Quiz
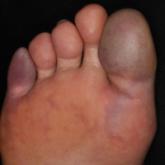
Painful Purple Toes
- Author:
- Chika Ohata, MD, PhD
- Taichi Imamura, MD
Article
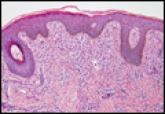
Brown Macule on the Waist
- Author:
- Chika Ohata, MD, PhD
Article
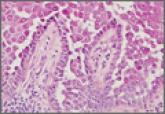
Hailey-Hailey Disease
- Author:
- Chika Ohata, MD, PhD
Hailey-Hailey disease typically presents as suprabasal blisters with a perivascular and interstitial lymphocytic infiltrate. The differential...
