User login
Vortioxetine for major depressive disorder
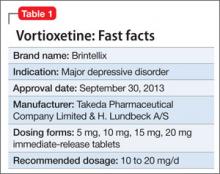
Vortioxetine is FDA-approved to treat major depressive disorder (MDD) (Table 1), having shown efficacy in relieving depressive symptoms in clinical trials.1 Vortioxetine’s mechanism of action enhances CNS serotonergic activity through inhibiting serotonin (5-HT) reuptake, agonizing the 5-HT1A receptor, partially agonizing the 5-HT1B receptor, and antagonizing the 5-HT3, 5-HT1D, and 5-HT7 receptors.
Clinical implications
It is hypothesized that depression is a heterogeneous disease caused by dysregulation of serotonin, norepinephrine, and dopamine, subsequently producing mood and neurovegetative symptoms of depression. Preclinical, in vivo studies indicate that vortioxetine enhances levels of serotonin, norepinephrine, dopamine, acetylcholine, and histamine in specific areas of the brain with the ability to improve depressive symptoms. Vortioxetine’s multimodal activity can be a useful alternative to other serotonergic antidepressants for some patients who are partial responders or non-responders to other treatment options. In addition, vortioxetine appears to have minimal effect on weight2 and sexual function—the latter being dose-dependent.3
How does it work?
Vortioxetine differs from other antidepressants in its multimodal activity (ie, affecting G-protein mode receptors, ion channel mode receptors, and neurotransmitter transporters). It inhibits the serotonin transporter (Ki = 1.6 nM), causing subsequent inhibition of serotonin reuptake into presynaptic neurons as well as selectively acting on the other subtypes of serotonergic receptors; however, activity on the norepinephrine transporter (Ki = 113 nM) and dopamine transporter (Ki > 1000 nM) is minimal. It is believed that mood-regulating effects of vortioxetine are caused by inhibition of serotonin reuptake, prolonged availability of serotonin to the postsynaptic neurons, its agonist activity on the 5-HT1A receptor (Ki = 15 nM), and partial agonist activity on the 5-HT1B receptor (Ki = 33 nM). Vortioxetine has strong affinity for the 5-HT3 receptor (Ki = 3.7 nM), which plays a role in modulation of centrally mediated nausea and vomiting. Positron emission tomography studies in humans determined that the occupancy of 5-HT transporter was 50% at 5 mg/d, 65% at 10 mg/d, and 80% at 20 mg/d.1,4 Human studies did not show that vortioxetine causes QTc prolongation.
Pharmacokinetics
Therapeutic activity of vortioxetine is thought to be due to the parent drug. It has a half-life of approximately 66 hours, and achieves steady state in 13.5 to 19 days. Bioavailability of vortioxetine is 75%; absorption does not depend on food; and 98% of drug is bound on plasma proteins.
Vortioxetine has linear pharmacokinetics, with maximum plasma concentration 7 to 11 hours after ingestion. The medication is metabolized primarily by oxidation through cytochrome P (CYP) 450: CYP2D6 (primary), CYP 3A4/5, CYP 2C19, CYP 2C9, CYP2A6, CYP2C8, and CYP2B6 with subsequent glucuronidation. This predisposes vortioxetine to potential pharmacokinetic drug-drug interaction warranting dose adjustment consideration when vortioxetine is coadministered with compounds inhibiting CYP2D6 or inducing CYP3A4 for ≥14 days, or for patients identified as poor 2D6 metabolizers.
In addition, coadministration of vortioxetine with serotonergic medications such as triptans, other antidepressants, and tramadol can cause potentially life-threatening serotonin syndrome, characterized by mental status changes, autonomic instability, neuromuscular aberrations, and GI symptoms. Concomitant use of vortioxetine and a nonsteroidal anti-inflammatory drug, aspirin, or warfarin can result in abnormal bleeding. Coadministration of vortioxetine with another highly protein-bound drug may increase or decrease the free concentration of either drug depending on the binding affinity of the drug for the protein.
