User login
Mortality After Therapeutic Hypothermia
There are over 350,000 cases of out‐of‐hospital cardiac arrest (OHCA) each year in the United States1, 2 and, with supportive therapy alone, only a fraction of victims survive to hospital discharge. Rapid intervention including cardiopulmonary resuscitation in the moments following arrest is critical to minimizing neurologic injury, morbidity, and mortality. In 2002, two small randomized controlled trials showed a survival benefit of therapeutic hypothermia (TH) when provided within 12 hours after return of circulation following an OHCA and, to date, TH remains one of the few interventions with proven mortality benefit after initial cardiopulmonary resuscitation.3, 4 Since 2003, TH has been incorporated into the American Heart Association practice guidelines57 and use of TH has steadily increased, but widespread clinical uptake remains low.8, 9
The initial studies that evaluated TH were small, with only 189 patients included in the TH arms of the 2 trials combined. To date, only a few studies have replicated this initial observation in real‐world settings, with little analysis of outcomes in US centers in particular.1013 Accordingly, we aimed to examine the real‐world experience with TH in the United States using a large administrative claims database of all California hospital admissions to describe utilization trends, hospital mortality, and volumeoutcome relationships associated with the intervention.
MATERIALS AND METHODS
Data
We identified all admissions to California hospitals during 19992008 based on discharge records from the California Office of Statewide Health Planning and Development. Our study period included cases of TH performed prior to the 2002 major clinical trials, since TH was in occasional use prior to the publication of these trials. The data was de‐identified and publicly available, and therefore exempt from review by the Institutional Review Board. In addition to hospital name, each discharge record included patient age, gender, admission year, International Classification of Disease, Ninth Revision (ICD‐9) code for presenting primary and secondary diagnoses, procedure codes, and disposition (discharge to home or rehabilitation, in‐hospital death). All California hospitals were included in the registry (n = 419). We defined teaching status for each hospital based on membership in the Council of Teaching Hospitals, as reported in the American Hospital Association's Annual Survey (n = 19 teaching hospitals).14
Setting and Participants
We used discharge diagnoses to identify patients who could be considered eligible for therapeutic hypothermia after cardiac arrest. We classified patients as eligible for therapeutic hypothermia after cardiac arrest based on ICD‐9 diagnosis codes that indicated the presence of both cardiac arrest and anoxic brain injury in the administrative diagnoses. Because of known imprecision in using billing codes to identify patients with cardiac arrest,15, 16 we broadly defined cardiac arrest to include those patients with ICD‐9 codes for cardiac arrest, ventricular fibrillation (VF), or ventricular tachycardia (VT) (see Supporting Table 1 in the online version of this article). We could not distinguish between out‐of‐hospital and in‐hospital cardiac arrest based on administrative diagnoses. To ensure that we included only patients with cardiac arrest complicated by neurologic insult, we required an ICD‐9 diagnosis of either anoxic brain injury, coma, or persistent vegetative state. Claims did not allow us to distinguish among initial cardiac arrest rhythms (VF vs pulseless VT vs asystole). Patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia were excluded.3, 17 We did not exclude patients based on coagulopathy (which is considered a contraindication to TH), since ICD‐9 coding did not allow us to determine the severity of the coagulopathy or whether it was a result of therapeutic hypothermia itself.
We used the ICD‐9 procedure code (99.81) for TH to first identify patients who underwent TH from 1999 to 2008. Since this code also applies to TH used during cardiac and neurosurgery, we examined each of these cases and excluded individuals who underwent cardiac surgery or neurosurgery during the hospitalization. As in our eligible for TH definition, we excluded patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia. Patients who underwent therapeutic hypothermia but for whom a specific procedure code was not recorded in the discharge abstractperhaps because the medical institution did not directly bill for the procedurecould not be identified.
Statistical Analysis
We used a multivariable logistic model to estimate differences in hospital mortality after cardiac arrest associated with use of therapeutic hypothermia. We conducted 2 specifications. In our baseline specification, we accounted for case‐mix differences between those who underwent TH and those who did not by adjusting for age, gender, year of admission, the number of Charlson‐Deyo comorbidities,1820 and hospital teaching status. Because mortality after cardiac arrest in centers that perform therapeutic hypothermia may be different from centers that do not, even for patients who do not undergo the procedure, we included indicators for volume tercile of therapeutic hypothermia cases performed. Volume of therapeutic hypothermia was defined at the hospital level as the total number of cases performed by that hospital from 1999 to 2008.
In order to explore how hospital teaching status, volume of therapeutic hypothermia procedures (broken into terciles), and year of admission affected the association between hospital mortality after cardiac arrest and therapeutic hypothermia, our baseline logistic model was expanded to include interactions between therapeutic hypothermia and each of these variables. The interaction between therapeutic hypothermia and year explored whether the effectiveness of the procedure changed over time, as case‐selection, method of therapeutic hypothermia (cold saline vs commercially available devices), and experience changed in California hospitals. For both specifications, we reported the odds ratio of hospital mortality among patients undergoing therapeutic hypothermia, as well as risk‐adjusted mortality for both TH and non‐TH groups.
STATA version 11 (STATA Corp, College Station, TX) was used for statistical analyses, and a 2‐sided P 0.05 was used.
RESULTS
Descriptive Data
Table 1 reports summary statistics for patients with cardiac arrest complicated by neurologic insult (anoxic brain injury, coma, or persistent vegetative state) between 1999 and 2008. Across all years, 204 patients were identified as undergoing TH. In comparison, 105 patients were identified as undergoing TH in 2008 alone. Patients who underwent TH were less likely to be male (30.7% vs 44.6% male, P < 0.01), were younger (63.9 15.0 years vs 67.3 15.7 years, P = 0.03), and had equivalent numbers of Charlson‐Deyo comorbidities (2.5 2.0 diagnoses vs 2.5 2.0 diagnoses, P = 0.89). Therapeutic hypothermia was more commonly employed at teaching hospitals (51/3709 [1.4%] vs 153/42,942 [0.4%], P < 0.01). There was a trend toward decreased unadjusted mortality among patients who underwent therapeutic hypothermia compared with those who did not (56.9% vs 62.8%, P = 0.08).
