally invasive hysterectomy rose from 28% to 80%!4
In New England, a 167-store grocery chain with 9,000 employees enrolled in the company’s self-funded health plan was able to reduce costs by identifying minimally invasive surgeons and steering members to them. The company covered 80% of surgical costs if minimally invasive surgery was performed, versus 70% for open surgery. Ultimately, the company’s per capita cost was 40% lower than all available benchmarks.4
We’re at a tipping point
The time has come for gynecologic surgeons to rejoin the revolution. Although we face many challenges, from limited experience and restricted access to training opportunities to lack of patient awareness of the benefits of minimally invasive surgery, it is imperative that we utilize minimally invasive approaches as often as possible. Educational opportunities are available, and third-party payers are beginning to demand it.
I believe it is only a matter of time before minimally invasive gynecologic procedures are the norm, not the exception.
You tell me that it’s evolution…
You say you want a revolution
Well, you know
We all want to change the world
—John Lennon
Gynecology was once a revolutionary specialty. Innovative and enterprising, its members were pioneers in operative laparoscopy, and they educated other surgical services on the merits and techniques of endoscopy.
Today the specialty lags behind others in both innovation and adoption of minimally invasive surgical techniques (FIGURE). By 2007, 95% of cholecystectomies were performed laparoscopically, as well as 90% of bariatric procedures and 70% of appendectomies. In contrast, only 20% of hysterectomies were performed using the laparoscopic approach. One reason for this discrepancy may be the extraordinary confidence that patients have in their gynecologist.
Consider the two sides of a coin that were revealed by the findings of an Internet survey of 526 women conducted by Russell Research and commissioned by the Patient Awareness Program of the AAGL. On one hand, investigators found that survey participants shared a steadfast opinion that their gynecologist would describe all available treatment choices, including the least traumatic and safest surgical procedures. Ninety-eight percent expected their physician to describe minimally invasive treatment options even if he or she was not proficient in them at the time—and to mention options that entail the least amount of pain. In addition, 94% of respondents expected their gynecologist to promote options with the lowest impact on lifestyle.1 Yet, on the other hand, although 517 of 528 (98%) respondents who were 18 years or older had experienced stress urinary incontinence, fibroids, or uterine prolapse, fewer than 40% were aware that a number of minimally invasive techniques could greatly reduce the need for hysterectomy. Fewer than 50% of respondents who suffered from menorrhagia were aware of endometrial ablation as a treatment, and only 21% realized that it could be performed in an office. Only 45% of women who had leiomyomata had heard of myomectomy. And one of every three women who had stress urinary incontinence was unaware of sling procedures. Fewer than 20% knew that sterilization could be performed in an office.1 Although these women expected to be thoroughly informed by their physician, their lack of awareness suggests the opposite.

Use of minimally invasive options for 4 common surgeries
Resident physicians need more hands-on experience
A barrier to adoption of minimally invasive surgical approaches is the limited access many residents have to training and experience. This barrier could become especially problematic as third-party payers gain awareness of the advantages of minimally invasive surgery and shift reimbursement accordingly. In association with the American Society of Reproductive Medicine (ASRM), AAGL is actively engaged in enhancing surgical education through its Fellowship in Minimally Invasive Gynecology and is designing programs to supplement resident education. It is also establishing centers of excellence through its professional interest partner, the Council on Gynecologic Endoscopy (CGE).
Although the solution to our problem seems simple—increase the number of gynecologists who perform minimally invasive gynecologic surgery—that is easier said than done. According to data from the Accreditation Council for Graduate Medical Education ( ACGME), one of every three graduating residents has limited experience in minimally invasive procedures, and 30% of residents perform an average of only 12 laparoscopic operations a year as the primary surgeon. The average is even lower for hysteroscopic procedures!2

In an article published earlier this year in the Journal of Minimally Invasive Gynecology, Jon I. Einarsson, MD, MPH, and colleagues explore attitudes toward hysterectomy among gynecologists in the United States.3 Although nearly three quarters of hysterectomies are performed using an open abdominal approach, only 8% of respondents said they would choose the abdominal approach for themselves or their spouse. Among respondents who reported the highest surgical volumes, the percentage likely to choose a laparoscopic approach was significantly higher. The main barriers to laparoscopic hysterectomy? Limited opportunity for training during residency, technical difficulty, personal surgical experience, and operating time.3
We need an advanced curriculum
We have made tremendous progress in postgraduate education, thanks to the efforts of AAGL and ASRM. Nevertheless, we lack a specific, unified curriculum to train and ultimately credential gynecology residents and fellows in minimally invasive surgery.
Once again, our specialty lags behind general surgery. As of July 2009, all residents performing general surgery are required to complete and pass a course, “Fundamentals of laparoscopic surgery” (FLS). This joint undertaking of the American College of Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons has established minimum standards for basic cognitive and technical skills required for laparoscopic surgery.
Using the FLS course as a model, AAGL is designing a structured core curriculum to educate not only residents and fellows, but practicing gynecologists as well. Like the FLS course, the AAGL curriculum will emphasize both didactic teaching and lab skills, but it will be even more extensive because it will include hysteroscopy.