Efficacy
Vortioxetine reduced depressive symptoms in 6 positive, 6- to 8-week, double-blind, placebo controlled and randomized studies and 1 maintenance study.1 Subjects were adults (Studies 1 to 5) and geriatric patients from age 64 to 88 who had ≥1 depressive episode before age 60 (Study 6). All met DSM-IV-TR criteria for MDD. Subjects with cognitive impairment scoring <24 on the Mini-Mental Status Examination and children were excluded. Depending on the study, response to the treatment was primarily measured on the Montgomery-Åsberg Depression Rating Scale (MADRS) or Hamilton Depression Rating Scale (HAM-D).
See Table 2 for a description of the positive studies, including dosages. In all studies, vortioxetine was superior to placebo at least one dosage for treating depression. In the 6- to 8-week placebo-controlled studies, an effect of vortioxetine based on the primary efficacy measure was generally observed starting at Week 2; that effect increased in subsequent weeks with the full antidepressant effect of vortioxetine generally not seen until study Week 4 or later.1
The maintenance treatment study included 639 patients who met DSM-IV-TR criteria for MDD. This study lasted for as long as 64 weeks. The first 12-week period was open-label, during which patients were treated with vortioxetine, 5 mg/d or 10 mg/d, with a possibility to adjust the dosage in the first 8 weeks. By the end of Week 12, 396 subjects achieved remission (MADRS <10), 75% of whom were taking vortioxetine, 10 mg/d. These patients were then randomly assigned to placebo or the dosage of vortioxetine to which they had responded, and continued the study for as long as 64 weeks. Time to relapse (MADRS total score ≥22) or an insufficient therapeutic response (as judged by the investigator) was the primary efficacy outcome, and demonstrated that vortioxetine was superior to placebo.
Tolerability
The tolerability of vortioxetine is comparable with other serotonergic antidepressants. In pooled analysis of pre-marketing studies, 5% to 8% of patients receiving vortioxetine (5 to 20 mg/d) discontinued treatment because of adverse effects (AEs), compared with 4% in the placebo group. Nausea was the most commonly reported AE leading to discontinuation and appeared to be dose dependent.
AEs, such as nausea, constipation, and vomiting, most commonly occurred in the first week of treatment, with a median duration of 2 weeks.5 In the 6- to 8-week trials, the most common AEs were nausea, constipation, and vomiting. In longer trials (24 to 64 weeks), the most common AE was nausea.
In 6- to 8-week placebo-controlled studies, vortioxetine was not associated with any clinically significant effect on vital signs or laboratory values in hematology, urinalysis, or serum chemistry (except sodium). Hyponatremia, the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), has occurred. The risk of developing SIADH and resultant hyponatremia is greater in geriatric patients and patients taking a diuretic.
Abruptly discontinuing vortioxetine can cause transient withdrawal symptoms, including headache and muscle tension, especially at a higher dosage (15 to 20 mg/d). Gradual tapering can reduce withdrawal symptoms.
Specific clinical issues
All antidepressants have a “black-box” warning about the potential for clinical worsening and increased suicidality early in treatment. Closely monitor patients for suicidal ideation and behaviors during the first months of treatment and with dosage changes.
Vortioxetine is categorized as pregnancy category C. Newborns exposed to a selective serotonin reuptake inhibitor (SSRI) in pregnancy may have an increased risk of persistent pulmonary hypertension during the neonatal period. When taken during the third trimester of pregnancy, SSRIs and serotonin-norepinephrine reuptake inhibitors can cause serious neonatal complications, including respiratory distress, cyanosis, apnea, and seizures, which may require longer hospitalization, respiratory support, or tube feeding for the infant. Consider risks and benefits of third-trimester use of an antidepressant. It is not known if vortioxetine is present in human breast milk.
Clinical studies on vortioxetine in pediatric patients have not been conducted.
No dosage adjustment is recommended on the basis of age for geriatric patients. No dose adjustment of vortioxetine is necessary on the basis of race, sex, ethnicity, renal function, or mild to moderate hepatic impairment. See Table 3 for practice points when prescribing vortioxetine. See Table 4 for contraindications to vortioxetine.