| Therapeutic Hypothermia | No Therapeutic Hypothermia | P Value | |
|---|---|---|---|
| |||
| No. observations | 204 | 46,629 | |
| No. cases in teaching hospitals | 51 | 3,658 | |
| No. cases in non‐teaching hospitals | 153 | 42,789 | |
| Age, y | 63.9 15.0 | 67.3 15.7 | 0.06 |
| Male | 30.7 | 44.6 | <0.01 |
| Hospital mortality, % | 56.9 | 62.8 | 0.08 |
| Comorbidities | |||
| No. Charlson‐Deyo comorbidities | 2.5 2.0 | 2.5 2.0 | 0.89 |
| Coronary artery disease, % | 48.0 | 38.0 | <0.01 |
| Acute myocardial infarction, % | 42.6 | 28.9 | <0.01 |
| Congestive heart failure, % | 27.9 | 35.3 | 0.03 |
| Hypertension, % | 36.3 | 33.2 | 0.83 |
| Acute renal failure, % | 33.3 | 26.6 | 0.03 |
| Diabetes mellitus, % | 30.9 | 23.0 | <0.01 |
| Chronic obstructive pulmonary disease, % | 10.3 | 19.3 | <0.01 |
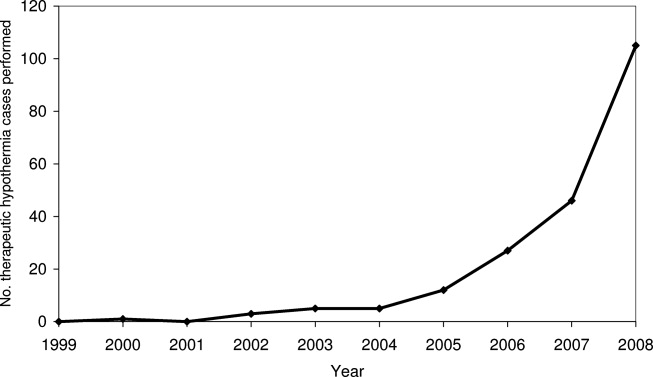
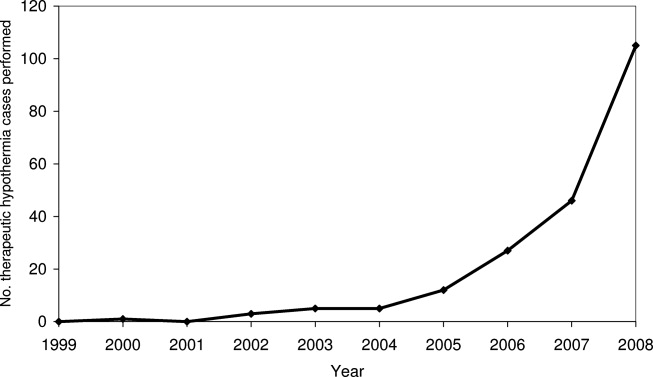
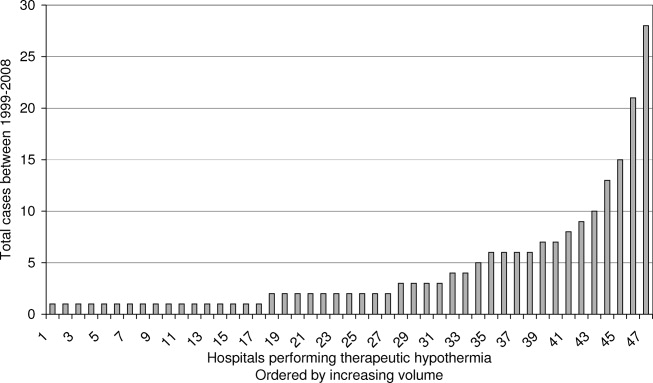
Figures 1 and 2 provide additional aggregate statistics on therapeutic hypothermia in California hospitals. Figure 1 plots the number of therapeutic hypothermia cases recorded in the administrative discharge registry between 1999 and 2008. Of the 204 total cases identified during this period, 178 (87.3%) were performed between 2006 and 2008. Figure 2 shows the distribution of TH cases across centers that performed therapeutic hypothermia (n = 47 hospitals, 11.3 % of all hospitals). Ten centers accounted for 124/204 (60.7%) of the total patients treated with TH after cardiac arrest; the top 3 centers accounted for 64 (31.4%) of the treated patients. Twenty‐seven hospitals were identified as performing therapeutic hypothermia on only 1 or 2 patients between 1999 and 2008.
Risk‐Adjusted Mortality
Table 2 presents the odds ratio of factors predicting in‐hospital mortality after cardiac arrest complicated by neurologic insult. Factors include use of TH after cardiac arrest, age, gender, year of admission, number of Charlson‐Deyo comorbidities, hospital teaching status, and volume tercile of hospitals that performed therapeutic hypothermia. Overall, patients who were older, male, and had greater comorbidities were statistically more likely to die after cardiac arrest complicated by neurologic insult. Regardless of whether they underwent TH, patients admitted to hospitals in the highest volume tercile of TH use were more likely to die after cardiac arrest. Adjusting for volume tercile, teaching hospital status was not independently associated with mortality after cardiac arrest. The adjusted odds ratio of mortality among patients undergoing therapeutic hypothermia was 0.80 (95% confidence interval [CI] 0.601.06, P = 0.11). The adjusted probability of inpatient mortality among patients undergoing therapeutic hypothermia was 57.5% (95% CI 50.764.3%) compared to those who did not 62.8% (95% CI 61.763.9%, P = 0.11).
| Variable | Odds Ratio of Hospital Mortality (95% CI) | P Value |
|---|---|---|
| ||
| No. observations | 46,651 | |
| Age* | ||
| 6569 | 1.19 (1.121.28) | <0.001 |
| 7074 | 1.29 (1.201.39) | <0.001 |
| 7579 | 1.55 (1.441.67) | <0.001 |
| 8084 | 1.79 (1.651.93) | <0.001 |
| 85 and over | 2.06 (1.892.25) | <0.001 |
| Male | 1.15 (1.101.21) | <0.001 |
| Teaching hospital | 1.13 (0.951.34) | 0.17 |
| No. Charlson‐Deyo comorbidities | 1.09 (1.081.10) | <0.001 |
| Year trend | 0.98 (0.970.99) | <0.001 |
| Volume tercile among hospitals performing TH | ||
| First tercile | 0.94 (0.791.12) | 0.48 |
| Second tercile | 1.03 (0.801.33) | 0.82 |
| Third tercile | 1.20 (1.051.36) | 0.006 |
| Therapeutic hypothermia | 0.80 (0.601.06) | 0.11 |
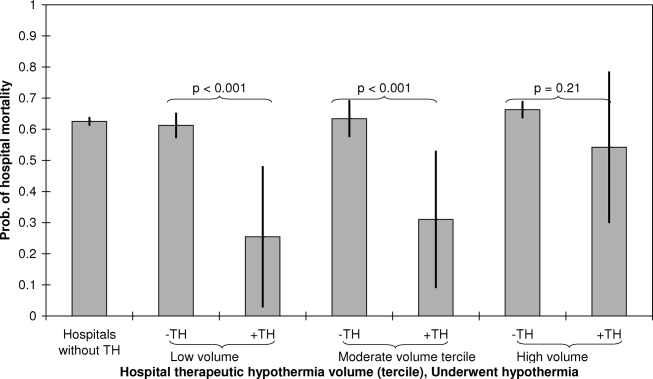
Figure 3 presents adjusted mortality after cardiac arrest in hospitals that did not perform TH, as well as adjusted mortality associated with TH for each volume tercile of hospitals that performed the procedure. Hospital mortality rates among patients not receiving TH after cardiac arrest were slightly higher in hospitals in the high volume tercile of TH (66.3%, 95% CI 63.868.8%) compared to hospitals in low and moderate volume terciles and to hospitals not performing TH (P < 0.001). Hospital mortality rates among low and moderate TH volume centers and in centers not performing TH were similar (62.3%, 61.3%, and 63.4%, respectively). Among both the low volume and moderate volume terciles, however, patients who underwent TH after cardiac arrest were significantly less likely to die in‐hospital compared to those who did not. For patients admitted to hospitals in the low volume tercile, those undergoing therapeutic hypothermia had an adjusted hospital mortality rate of 25.5% (95% CI 3.047.9%) compared to those who did not undergo TH (adjusted mortality 61.3%, 95% CI 57.465.1%), P < 0.001. In the moderate volume tercile, patients receiving therapeutic hypothermia had an adjusted hospital mortality rate of 31.0% (95% CI 9.2%52.8%) compared to 63.4% (95% CI 57.769.1%), P < 0.001, among those not undergoing the procedure. There was no statistically significant difference in adjusted mortality between those who underwent TH and those who did not, in hospitals in the highest volume tercile (P = 0.211). In addition to examining how volume of therapeutic hypothermia performed by hospitals affected the association between TH and hospital mortality, we also examined whether year of admission and teaching hospital independently modified the association. Neither year of admission nor teaching hospital statistically significantly affected the association between therapeutic hypothermia and hospital mortality after cardiac arrest at the P < 0.10 level.