What we are doing now
After analyzing the needs of patients and providers around the world and acknowledging the lack of national standards to improve outcomes, AAGL and CGE have launched two other ambitious programs:
- a registry of physicians that includes operative experience and complication rate, as reviewed by their peers. This system lists physicians by level of practice, according to complexity of procedures, and by proactive experience and specialization
- a center of excellence in minimally invasive gynecology. The CGE recognizes that the achievement of best-in-class surgical outcomes requires not only an expert minimally invasive gynecologic surgeon but also an integrated, multidisciplinary surgical facility with systems and procedures to maximize quality, cost-effectiveness, and safety. For example, it is the vision of AAGL President C. Y. Liu that complex endometriosis surgery will one day be performed only by competent and experienced laparoscopic surgeons in endometriosis surgery centers.
Another initiative, launched in 2009, is the American Institute of Minimally Invasive Surgery (AIMIS). This not-for-profit organization offers member surgeons and hospitals not only accreditation and recognition, but also a number of useful products and programs, including:
- education
- guidance on technology
- business development
- insurance
- marketing strategies
- financial services
- consultation in practice management.
AIMIS is not a medical society but a national institute of which surgeons and hospitals become affiliated members.
Minimally invasive approach reduces costs, too
Rising health care costs have focused attention on ways to improve quality of care and contain costs at the same time, and minimally invasive surgery has emerged as a means of doing both. A recent study of 15,404 patients compared total abdominal hysterectomy with the minimally invasive alternatives (vaginal and laparoscopic hysterectomy) and found that the latter shortened hospitalization, reduced infection, and decreased the cost by $4,000 for the vaginal approach and $2,000 for laparoscopy.4 A study of more than 11,000 patients demonstrated a 52% reduction in the rate of hospital-acquired infection when a minimally invasive approach was utilized rather than open abdominal surgery.4
Some initiatives focus on the patient as a way of increasing utilization of minimally invasive approaches. For example, in Colorado, a 6,000-member self-funded medical plan launched a value-based program for patients undergoing colectomy, cholecystectomy, hysterectomy, appendectomy, and bariatric surgery. The program educated patients about minimally invasive options and encouraged them to seek consultation with a minimally invasive surgeon. Members who chose a minimally invasive procedure saw their copayment go down significantly. In addition, any surgeon who decided to perform one of these five procedures using an open approach was required to obtain preauthorization. Referral physicians were notified of the initiative, and minimally invasive surgeons were identified on the plan’s Web site.4
After 2 years, the plan saved nearly $1 million in direct hospital and surgeon claim costs. (Indirect savings from reduced need for prescription drugs and fewer complications were not included in this estimate. Nor were the economic advantages gained from the patient’s faster return to work and increased productivity.) After only 1 year, the utilization of minimally invasive hysterectomy rose from 28% to 80%!4
In New England, a 167-store grocery chain with 9,000 employees enrolled in the company’s self-funded health plan was able to reduce costs by identifying minimally invasive surgeons and steering members to them. The company covered 80% of surgical costs if minimally invasive surgery was performed, versus 70% for open surgery. Ultimately, the company’s per capita cost was 40% lower than all available benchmarks.4
We’re at a tipping point
The time has come for gynecologic surgeons to rejoin the revolution. Although we face many challenges, from limited experience and restricted access to training opportunities to lack of patient awareness of the benefits of minimally invasive surgery, it is imperative that we utilize minimally invasive approaches as often as possible. Educational opportunities are available, and third-party payers are beginning to demand it.
I believe it is only a matter of time before minimally invasive gynecologic procedures are the norm, not the exception.
You tell me that it’s evolution…
1. AAGL study finds women still in the dark about minimally invasive treatments for pelvic health disorders [press release]. Cypress, Calif: AAGL; 2008.
2. Accreditation Council for Graduate Medical Education. Obstetrics and gynecology case logs. National data report. 2008–2009. http://www.acgme.org/acWebsite/RRC_220/ObGynNatData0809.pdf. Accessed May 6, 2010.
3. Einarsson JI, Matteson KA, Schulkin J, Chavan NR, Sangi-Haghpeykar H. Minimally invasive hysterectomies—a survey on attitudes and barriers among practicing gynecologists. JMIG. 2010;17(2):167-175
4. Detweiler K, Hayes P, Cardinal A. Targeting surgery to reduce costs for employers. Employee Benefit Adviser. 2009. http://eba.benefitnews.com/news/targeting-surgery-to-reduce-costs-for-employers-2682543-1.html. Accessed May 6, 2010.
This commentary is based on the author’s 2008 presidential address to the 37th World Congress and Annual Meeting of the AAGL, which took place October 30 in Las Vegas, Nevada.
1. AAGL study finds women still in the dark about minimally invasive treatments for pelvic health disorders [press release]. Cypress, Calif: AAGL; 2008.
2. Accreditation Council for Graduate Medical Education. Obstetrics and gynecology case logs. National data report. 2008–2009. http://www.acgme.org/acWebsite/RRC_220/ObGynNatData0809.pdf. Accessed May 6, 2010.
3. Einarsson JI, Matteson KA, Schulkin J, Chavan NR, Sangi-Haghpeykar H. Minimally invasive hysterectomies—a survey on attitudes and barriers among practicing gynecologists. JMIG. 2010;17(2):167-175
4. Detweiler K, Hayes P, Cardinal A. Targeting surgery to reduce costs for employers. Employee Benefit Adviser. 2009. http://eba.benefitnews.com/news/targeting-surgery-to-reduce-costs-for-employers-2682543-1.html. Accessed May 6, 2010.
This commentary is based on the author’s 2008 presidential address to the 37th World Congress and Annual Meeting of the AAGL, which took place October 30 in Las Vegas, Nevada.