Dosing
The recommended starting dosage is 10 mg, administered orally once daily without regard to meals. Dosage should then be increased to 20 mg/d, as clinically warranted and tolerated. Consider a dosage decrease to 5 mg/d in patients who do not tolerate higher dosages or require drug adjustment because of drug-drug interaction or poor 2D6 metabolizer status.
Bottom Line
FDA-approved for major depressive disorder in adults, vortioxetine reduced depressive symptoms in 6 positive, double-blind, placebo-controlled, and randomized studies. The multimodal activity of vortioxeine can be a useful alternative to serotonergic antidepressants for some patients who are partial responders or nonresponders. Tolerability is comparable with other serotonergic antidepressants.
Related Resources
- Alam MY, Jacobsen PL, Chen Y, et al. Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2014; 29(1):36-44.
- Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29(3):217-226.
Drug Brand Names
Linezolid • Zyvox Vortioxetine • Brintellix
Methylene blue • Urolene Blue Warfarin • Coumadin
Tramadol • Ultram
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Vortioxetine [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2013.
2. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259-1272.
3. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a comprehensive review and meta-analysis. J Clin Psychopharmacol. 2009; 29(3):259-266.
4. Chen G, Lee R, Højer A, et al. Pharmacokinetic drug interactions involving vortioxetine (LU AA 21004), a multimodal antidepressant. Clin Drug Invetig. 2013; 33(10):727-736.
5. Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Prac. 2014;68(1):60-82.
Vortioxetine is FDA-approved to treat major depressive disorder (MDD) (Table 1), having shown efficacy in relieving depressive symptoms in clinical trials.1 Vortioxetine’s mechanism of action enhances CNS serotonergic activity through inhibiting serotonin (5-HT) reuptake, agonizing the 5-HT1A receptor, partially agonizing the 5-HT1B receptor, and antagonizing the 5-HT3, 5-HT1D, and 5-HT7 receptors.
Clinical implications
It is hypothesized that depression is a heterogeneous disease caused by dysregulation of serotonin, norepinephrine, and dopamine, subsequently producing mood and neurovegetative symptoms of depression. Preclinical, in vivo studies indicate that vortioxetine enhances levels of serotonin, norepinephrine, dopamine, acetylcholine, and histamine in specific areas of the brain with the ability to improve depressive symptoms. Vortioxetine’s multimodal activity can be a useful alternative to other serotonergic antidepressants for some patients who are partial responders or non-responders to other treatment options. In addition, vortioxetine appears to have minimal effect on weight2 and sexual function—the latter being dose-dependent.3
How does it work?
Vortioxetine differs from other antidepressants in its multimodal activity (ie, affecting G-protein mode receptors, ion channel mode receptors, and neurotransmitter transporters). It inhibits the serotonin transporter (Ki = 1.6 nM), causing subsequent inhibition of serotonin reuptake into presynaptic neurons as well as selectively acting on the other subtypes of serotonergic receptors; however, activity on the norepinephrine transporter (Ki = 113 nM) and dopamine transporter (Ki > 1000 nM) is minimal. It is believed that mood-regulating effects of vortioxetine are caused by inhibition of serotonin reuptake, prolonged availability of serotonin to the postsynaptic neurons, its agonist activity on the 5-HT1A receptor (Ki = 15 nM), and partial agonist activity on the 5-HT1B receptor (Ki = 33 nM). Vortioxetine has strong affinity for the 5-HT3 receptor (Ki = 3.7 nM), which plays a role in modulation of centrally mediated nausea and vomiting. Positron emission tomography studies in humans determined that the occupancy of 5-HT transporter was 50% at 5 mg/d, 65% at 10 mg/d, and 80% at 20 mg/d.1,4 Human studies did not show that vortioxetine causes QTc prolongation.
Pharmacokinetics
Therapeutic activity of vortioxetine is thought to be due to the parent drug. It has a half-life of approximately 66 hours, and achieves steady state in 13.5 to 19 days. Bioavailability of vortioxetine is 75%; absorption does not depend on food; and 98% of drug is bound on plasma proteins.