DISCUSSION
In an administrative database of all admissions to California hospitals, we demonstrated that use of TH increased steadily since the publication of the initial clinical trials in 2002. The absolute level of TH utilization in our study undoubtedly represents a significant underestimation of actual TH utilization, however, our study does provide an assessment of the utilization trends over time. The bulk of TH use appears to be performed in a small group of high volume centers, and 89% of California hospitals did not perform TH during the study period (as assessed by procedure billing codes). Additionally, within the limitations of a retrospective, administrative claims‐based study design, TH appears to be associated with a similar in‐hospital mortality rate to that seen in clinical trials.3, 4 In exploratory analyses, there appears to be a particular benefit of TH in low and moderate volume centers, though these findings should be considered hypothesis‐generating.
Despite the body of evidence in favor of TH, utilization in our study and others appears quite low. In a 2005 survey of physicians, 87% of respondents had never used therapeutic hypothermia, citing inadequate data, technical limitations, and lack of incorporation in the Advanced Cardiac Life Support (ACLS) protocol as principal justifications.8 Other surveys have shown similar results and noted that critical care physicians and those working in large medical centers were more likely to adopt the therapy.9 Advocates of the therapy have suggested that an explicit hospital‐based plan developed by key stakeholders can help facilitate implementation.21 Accordingly, there is growing interest in developing centers of expertise in highly intensive therapies such as TH. For instance, the New York City Emergency Medical Service has begun to explore a protocol to divert TH candidates to specialized centers.22, 23 Some favorable results have been reported in individual hospitals and local hospital systems.2428
Our data suggest that TH is associated with an in‐hospital mortality rate that is comparable to that reported in the clinical trials. For example, in a 2009 meta‐analysis of 4 clinical trials and 1 abstract (481 patients in total), TH was associated with a 35% relative mortality benefit as compared to standard post‐resuscitation care.29 It has been estimated that broad TH implementation could save thousands of lives30 and many authors have advocated for its use and outlined explicit protocols for implementation.17 Furthermore, TH appears to be cost‐effective in line with other accepted therapies. Assuming the Hypothermia After Cardiac Arrest (HACA) trial inclusion criteria, even at extreme estimates for costs, the cost‐effectiveness of hypothermia remains less than $100,000 per quality‐adjusted life year.31
There are important limitations of this study. Our use of administrative claims data certainly underestimates the level of TH utilization, since we could only identify cases in which TH was included in the billing codes for the hospitalization. Hospitals may vary in utilization of this particular billing code for TH in ways that bias our estimated associations. The ICD‐9 code 99.81 for therapeutic hypothermia was also not developed for post‐cardiac arrest TH specifically, so use of the code may actually lag clinical utilization. Although the observed trend in TH utilization is likely mainly due to a true increase in utilization, it is possible that some of the observed increase is due to an increase in utilization of TH procedure billing codes. Our TH utilization estimates should be construed as a lower bound of the actual rates. Additionally, although the estimated real‐world mortality benefit of TH may be comparable to that of clinical trials, the equivalence of patients in our sample to those in published randomized trials is uncertain. Similarly, even after adjusting for age, gender, year of admission, comorbidities, hospital teaching status, and TH volume, there are likely many unmeasured variables that influence mortality in both the TH and comparison groups. There are also likely patients included in our comparison group who had both cardiac arrest or ventricular tachycardia and anoxic brain injury, but who were not candidates for TH as the episode of cardiac arrest followed rather than preceded the anoxic brain injury. Since we lack detailed clinical data about the TH cases (initial rhythm, time before return of circulation, preexisting disease states, etc.), we are unable to match controls directly to cases. Additionally, we lack data to assess neurologic recovery or quality of life after arrest.
The observation that a mortality benefit in our study could be detected only in low and moderate volume centers requires further exploration. Indeed, one might expect that high volume centers may have better outcomes with TH as a result of more robust infrastructure, technical experience, and available resources. Our finding that mortality benefits of TH appear concentrated in centers with low to moderate volume of TH utilization suggest at least 1 of 2 possibilities. First, low and moderate volume centers may perform TH in a subset of patients who benefit most from the intervention or, alternatively, in the most viable cardiac arrest cases (those who may fare well with or without the therapy). Consequently, we may observe relatively favorable outcomes in this group due to this selection bias. Second, high volume centersdespite having more expertisemay also attract patients at higher mortality risk due to referral bias. This would lead us to estimate lower mortality benefits associated with TH in these high volume centers. Indeed, greater observed mortality at high volume centers regardless of TH status suggests that overall acuity is higher at high volume centers. While our inferences are greatly affected by issues of case selection and referral bias, it also important to consider the possibility that the estimated mortality benefit of TH in higher volume centers is lower because of the selection of patients who do not meet current guidelines for treatment with TH. Distinguishing whether the selection of patients undergoing TH at high volume centers is appropriate or inappropriate based on current guidelines is an important issue that merits further research with datasets with more refined patient clinical information.
In summary, therapeutic hypothermia utilization is low, but the rate of implementation has increased since the publication of the initial clinical trials in 2002. The bulk of TH utilization appears limited to a subset of high volume centers, and most centers in California appear to have not used the therapy. Real‐world in‐hospital mortality associated with TH is comparable to that reported in randomized clinical trials.
Acknowledgements
Disclosures: Dr Romley received support from NIH grant R03AG031990‐A1. Dr Noseworthy received support from the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology granted by the Heart Rhythm Society. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources. The contents of this article have not been published in any other peer‐reviewed media, and the manuscript is not under review elsewhere. All authors listed have contributed sufficiently to this project to be included as authors. The authors have no conflict of interest, financial or otherwise.
- , , , et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163.
- , . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- , , , et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563.
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556.
- , , , et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 7.5: postresuscitation support. Circulation. 2005;112(suppl I):IV‐84–IV‐88.
- , , , et al. Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(suppl 1):e93–e174.
- , , , et al. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186.
- , , , et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940.
- , , , et al. Adverse events and their relation to mortality in out‐of‐hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med.39(1):57–64.
- , , , et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934.
- . Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–1047.
- , , , et al. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–214.
- American Hospital Association (AHA). American Hospital Association 2001 Annual Survey. Chicago, IL: Health Forum, LLC.
- , , . A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296.
- . Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604.
- . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–1264.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- , , , et al. Practical implementation of therapeutic hypothermia after cardiac arrest. Hosp Pract (Minneap). 2009;37(1):71–83.
- . Paramedics will employ new therapy in cardiac arrest cases. New York Times. August 3, 2010:A18.
- , , , et al. Resuscitation center designation: recommendations for emergency medical services practices. Prehosp Emerg Care. 2010;14(1):51–61.
- , , , et al. Implementation of a hospital‐wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol. 2010;9(4):216–220.
- , , , et al. Therapeutic hypothermia protocol in a community emergency department. West J Emerg Med. 2010;11(4):367–372.
- , , , et al. Implementation of a post‐cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out‐of‐hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366.
- , , , et al. Improved out‐of‐hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med. 2010;56(4):348–357.
- , , , et al. Community‐based application of mild therapeutic hypothermia for survivors of cardiac arrest. South Med J. 2010;103(4):295–300.
- , , , et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009(4):CD004128.
- , . Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22(4):711–728.
- , , , et al. Cost‐effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–428.
There are over 350,000 cases of out‐of‐hospital cardiac arrest (OHCA) each year in the United States1, 2 and, with supportive therapy alone, only a fraction of victims survive to hospital discharge. Rapid intervention including cardiopulmonary resuscitation in the moments following arrest is critical to minimizing neurologic injury, morbidity, and mortality. In 2002, two small randomized controlled trials showed a survival benefit of therapeutic hypothermia (TH) when provided within 12 hours after return of circulation following an OHCA and, to date, TH remains one of the few interventions with proven mortality benefit after initial cardiopulmonary resuscitation.3, 4 Since 2003, TH has been incorporated into the American Heart Association practice guidelines57 and use of TH has steadily increased, but widespread clinical uptake remains low.8, 9
The initial studies that evaluated TH were small, with only 189 patients included in the TH arms of the 2 trials combined. To date, only a few studies have replicated this initial observation in real‐world settings, with little analysis of outcomes in US centers in particular.1013 Accordingly, we aimed to examine the real‐world experience with TH in the United States using a large administrative claims database of all California hospital admissions to describe utilization trends, hospital mortality, and volumeoutcome relationships associated with the intervention.
MATERIALS AND METHODS
Data
We identified all admissions to California hospitals during 19992008 based on discharge records from the California Office of Statewide Health Planning and Development. Our study period included cases of TH performed prior to the 2002 major clinical trials, since TH was in occasional use prior to the publication of these trials. The data was de‐identified and publicly available, and therefore exempt from review by the Institutional Review Board. In addition to hospital name, each discharge record included patient age, gender, admission year, International Classification of Disease, Ninth Revision (ICD‐9) code for presenting primary and secondary diagnoses, procedure codes, and disposition (discharge to home or rehabilitation, in‐hospital death). All California hospitals were included in the registry (n = 419). We defined teaching status for each hospital based on membership in the Council of Teaching Hospitals, as reported in the American Hospital Association's Annual Survey (n = 19 teaching hospitals).14
Setting and Participants
We used discharge diagnoses to identify patients who could be considered eligible for therapeutic hypothermia after cardiac arrest. We classified patients as eligible for therapeutic hypothermia after cardiac arrest based on ICD‐9 diagnosis codes that indicated the presence of both cardiac arrest and anoxic brain injury in the administrative diagnoses. Because of known imprecision in using billing codes to identify patients with cardiac arrest,15, 16 we broadly defined cardiac arrest to include those patients with ICD‐9 codes for cardiac arrest, ventricular fibrillation (VF), or ventricular tachycardia (VT) (see Supporting Table 1 in the online version of this article). We could not distinguish between out‐of‐hospital and in‐hospital cardiac arrest based on administrative diagnoses. To ensure that we included only patients with cardiac arrest complicated by neurologic insult, we required an ICD‐9 diagnosis of either anoxic brain injury, coma, or persistent vegetative state. Claims did not allow us to distinguish among initial cardiac arrest rhythms (VF vs pulseless VT vs asystole). Patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia were excluded.3, 17 We did not exclude patients based on coagulopathy (which is considered a contraindication to TH), since ICD‐9 coding did not allow us to determine the severity of the coagulopathy or whether it was a result of therapeutic hypothermia itself.
We used the ICD‐9 procedure code (99.81) for TH to first identify patients who underwent TH from 1999 to 2008. Since this code also applies to TH used during cardiac and neurosurgery, we examined each of these cases and excluded individuals who underwent cardiac surgery or neurosurgery during the hospitalization. As in our eligible for TH definition, we excluded patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia. Patients who underwent therapeutic hypothermia but for whom a specific procedure code was not recorded in the discharge abstractperhaps because the medical institution did not directly bill for the procedurecould not be identified.
Statistical Analysis
We used a multivariable logistic model to estimate differences in hospital mortality after cardiac arrest associated with use of therapeutic hypothermia. We conducted 2 specifications. In our baseline specification, we accounted for case‐mix differences between those who underwent TH and those who did not by adjusting for age, gender, year of admission, the number of Charlson‐Deyo comorbidities,1820 and hospital teaching status. Because mortality after cardiac arrest in centers that perform therapeutic hypothermia may be different from centers that do not, even for patients who do not undergo the procedure, we included indicators for volume tercile of therapeutic hypothermia cases performed. Volume of therapeutic hypothermia was defined at the hospital level as the total number of cases performed by that hospital from 1999 to 2008.
In order to explore how hospital teaching status, volume of therapeutic hypothermia procedures (broken into terciles), and year of admission affected the association between hospital mortality after cardiac arrest and therapeutic hypothermia, our baseline logistic model was expanded to include interactions between therapeutic hypothermia and each of these variables. The interaction between therapeutic hypothermia and year explored whether the effectiveness of the procedure changed over time, as case‐selection, method of therapeutic hypothermia (cold saline vs commercially available devices), and experience changed in California hospitals. For both specifications, we reported the odds ratio of hospital mortality among patients undergoing therapeutic hypothermia, as well as risk‐adjusted mortality for both TH and non‐TH groups.
STATA version 11 (STATA Corp, College Station, TX) was used for statistical analyses, and a 2‐sided P 0.05 was used.
RESULTS
Descriptive Data
Table 1 reports summary statistics for patients with cardiac arrest complicated by neurologic insult (anoxic brain injury, coma, or persistent vegetative state) between 1999 and 2008. Across all years, 204 patients were identified as undergoing TH. In comparison, 105 patients were identified as undergoing TH in 2008 alone. Patients who underwent TH were less likely to be male (30.7% vs 44.6% male, P < 0.01), were younger (63.9 15.0 years vs 67.3 15.7 years, P = 0.03), and had equivalent numbers of Charlson‐Deyo comorbidities (2.5 2.0 diagnoses vs 2.5 2.0 diagnoses, P = 0.89). Therapeutic hypothermia was more commonly employed at teaching hospitals (51/3709 [1.4%] vs 153/42,942 [0.4%], P < 0.01). There was a trend toward decreased unadjusted mortality among patients who underwent therapeutic hypothermia compared with those who did not (56.9% vs 62.8%, P = 0.08).
| Therapeutic Hypothermia | No Therapeutic Hypothermia | P Value | |
|---|---|---|---|
| |||
| No. observations | 204 | 46,629 | |
| No. cases in teaching hospitals | 51 | 3,658 | |
| No. cases in non‐teaching hospitals | 153 | 42,789 | |
| Age, y | 63.9 15.0 | 67.3 15.7 | 0.06 |
| Male | 30.7 | 44.6 | <0.01 |
| Hospital mortality, % | 56.9 | 62.8 | 0.08 |
| Comorbidities | |||
| No. Charlson‐Deyo comorbidities | 2.5 2.0 | 2.5 2.0 | 0.89 |
| Coronary artery disease, % | 48.0 | 38.0 | <0.01 |
| Acute myocardial infarction, % | 42.6 | 28.9 | <0.01 |
| Congestive heart failure, % | 27.9 | 35.3 | 0.03 |
| Hypertension, % | 36.3 | 33.2 | 0.83 |
| Acute renal failure, % | 33.3 | 26.6 | 0.03 |
| Diabetes mellitus, % | 30.9 | 23.0 | <0.01 |
| Chronic obstructive pulmonary disease, % | 10.3 | 19.3 | <0.01 |
Figures 1 and 2 provide additional aggregate statistics on therapeutic hypothermia in California hospitals. Figure 1 plots the number of therapeutic hypothermia cases recorded in the administrative discharge registry between 1999 and 2008. Of the 204 total cases identified during this period, 178 (87.3%) were performed between 2006 and 2008. Figure 2 shows the distribution of TH cases across centers that performed therapeutic hypothermia (n = 47 hospitals, 11.3 % of all hospitals). Ten centers accounted for 124/204 (60.7%) of the total patients treated with TH after cardiac arrest; the top 3 centers accounted for 64 (31.4%) of the treated patients. Twenty‐seven hospitals were identified as performing therapeutic hypothermia on only 1 or 2 patients between 1999 and 2008.