Vortioxetine has linear pharmacokinetics, with maximum plasma concentration 7 to 11 hours after ingestion. The medication is metabolized primarily by oxidation through cytochrome P (CYP) 450: CYP2D6 (primary), CYP 3A4/5, CYP 2C19, CYP 2C9, CYP2A6, CYP2C8, and CYP2B6 with subsequent glucuronidation. This predisposes vortioxetine to potential pharmacokinetic drug-drug interaction warranting dose adjustment consideration when vortioxetine is coadministered with compounds inhibiting CYP2D6 or inducing CYP3A4 for ≥14 days, or for patients identified as poor 2D6 metabolizers.
In addition, coadministration of vortioxetine with serotonergic medications such as triptans, other antidepressants, and tramadol can cause potentially life-threatening serotonin syndrome, characterized by mental status changes, autonomic instability, neuromuscular aberrations, and GI symptoms. Concomitant use of vortioxetine and a nonsteroidal anti-inflammatory drug, aspirin, or warfarin can result in abnormal bleeding. Coadministration of vortioxetine with another highly protein-bound drug may increase or decrease the free concentration of either drug depending on the binding affinity of the drug for the protein.
Efficacy
Vortioxetine reduced depressive symptoms in 6 positive, 6- to 8-week, double-blind, placebo controlled and randomized studies and 1 maintenance study.1 Subjects were adults (Studies 1 to 5) and geriatric patients from age 64 to 88 who had ≥1 depressive episode before age 60 (Study 6). All met DSM-IV-TR criteria for MDD. Subjects with cognitive impairment scoring <24 on the Mini-Mental Status Examination and children were excluded. Depending on the study, response to the treatment was primarily measured on the Montgomery-Åsberg Depression Rating Scale (MADRS) or Hamilton Depression Rating Scale (HAM-D).
See Table 2 for a description of the positive studies, including dosages. In all studies, vortioxetine was superior to placebo at least one dosage for treating depression. In the 6- to 8-week placebo-controlled studies, an effect of vortioxetine based on the primary efficacy measure was generally observed starting at Week 2; that effect increased in subsequent weeks with the full antidepressant effect of vortioxetine generally not seen until study Week 4 or later.1
The maintenance treatment study included 639 patients who met DSM-IV-TR criteria for MDD. This study lasted for as long as 64 weeks. The first 12-week period was open-label, during which patients were treated with vortioxetine, 5 mg/d or 10 mg/d, with a possibility to adjust the dosage in the first 8 weeks. By the end of Week 12, 396 subjects achieved remission (MADRS <10), 75% of whom were taking vortioxetine, 10 mg/d. These patients were then randomly assigned to placebo or the dosage of vortioxetine to which they had responded, and continued the study for as long as 64 weeks. Time to relapse (MADRS total score ≥22) or an insufficient therapeutic response (as judged by the investigator) was the primary efficacy outcome, and demonstrated that vortioxetine was superior to placebo.
Tolerability
The tolerability of vortioxetine is comparable with other serotonergic antidepressants. In pooled analysis of pre-marketing studies, 5% to 8% of patients receiving vortioxetine (5 to 20 mg/d) discontinued treatment because of adverse effects (AEs), compared with 4% in the placebo group. Nausea was the most commonly reported AE leading to discontinuation and appeared to be dose dependent.
AEs, such as nausea, constipation, and vomiting, most commonly occurred in the first week of treatment, with a median duration of 2 weeks.5 In the 6- to 8-week trials, the most common AEs were nausea, constipation, and vomiting. In longer trials (24 to 64 weeks), the most common AE was nausea.
In 6- to 8-week placebo-controlled studies, vortioxetine was not associated with any clinically significant effect on vital signs or laboratory values in hematology, urinalysis, or serum chemistry (except sodium). Hyponatremia, the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), has occurred. The risk of developing SIADH and resultant hyponatremia is greater in geriatric patients and patients taking a diuretic.