Risk‐Adjusted Mortality
Table 2 presents the odds ratio of factors predicting in‐hospital mortality after cardiac arrest complicated by neurologic insult. Factors include use of TH after cardiac arrest, age, gender, year of admission, number of Charlson‐Deyo comorbidities, hospital teaching status, and volume tercile of hospitals that performed therapeutic hypothermia. Overall, patients who were older, male, and had greater comorbidities were statistically more likely to die after cardiac arrest complicated by neurologic insult. Regardless of whether they underwent TH, patients admitted to hospitals in the highest volume tercile of TH use were more likely to die after cardiac arrest. Adjusting for volume tercile, teaching hospital status was not independently associated with mortality after cardiac arrest. The adjusted odds ratio of mortality among patients undergoing therapeutic hypothermia was 0.80 (95% confidence interval [CI] 0.601.06, P = 0.11). The adjusted probability of inpatient mortality among patients undergoing therapeutic hypothermia was 57.5% (95% CI 50.764.3%) compared to those who did not 62.8% (95% CI 61.763.9%, P = 0.11).
| Variable | Odds Ratio of Hospital Mortality (95% CI) | P Value |
|---|---|---|
| ||
| No. observations | 46,651 | |
| Age* | ||
| 6569 | 1.19 (1.121.28) | <0.001 |
| 7074 | 1.29 (1.201.39) | <0.001 |
| 7579 | 1.55 (1.441.67) | <0.001 |
| 8084 | 1.79 (1.651.93) | <0.001 |
| 85 and over | 2.06 (1.892.25) | <0.001 |
| Male | 1.15 (1.101.21) | <0.001 |
| Teaching hospital | 1.13 (0.951.34) | 0.17 |
| No. Charlson‐Deyo comorbidities | 1.09 (1.081.10) | <0.001 |
| Year trend | 0.98 (0.970.99) | <0.001 |
| Volume tercile among hospitals performing TH | ||
| First tercile | 0.94 (0.791.12) | 0.48 |
| Second tercile | 1.03 (0.801.33) | 0.82 |
| Third tercile | 1.20 (1.051.36) | 0.006 |
| Therapeutic hypothermia | 0.80 (0.601.06) | 0.11 |
Figure 3 presents adjusted mortality after cardiac arrest in hospitals that did not perform TH, as well as adjusted mortality associated with TH for each volume tercile of hospitals that performed the procedure. Hospital mortality rates among patients not receiving TH after cardiac arrest were slightly higher in hospitals in the high volume tercile of TH (66.3%, 95% CI 63.868.8%) compared to hospitals in low and moderate volume terciles and to hospitals not performing TH (P < 0.001). Hospital mortality rates among low and moderate TH volume centers and in centers not performing TH were similar (62.3%, 61.3%, and 63.4%, respectively). Among both the low volume and moderate volume terciles, however, patients who underwent TH after cardiac arrest were significantly less likely to die in‐hospital compared to those who did not. For patients admitted to hospitals in the low volume tercile, those undergoing therapeutic hypothermia had an adjusted hospital mortality rate of 25.5% (95% CI 3.047.9%) compared to those who did not undergo TH (adjusted mortality 61.3%, 95% CI 57.465.1%), P < 0.001. In the moderate volume tercile, patients receiving therapeutic hypothermia had an adjusted hospital mortality rate of 31.0% (95% CI 9.2%52.8%) compared to 63.4% (95% CI 57.769.1%), P < 0.001, among those not undergoing the procedure. There was no statistically significant difference in adjusted mortality between those who underwent TH and those who did not, in hospitals in the highest volume tercile (P = 0.211). In addition to examining how volume of therapeutic hypothermia performed by hospitals affected the association between TH and hospital mortality, we also examined whether year of admission and teaching hospital independently modified the association. Neither year of admission nor teaching hospital statistically significantly affected the association between therapeutic hypothermia and hospital mortality after cardiac arrest at the P < 0.10 level.

DISCUSSION
In an administrative database of all admissions to California hospitals, we demonstrated that use of TH increased steadily since the publication of the initial clinical trials in 2002. The absolute level of TH utilization in our study undoubtedly represents a significant underestimation of actual TH utilization, however, our study does provide an assessment of the utilization trends over time. The bulk of TH use appears to be performed in a small group of high volume centers, and 89% of California hospitals did not perform TH during the study period (as assessed by procedure billing codes). Additionally, within the limitations of a retrospective, administrative claims‐based study design, TH appears to be associated with a similar in‐hospital mortality rate to that seen in clinical trials.3, 4 In exploratory analyses, there appears to be a particular benefit of TH in low and moderate volume centers, though these findings should be considered hypothesis‐generating.
Despite the body of evidence in favor of TH, utilization in our study and others appears quite low. In a 2005 survey of physicians, 87% of respondents had never used therapeutic hypothermia, citing inadequate data, technical limitations, and lack of incorporation in the Advanced Cardiac Life Support (ACLS) protocol as principal justifications.8 Other surveys have shown similar results and noted that critical care physicians and those working in large medical centers were more likely to adopt the therapy.9 Advocates of the therapy have suggested that an explicit hospital‐based plan developed by key stakeholders can help facilitate implementation.21 Accordingly, there is growing interest in developing centers of expertise in highly intensive therapies such as TH. For instance, the New York City Emergency Medical Service has begun to explore a protocol to divert TH candidates to specialized centers.22, 23 Some favorable results have been reported in individual hospitals and local hospital systems.2428
Our data suggest that TH is associated with an in‐hospital mortality rate that is comparable to that reported in the clinical trials. For example, in a 2009 meta‐analysis of 4 clinical trials and 1 abstract (481 patients in total), TH was associated with a 35% relative mortality benefit as compared to standard post‐resuscitation care.29 It has been estimated that broad TH implementation could save thousands of lives30 and many authors have advocated for its use and outlined explicit protocols for implementation.17 Furthermore, TH appears to be cost‐effective in line with other accepted therapies. Assuming the Hypothermia After Cardiac Arrest (HACA) trial inclusion criteria, even at extreme estimates for costs, the cost‐effectiveness of hypothermia remains less than $100,000 per quality‐adjusted life year.31
There are important limitations of this study. Our use of administrative claims data certainly underestimates the level of TH utilization, since we could only identify cases in which TH was included in the billing codes for the hospitalization. Hospitals may vary in utilization of this particular billing code for TH in ways that bias our estimated associations. The ICD‐9 code 99.81 for therapeutic hypothermia was also not developed for post‐cardiac arrest TH specifically, so use of the code may actually lag clinical utilization. Although the observed trend in TH utilization is likely mainly due to a true increase in utilization, it is possible that some of the observed increase is due to an increase in utilization of TH procedure billing codes. Our TH utilization estimates should be construed as a lower bound of the actual rates. Additionally, although the estimated real‐world mortality benefit of TH may be comparable to that of clinical trials, the equivalence of patients in our sample to those in published randomized trials is uncertain. Similarly, even after adjusting for age, gender, year of admission, comorbidities, hospital teaching status, and TH volume, there are likely many unmeasured variables that influence mortality in both the TH and comparison groups. There are also likely patients included in our comparison group who had both cardiac arrest or ventricular tachycardia and anoxic brain injury, but who were not candidates for TH as the episode of cardiac arrest followed rather than preceded the anoxic brain injury. Since we lack detailed clinical data about the TH cases (initial rhythm, time before return of circulation, preexisting disease states, etc.), we are unable to match controls directly to cases. Additionally, we lack data to assess neurologic recovery or quality of life after arrest.
The observation that a mortality benefit in our study could be detected only in low and moderate volume centers requires further exploration. Indeed, one might expect that high volume centers may have better outcomes with TH as a result of more robust infrastructure, technical experience, and available resources. Our finding that mortality benefits of TH appear concentrated in centers with low to moderate volume of TH utilization suggest at least 1 of 2 possibilities. First, low and moderate volume centers may perform TH in a subset of patients who benefit most from the intervention or, alternatively, in the most viable cardiac arrest cases (those who may fare well with or without the therapy). Consequently, we may observe relatively favorable outcomes in this group due to this selection bias. Second, high volume centersdespite having more expertisemay also attract patients at higher mortality risk due to referral bias. This would lead us to estimate lower mortality benefits associated with TH in these high volume centers. Indeed, greater observed mortality at high volume centers regardless of TH status suggests that overall acuity is higher at high volume centers. While our inferences are greatly affected by issues of case selection and referral bias, it also important to consider the possibility that the estimated mortality benefit of TH in higher volume centers is lower because of the selection of patients who do not meet current guidelines for treatment with TH. Distinguishing whether the selection of patients undergoing TH at high volume centers is appropriate or inappropriate based on current guidelines is an important issue that merits further research with datasets with more refined patient clinical information.
In summary, therapeutic hypothermia utilization is low, but the rate of implementation has increased since the publication of the initial clinical trials in 2002. The bulk of TH utilization appears limited to a subset of high volume centers, and most centers in California appear to have not used the therapy. Real‐world in‐hospital mortality associated with TH is comparable to that reported in randomized clinical trials.
Acknowledgements
Disclosures: Dr Romley received support from NIH grant R03AG031990‐A1. Dr Noseworthy received support from the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology granted by the Heart Rhythm Society. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources. The contents of this article have not been published in any other peer‐reviewed media, and the manuscript is not under review elsewhere. All authors listed have contributed sufficiently to this project to be included as authors. The authors have no conflict of interest, financial or otherwise.
There are over 350,000 cases of out‐of‐hospital cardiac arrest (OHCA) each year in the United States1, 2 and, with supportive therapy alone, only a fraction of victims survive to hospital discharge. Rapid intervention including cardiopulmonary resuscitation in the moments following arrest is critical to minimizing neurologic injury, morbidity, and mortality. In 2002, two small randomized controlled trials showed a survival benefit of therapeutic hypothermia (TH) when provided within 12 hours after return of circulation following an OHCA and, to date, TH remains one of the few interventions with proven mortality benefit after initial cardiopulmonary resuscitation.3, 4 Since 2003, TH has been incorporated into the American Heart Association practice guidelines57 and use of TH has steadily increased, but widespread clinical uptake remains low.8, 9
The initial studies that evaluated TH were small, with only 189 patients included in the TH arms of the 2 trials combined. To date, only a few studies have replicated this initial observation in real‐world settings, with little analysis of outcomes in US centers in particular.1013 Accordingly, we aimed to examine the real‐world experience with TH in the United States using a large administrative claims database of all California hospital admissions to describe utilization trends, hospital mortality, and volumeoutcome relationships associated with the intervention.
MATERIALS AND METHODS
Data
We identified all admissions to California hospitals during 19992008 based on discharge records from the California Office of Statewide Health Planning and Development. Our study period included cases of TH performed prior to the 2002 major clinical trials, since TH was in occasional use prior to the publication of these trials. The data was de‐identified and publicly available, and therefore exempt from review by the Institutional Review Board. In addition to hospital name, each discharge record included patient age, gender, admission year, International Classification of Disease, Ninth Revision (ICD‐9) code for presenting primary and secondary diagnoses, procedure codes, and disposition (discharge to home or rehabilitation, in‐hospital death). All California hospitals were included in the registry (n = 419). We defined teaching status for each hospital based on membership in the Council of Teaching Hospitals, as reported in the American Hospital Association's Annual Survey (n = 19 teaching hospitals).14
Setting and Participants
We used discharge diagnoses to identify patients who could be considered eligible for therapeutic hypothermia after cardiac arrest. We classified patients as eligible for therapeutic hypothermia after cardiac arrest based on ICD‐9 diagnosis codes that indicated the presence of both cardiac arrest and anoxic brain injury in the administrative diagnoses. Because of known imprecision in using billing codes to identify patients with cardiac arrest,15, 16 we broadly defined cardiac arrest to include those patients with ICD‐9 codes for cardiac arrest, ventricular fibrillation (VF), or ventricular tachycardia (VT) (see Supporting Table 1 in the online version of this article). We could not distinguish between out‐of‐hospital and in‐hospital cardiac arrest based on administrative diagnoses. To ensure that we included only patients with cardiac arrest complicated by neurologic insult, we required an ICD‐9 diagnosis of either anoxic brain injury, coma, or persistent vegetative state. Claims did not allow us to distinguish among initial cardiac arrest rhythms (VF vs pulseless VT vs asystole). Patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia were excluded.3, 17 We did not exclude patients based on coagulopathy (which is considered a contraindication to TH), since ICD‐9 coding did not allow us to determine the severity of the coagulopathy or whether it was a result of therapeutic hypothermia itself.
We used the ICD‐9 procedure code (99.81) for TH to first identify patients who underwent TH from 1999 to 2008. Since this code also applies to TH used during cardiac and neurosurgery, we examined each of these cases and excluded individuals who underwent cardiac surgery or neurosurgery during the hospitalization. As in our eligible for TH definition, we excluded patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia. Patients who underwent therapeutic hypothermia but for whom a specific procedure code was not recorded in the discharge abstractperhaps because the medical institution did not directly bill for the procedurecould not be identified.
Statistical Analysis
We used a multivariable logistic model to estimate differences in hospital mortality after cardiac arrest associated with use of therapeutic hypothermia. We conducted 2 specifications. In our baseline specification, we accounted for case‐mix differences between those who underwent TH and those who did not by adjusting for age, gender, year of admission, the number of Charlson‐Deyo comorbidities,1820 and hospital teaching status. Because mortality after cardiac arrest in centers that perform therapeutic hypothermia may be different from centers that do not, even for patients who do not undergo the procedure, we included indicators for volume tercile of therapeutic hypothermia cases performed. Volume of therapeutic hypothermia was defined at the hospital level as the total number of cases performed by that hospital from 1999 to 2008.
In order to explore how hospital teaching status, volume of therapeutic hypothermia procedures (broken into terciles), and year of admission affected the association between hospital mortality after cardiac arrest and therapeutic hypothermia, our baseline logistic model was expanded to include interactions between therapeutic hypothermia and each of these variables. The interaction between therapeutic hypothermia and year explored whether the effectiveness of the procedure changed over time, as case‐selection, method of therapeutic hypothermia (cold saline vs commercially available devices), and experience changed in California hospitals. For both specifications, we reported the odds ratio of hospital mortality among patients undergoing therapeutic hypothermia, as well as risk‐adjusted mortality for both TH and non‐TH groups.
STATA version 11 (STATA Corp, College Station, TX) was used for statistical analyses, and a 2‐sided P 0.05 was used.