Abruptly discontinuing vortioxetine can cause transient withdrawal symptoms, including headache and muscle tension, especially at a higher dosage (15 to 20 mg/d). Gradual tapering can reduce withdrawal symptoms.
Specific clinical issues
All antidepressants have a “black-box” warning about the potential for clinical worsening and increased suicidality early in treatment. Closely monitor patients for suicidal ideation and behaviors during the first months of treatment and with dosage changes.
Vortioxetine is categorized as pregnancy category C. Newborns exposed to a selective serotonin reuptake inhibitor (SSRI) in pregnancy may have an increased risk of persistent pulmonary hypertension during the neonatal period. When taken during the third trimester of pregnancy, SSRIs and serotonin-norepinephrine reuptake inhibitors can cause serious neonatal complications, including respiratory distress, cyanosis, apnea, and seizures, which may require longer hospitalization, respiratory support, or tube feeding for the infant. Consider risks and benefits of third-trimester use of an antidepressant. It is not known if vortioxetine is present in human breast milk.
Clinical studies on vortioxetine in pediatric patients have not been conducted.
No dosage adjustment is recommended on the basis of age for geriatric patients. No dose adjustment of vortioxetine is necessary on the basis of race, sex, ethnicity, renal function, or mild to moderate hepatic impairment. See Table 3 for practice points when prescribing vortioxetine. See Table 4 for contraindications to vortioxetine.
Dosing
The recommended starting dosage is 10 mg, administered orally once daily without regard to meals. Dosage should then be increased to 20 mg/d, as clinically warranted and tolerated. Consider a dosage decrease to 5 mg/d in patients who do not tolerate higher dosages or require drug adjustment because of drug-drug interaction or poor 2D6 metabolizer status.
Bottom Line
FDA-approved for major depressive disorder in adults, vortioxetine reduced depressive symptoms in 6 positive, double-blind, placebo-controlled, and randomized studies. The multimodal activity of vortioxeine can be a useful alternative to serotonergic antidepressants for some patients who are partial responders or nonresponders. Tolerability is comparable with other serotonergic antidepressants.
Related Resources
- Alam MY, Jacobsen PL, Chen Y, et al. Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2014; 29(1):36-44.
- Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29(3):217-226.
Drug Brand Names
Linezolid • Zyvox Vortioxetine • Brintellix
Methylene blue • Urolene Blue Warfarin • Coumadin
Tramadol • Ultram
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Vortioxetine is FDA-approved to treat major depressive disorder (MDD) (Table 1), having shown efficacy in relieving depressive symptoms in clinical trials.1 Vortioxetine’s mechanism of action enhances CNS serotonergic activity through inhibiting serotonin (5-HT) reuptake, agonizing the 5-HT1A receptor, partially agonizing the 5-HT1B receptor, and antagonizing the 5-HT3, 5-HT1D, and 5-HT7 receptors.
Clinical implications
It is hypothesized that depression is a heterogeneous disease caused by dysregulation of serotonin, norepinephrine, and dopamine, subsequently producing mood and neurovegetative symptoms of depression. Preclinical, in vivo studies indicate that vortioxetine enhances levels of serotonin, norepinephrine, dopamine, acetylcholine, and histamine in specific areas of the brain with the ability to improve depressive symptoms. Vortioxetine’s multimodal activity can be a useful alternative to other serotonergic antidepressants for some patients who are partial responders or non-responders to other treatment options. In addition, vortioxetine appears to have minimal effect on weight2 and sexual function—the latter being dose-dependent.3
How does it work?
Vortioxetine differs from other antidepressants in its multimodal activity (ie, affecting G-protein mode receptors, ion channel mode receptors, and neurotransmitter transporters). It inhibits the serotonin transporter (Ki = 1.6 nM), causing subsequent inhibition of serotonin reuptake into presynaptic neurons as well as selectively acting on the other subtypes of serotonergic receptors; however, activity on the norepinephrine transporter (Ki = 113 nM) and dopamine transporter (Ki > 1000 nM) is minimal. It is believed that mood-regulating effects of vortioxetine are caused by inhibition of serotonin reuptake, prolonged availability of serotonin to the postsynaptic neurons, its agonist activity on the 5-HT1A receptor (Ki = 15 nM), and partial agonist activity on the 5-HT1B receptor (Ki = 33 nM). Vortioxetine has strong affinity for the 5-HT3 receptor (Ki = 3.7 nM), which plays a role in modulation of centrally mediated nausea and vomiting. Positron emission tomography studies in humans determined that the occupancy of 5-HT transporter was 50% at 5 mg/d, 65% at 10 mg/d, and 80% at 20 mg/d.1,4 Human studies did not show that vortioxetine causes QTc prolongation.
Pharmacokinetics
Therapeutic activity of vortioxetine is thought to be due to the parent drug. It has a half-life of approximately 66 hours, and achieves steady state in 13.5 to 19 days. Bioavailability of vortioxetine is 75%; absorption does not depend on food; and 98% of drug is bound on plasma proteins.
Vortioxetine has linear pharmacokinetics, with maximum plasma concentration 7 to 11 hours after ingestion. The medication is metabolized primarily by oxidation through cytochrome P (CYP) 450: CYP2D6 (primary), CYP 3A4/5, CYP 2C19, CYP 2C9, CYP2A6, CYP2C8, and CYP2B6 with subsequent glucuronidation. This predisposes vortioxetine to potential pharmacokinetic drug-drug interaction warranting dose adjustment consideration when vortioxetine is coadministered with compounds inhibiting CYP2D6 or inducing CYP3A4 for ≥14 days, or for patients identified as poor 2D6 metabolizers.
In addition, coadministration of vortioxetine with serotonergic medications such as triptans, other antidepressants, and tramadol can cause potentially life-threatening serotonin syndrome, characterized by mental status changes, autonomic instability, neuromuscular aberrations, and GI symptoms. Concomitant use of vortioxetine and a nonsteroidal anti-inflammatory drug, aspirin, or warfarin can result in abnormal bleeding. Coadministration of vortioxetine with another highly protein-bound drug may increase or decrease the free concentration of either drug depending on the binding affinity of the drug for the protein.
Efficacy
Vortioxetine reduced depressive symptoms in 6 positive, 6- to 8-week, double-blind, placebo controlled and randomized studies and 1 maintenance study.1 Subjects were adults (Studies 1 to 5) and geriatric patients from age 64 to 88 who had ≥1 depressive episode before age 60 (Study 6). All met DSM-IV-TR criteria for MDD. Subjects with cognitive impairment scoring <24 on the Mini-Mental Status Examination and children were excluded. Depending on the study, response to the treatment was primarily measured on the Montgomery-Åsberg Depression Rating Scale (MADRS) or Hamilton Depression Rating Scale (HAM-D).
See Table 2 for a description of the positive studies, including dosages. In all studies, vortioxetine was superior to placebo at least one dosage for treating depression. In the 6- to 8-week placebo-controlled studies, an effect of vortioxetine based on the primary efficacy measure was generally observed starting at Week 2; that effect increased in subsequent weeks with the full antidepressant effect of vortioxetine generally not seen until study Week 4 or later.1
The maintenance treatment study included 639 patients who met DSM-IV-TR criteria for MDD. This study lasted for as long as 64 weeks. The first 12-week period was open-label, during which patients were treated with vortioxetine, 5 mg/d or 10 mg/d, with a possibility to adjust the dosage in the first 8 weeks. By the end of Week 12, 396 subjects achieved remission (MADRS <10), 75% of whom were taking vortioxetine, 10 mg/d. These patients were then randomly assigned to placebo or the dosage of vortioxetine to which they had responded, and continued the study for as long as 64 weeks. Time to relapse (MADRS total score ≥22) or an insufficient therapeutic response (as judged by the investigator) was the primary efficacy outcome, and demonstrated that vortioxetine was superior to placebo.
Tolerability
The tolerability of vortioxetine is comparable with other serotonergic antidepressants. In pooled analysis of pre-marketing studies, 5% to 8% of patients receiving vortioxetine (5 to 20 mg/d) discontinued treatment because of adverse effects (AEs), compared with 4% in the placebo group. Nausea was the most commonly reported AE leading to discontinuation and appeared to be dose dependent.