RESULTS
Descriptive Data
Table 1 reports summary statistics for patients with cardiac arrest complicated by neurologic insult (anoxic brain injury, coma, or persistent vegetative state) between 1999 and 2008. Across all years, 204 patients were identified as undergoing TH. In comparison, 105 patients were identified as undergoing TH in 2008 alone. Patients who underwent TH were less likely to be male (30.7% vs 44.6% male, P < 0.01), were younger (63.9 15.0 years vs 67.3 15.7 years, P = 0.03), and had equivalent numbers of Charlson‐Deyo comorbidities (2.5 2.0 diagnoses vs 2.5 2.0 diagnoses, P = 0.89). Therapeutic hypothermia was more commonly employed at teaching hospitals (51/3709 [1.4%] vs 153/42,942 [0.4%], P < 0.01). There was a trend toward decreased unadjusted mortality among patients who underwent therapeutic hypothermia compared with those who did not (56.9% vs 62.8%, P = 0.08).
| Therapeutic Hypothermia | No Therapeutic Hypothermia | P Value | |
|---|---|---|---|
| |||
| No. observations | 204 | 46,629 | |
| No. cases in teaching hospitals | 51 | 3,658 | |
| No. cases in non‐teaching hospitals | 153 | 42,789 | |
| Age, y | 63.9 15.0 | 67.3 15.7 | 0.06 |
| Male | 30.7 | 44.6 | <0.01 |
| Hospital mortality, % | 56.9 | 62.8 | 0.08 |
| Comorbidities | |||
| No. Charlson‐Deyo comorbidities | 2.5 2.0 | 2.5 2.0 | 0.89 |
| Coronary artery disease, % | 48.0 | 38.0 | <0.01 |
| Acute myocardial infarction, % | 42.6 | 28.9 | <0.01 |
| Congestive heart failure, % | 27.9 | 35.3 | 0.03 |
| Hypertension, % | 36.3 | 33.2 | 0.83 |
| Acute renal failure, % | 33.3 | 26.6 | 0.03 |
| Diabetes mellitus, % | 30.9 | 23.0 | <0.01 |
| Chronic obstructive pulmonary disease, % | 10.3 | 19.3 | <0.01 |
Figures 1 and 2 provide additional aggregate statistics on therapeutic hypothermia in California hospitals. Figure 1 plots the number of therapeutic hypothermia cases recorded in the administrative discharge registry between 1999 and 2008. Of the 204 total cases identified during this period, 178 (87.3%) were performed between 2006 and 2008. Figure 2 shows the distribution of TH cases across centers that performed therapeutic hypothermia (n = 47 hospitals, 11.3 % of all hospitals). Ten centers accounted for 124/204 (60.7%) of the total patients treated with TH after cardiac arrest; the top 3 centers accounted for 64 (31.4%) of the treated patients. Twenty‐seven hospitals were identified as performing therapeutic hypothermia on only 1 or 2 patients between 1999 and 2008.


Risk‐Adjusted Mortality
Table 2 presents the odds ratio of factors predicting in‐hospital mortality after cardiac arrest complicated by neurologic insult. Factors include use of TH after cardiac arrest, age, gender, year of admission, number of Charlson‐Deyo comorbidities, hospital teaching status, and volume tercile of hospitals that performed therapeutic hypothermia. Overall, patients who were older, male, and had greater comorbidities were statistically more likely to die after cardiac arrest complicated by neurologic insult. Regardless of whether they underwent TH, patients admitted to hospitals in the highest volume tercile of TH use were more likely to die after cardiac arrest. Adjusting for volume tercile, teaching hospital status was not independently associated with mortality after cardiac arrest. The adjusted odds ratio of mortality among patients undergoing therapeutic hypothermia was 0.80 (95% confidence interval [CI] 0.601.06, P = 0.11). The adjusted probability of inpatient mortality among patients undergoing therapeutic hypothermia was 57.5% (95% CI 50.764.3%) compared to those who did not 62.8% (95% CI 61.763.9%, P = 0.11).
| Variable | Odds Ratio of Hospital Mortality (95% CI) | P Value |
|---|---|---|
| ||
| No. observations | 46,651 | |
| Age* | ||
| 6569 | 1.19 (1.121.28) | <0.001 |
| 7074 | 1.29 (1.201.39) | <0.001 |
| 7579 | 1.55 (1.441.67) | <0.001 |
| 8084 | 1.79 (1.651.93) | <0.001 |
| 85 and over | 2.06 (1.892.25) | <0.001 |
| Male | 1.15 (1.101.21) | <0.001 |
| Teaching hospital | 1.13 (0.951.34) | 0.17 |
| No. Charlson‐Deyo comorbidities | 1.09 (1.081.10) | <0.001 |
| Year trend | 0.98 (0.970.99) | <0.001 |
| Volume tercile among hospitals performing TH | ||
| First tercile | 0.94 (0.791.12) | 0.48 |
| Second tercile | 1.03 (0.801.33) | 0.82 |
| Third tercile | 1.20 (1.051.36) | 0.006 |
| Therapeutic hypothermia | 0.80 (0.601.06) | 0.11 |
Figure 3 presents adjusted mortality after cardiac arrest in hospitals that did not perform TH, as well as adjusted mortality associated with TH for each volume tercile of hospitals that performed the procedure. Hospital mortality rates among patients not receiving TH after cardiac arrest were slightly higher in hospitals in the high volume tercile of TH (66.3%, 95% CI 63.868.8%) compared to hospitals in low and moderate volume terciles and to hospitals not performing TH (P < 0.001). Hospital mortality rates among low and moderate TH volume centers and in centers not performing TH were similar (62.3%, 61.3%, and 63.4%, respectively). Among both the low volume and moderate volume terciles, however, patients who underwent TH after cardiac arrest were significantly less likely to die in‐hospital compared to those who did not. For patients admitted to hospitals in the low volume tercile, those undergoing therapeutic hypothermia had an adjusted hospital mortality rate of 25.5% (95% CI 3.047.9%) compared to those who did not undergo TH (adjusted mortality 61.3%, 95% CI 57.465.1%), P < 0.001. In the moderate volume tercile, patients receiving therapeutic hypothermia had an adjusted hospital mortality rate of 31.0% (95% CI 9.2%52.8%) compared to 63.4% (95% CI 57.769.1%), P < 0.001, among those not undergoing the procedure. There was no statistically significant difference in adjusted mortality between those who underwent TH and those who did not, in hospitals in the highest volume tercile (P = 0.211). In addition to examining how volume of therapeutic hypothermia performed by hospitals affected the association between TH and hospital mortality, we also examined whether year of admission and teaching hospital independently modified the association. Neither year of admission nor teaching hospital statistically significantly affected the association between therapeutic hypothermia and hospital mortality after cardiac arrest at the P < 0.10 level.

DISCUSSION
In an administrative database of all admissions to California hospitals, we demonstrated that use of TH increased steadily since the publication of the initial clinical trials in 2002. The absolute level of TH utilization in our study undoubtedly represents a significant underestimation of actual TH utilization, however, our study does provide an assessment of the utilization trends over time. The bulk of TH use appears to be performed in a small group of high volume centers, and 89% of California hospitals did not perform TH during the study period (as assessed by procedure billing codes). Additionally, within the limitations of a retrospective, administrative claims‐based study design, TH appears to be associated with a similar in‐hospital mortality rate to that seen in clinical trials.3, 4 In exploratory analyses, there appears to be a particular benefit of TH in low and moderate volume centers, though these findings should be considered hypothesis‐generating.
Despite the body of evidence in favor of TH, utilization in our study and others appears quite low. In a 2005 survey of physicians, 87% of respondents had never used therapeutic hypothermia, citing inadequate data, technical limitations, and lack of incorporation in the Advanced Cardiac Life Support (ACLS) protocol as principal justifications.8 Other surveys have shown similar results and noted that critical care physicians and those working in large medical centers were more likely to adopt the therapy.9 Advocates of the therapy have suggested that an explicit hospital‐based plan developed by key stakeholders can help facilitate implementation.21 Accordingly, there is growing interest in developing centers of expertise in highly intensive therapies such as TH. For instance, the New York City Emergency Medical Service has begun to explore a protocol to divert TH candidates to specialized centers.22, 23 Some favorable results have been reported in individual hospitals and local hospital systems.2428
Our data suggest that TH is associated with an in‐hospital mortality rate that is comparable to that reported in the clinical trials. For example, in a 2009 meta‐analysis of 4 clinical trials and 1 abstract (481 patients in total), TH was associated with a 35% relative mortality benefit as compared to standard post‐resuscitation care.29 It has been estimated that broad TH implementation could save thousands of lives30 and many authors have advocated for its use and outlined explicit protocols for implementation.17 Furthermore, TH appears to be cost‐effective in line with other accepted therapies. Assuming the Hypothermia After Cardiac Arrest (HACA) trial inclusion criteria, even at extreme estimates for costs, the cost‐effectiveness of hypothermia remains less than $100,000 per quality‐adjusted life year.31
There are important limitations of this study. Our use of administrative claims data certainly underestimates the level of TH utilization, since we could only identify cases in which TH was included in the billing codes for the hospitalization. Hospitals may vary in utilization of this particular billing code for TH in ways that bias our estimated associations. The ICD‐9 code 99.81 for therapeutic hypothermia was also not developed for post‐cardiac arrest TH specifically, so use of the code may actually lag clinical utilization. Although the observed trend in TH utilization is likely mainly due to a true increase in utilization, it is possible that some of the observed increase is due to an increase in utilization of TH procedure billing codes. Our TH utilization estimates should be construed as a lower bound of the actual rates. Additionally, although the estimated real‐world mortality benefit of TH may be comparable to that of clinical trials, the equivalence of patients in our sample to those in published randomized trials is uncertain. Similarly, even after adjusting for age, gender, year of admission, comorbidities, hospital teaching status, and TH volume, there are likely many unmeasured variables that influence mortality in both the TH and comparison groups. There are also likely patients included in our comparison group who had both cardiac arrest or ventricular tachycardia and anoxic brain injury, but who were not candidates for TH as the episode of cardiac arrest followed rather than preceded the anoxic brain injury. Since we lack detailed clinical data about the TH cases (initial rhythm, time before return of circulation, preexisting disease states, etc.), we are unable to match controls directly to cases. Additionally, we lack data to assess neurologic recovery or quality of life after arrest.
The observation that a mortality benefit in our study could be detected only in low and moderate volume centers requires further exploration. Indeed, one might expect that high volume centers may have better outcomes with TH as a result of more robust infrastructure, technical experience, and available resources. Our finding that mortality benefits of TH appear concentrated in centers with low to moderate volume of TH utilization suggest at least 1 of 2 possibilities. First, low and moderate volume centers may perform TH in a subset of patients who benefit most from the intervention or, alternatively, in the most viable cardiac arrest cases (those who may fare well with or without the therapy). Consequently, we may observe relatively favorable outcomes in this group due to this selection bias. Second, high volume centersdespite having more expertisemay also attract patients at higher mortality risk due to referral bias. This would lead us to estimate lower mortality benefits associated with TH in these high volume centers. Indeed, greater observed mortality at high volume centers regardless of TH status suggests that overall acuity is higher at high volume centers. While our inferences are greatly affected by issues of case selection and referral bias, it also important to consider the possibility that the estimated mortality benefit of TH in higher volume centers is lower because of the selection of patients who do not meet current guidelines for treatment with TH. Distinguishing whether the selection of patients undergoing TH at high volume centers is appropriate or inappropriate based on current guidelines is an important issue that merits further research with datasets with more refined patient clinical information.
In summary, therapeutic hypothermia utilization is low, but the rate of implementation has increased since the publication of the initial clinical trials in 2002. The bulk of TH utilization appears limited to a subset of high volume centers, and most centers in California appear to have not used the therapy. Real‐world in‐hospital mortality associated with TH is comparable to that reported in randomized clinical trials.
Acknowledgements
Disclosures: Dr Romley received support from NIH grant R03AG031990‐A1. Dr Noseworthy received support from the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology granted by the Heart Rhythm Society. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources. The contents of this article have not been published in any other peer‐reviewed media, and the manuscript is not under review elsewhere. All authors listed have contributed sufficiently to this project to be included as authors. The authors have no conflict of interest, financial or otherwise.
- , , , et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163.
- , . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- , , , et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563.
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556.
- , , , et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 7.5: postresuscitation support. Circulation. 2005;112(suppl I):IV‐84–IV‐88.
- , , , et al. Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(suppl 1):e93–e174.
- , , , et al. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186.
- , , , et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940.
- , , , et al. Adverse events and their relation to mortality in out‐of‐hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med.39(1):57–64.
- , , , et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934.
- . Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–1047.
- , , , et al. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–214.
- American Hospital Association (AHA). American Hospital Association 2001 Annual Survey. Chicago, IL: Health Forum, LLC.
- , , . A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296.
- . Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604.
- . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–1264.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- , , , et al. Practical implementation of therapeutic hypothermia after cardiac arrest. Hosp Pract (Minneap). 2009;37(1):71–83.
- . Paramedics will employ new therapy in cardiac arrest cases. New York Times. August 3, 2010:A18.
- , , , et al. Resuscitation center designation: recommendations for emergency medical services practices. Prehosp Emerg Care. 2010;14(1):51–61.
- , , , et al. Implementation of a hospital‐wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol. 2010;9(4):216–220.
- , , , et al. Therapeutic hypothermia protocol in a community emergency department. West J Emerg Med. 2010;11(4):367–372.
- , , , et al. Implementation of a post‐cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out‐of‐hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366.
- , , , et al. Improved out‐of‐hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med. 2010;56(4):348–357.
- , , , et al. Community‐based application of mild therapeutic hypothermia for survivors of cardiac arrest. South Med J. 2010;103(4):295–300.
- , , , et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009(4):CD004128.
- , . Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22(4):711–728.
- , , , et al. Cost‐effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–428.
- , , , et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163.
- , . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- , , , et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563.
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556.
- , , , et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 7.5: postresuscitation support. Circulation. 2005;112(suppl I):IV‐84–IV‐88.
- , , , et al. Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(suppl 1):e93–e174.
- , , , et al. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186.
- , , , et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940.
- , , , et al. Adverse events and their relation to mortality in out‐of‐hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med.39(1):57–64.
- , , , et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934.
- . Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–1047.
- , , , et al. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–214.
- American Hospital Association (AHA). American Hospital Association 2001 Annual Survey. Chicago, IL: Health Forum, LLC.
- , , . A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296.
- . Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604.
- . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–1264.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- , , , et al. Practical implementation of therapeutic hypothermia after cardiac arrest. Hosp Pract (Minneap). 2009;37(1):71–83.
- . Paramedics will employ new therapy in cardiac arrest cases. New York Times. August 3, 2010:A18.
- , , , et al. Resuscitation center designation: recommendations for emergency medical services practices. Prehosp Emerg Care. 2010;14(1):51–61.
- , , , et al. Implementation of a hospital‐wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol. 2010;9(4):216–220.
- , , , et al. Therapeutic hypothermia protocol in a community emergency department. West J Emerg Med. 2010;11(4):367–372.
- , , , et al. Implementation of a post‐cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out‐of‐hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366.
- , , , et al. Improved out‐of‐hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med. 2010;56(4):348–357.
- , , , et al. Community‐based application of mild therapeutic hypothermia for survivors of cardiac arrest. South Med J. 2010;103(4):295–300.
- , , , et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009(4):CD004128.
- , . Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22(4):711–728.
- , , , et al. Cost‐effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–428.
Copyright © 2012 Society of Hospital Medicine