AEs, such as nausea, constipation, and vomiting, most commonly occurred in the first week of treatment, with a median duration of 2 weeks.5 In the 6- to 8-week trials, the most common AEs were nausea, constipation, and vomiting. In longer trials (24 to 64 weeks), the most common AE was nausea.
In 6- to 8-week placebo-controlled studies, vortioxetine was not associated with any clinically significant effect on vital signs or laboratory values in hematology, urinalysis, or serum chemistry (except sodium). Hyponatremia, the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), has occurred. The risk of developing SIADH and resultant hyponatremia is greater in geriatric patients and patients taking a diuretic.
Abruptly discontinuing vortioxetine can cause transient withdrawal symptoms, including headache and muscle tension, especially at a higher dosage (15 to 20 mg/d). Gradual tapering can reduce withdrawal symptoms.
Specific clinical issues
All antidepressants have a “black-box” warning about the potential for clinical worsening and increased suicidality early in treatment. Closely monitor patients for suicidal ideation and behaviors during the first months of treatment and with dosage changes.
Vortioxetine is categorized as pregnancy category C. Newborns exposed to a selective serotonin reuptake inhibitor (SSRI) in pregnancy may have an increased risk of persistent pulmonary hypertension during the neonatal period. When taken during the third trimester of pregnancy, SSRIs and serotonin-norepinephrine reuptake inhibitors can cause serious neonatal complications, including respiratory distress, cyanosis, apnea, and seizures, which may require longer hospitalization, respiratory support, or tube feeding for the infant. Consider risks and benefits of third-trimester use of an antidepressant. It is not known if vortioxetine is present in human breast milk.
Clinical studies on vortioxetine in pediatric patients have not been conducted.
No dosage adjustment is recommended on the basis of age for geriatric patients. No dose adjustment of vortioxetine is necessary on the basis of race, sex, ethnicity, renal function, or mild to moderate hepatic impairment. See Table 3 for practice points when prescribing vortioxetine. See Table 4 for contraindications to vortioxetine.
Dosing
The recommended starting dosage is 10 mg, administered orally once daily without regard to meals. Dosage should then be increased to 20 mg/d, as clinically warranted and tolerated. Consider a dosage decrease to 5 mg/d in patients who do not tolerate higher dosages or require drug adjustment because of drug-drug interaction or poor 2D6 metabolizer status.
Bottom Line
FDA-approved for major depressive disorder in adults, vortioxetine reduced depressive symptoms in 6 positive, double-blind, placebo-controlled, and randomized studies. The multimodal activity of vortioxeine can be a useful alternative to serotonergic antidepressants for some patients who are partial responders or nonresponders. Tolerability is comparable with other serotonergic antidepressants.
Related Resources
- Alam MY, Jacobsen PL, Chen Y, et al. Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2014; 29(1):36-44.
- Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29(3):217-226.
Drug Brand Names
Linezolid • Zyvox Vortioxetine • Brintellix
Methylene blue • Urolene Blue Warfarin • Coumadin
Tramadol • Ultram
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Vortioxetine [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2013.
2. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259-1272.
3. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a comprehensive review and meta-analysis. J Clin Psychopharmacol. 2009; 29(3):259-266.
4. Chen G, Lee R, Højer A, et al. Pharmacokinetic drug interactions involving vortioxetine (LU AA 21004), a multimodal antidepressant. Clin Drug Invetig. 2013; 33(10):727-736.
5. Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Prac. 2014;68(1):60-82.
1. Vortioxetine [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2013.
2. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259-1272.
3. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a comprehensive review and meta-analysis. J Clin Psychopharmacol. 2009; 29(3):259-266.
4. Chen G, Lee R, Højer A, et al. Pharmacokinetic drug interactions involving vortioxetine (LU AA 21004), a multimodal antidepressant. Clin Drug Invetig. 2013; 33(10):727-736.
5. Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Prac. 2014;68(1):60-82.