User login
Safe Opioid Prescribing for Acute Noncancer Pain in Hospitalized Adults: A Systematic Review of Existing Guidelines
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
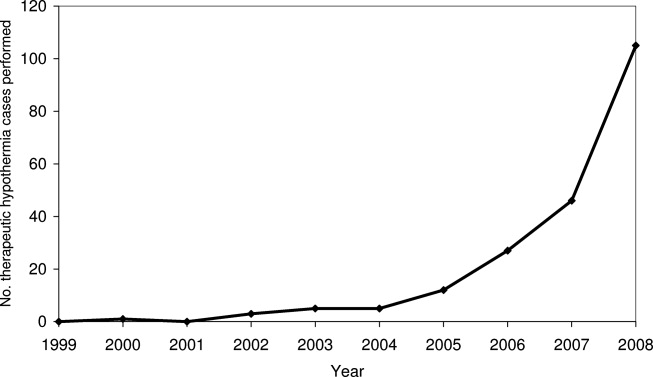
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
© 2018 Society of Hospital Medicine
Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement From the Society of Hospital Medicine
Since the initial reports of an emerging opioid epidemic in the early 2000s, intense focus on improving opioid prescribing in outpatient settings has culminated in new guidelines for chronic pain.1,2 Although opioid stewardship in the setting of chronic pain is of paramount importance in curbing the ongoing epidemic, long-term prescription opioid use often begins with treatment of acute pain.1 In addition to differences in recommended management strategies for acute and chronic pain, there are unique aspects and challenges to pain management in the acute-care setting.
Opioids are commonly used for the treatment of acute pain in hospitalized patients, often at high doses.3 Recent reports highlight that hospital use of opioids impacts downstream use.4-6 Additionally, opioid prescribing practices vary between hospital-based providers and hospitals,3,7 highlighting the need for prescribing standards and guidance. To our knowledge, there are no existing guidelines for improving the safety of opioid use in hospitalized patients outside of the intensive care or immediate perioperative settings.
The Society of Hospital Medicine (SHM) convened a working group to systematically review existing guidelines and develop a consensus statement to assist clinicians in safe opioid use for acute, noncancer pain in hospitalized adults.
Consensus Statement Purpose and Scope
The purpose of this Consensus Statement is to present clinical recommendations on the safe use of opioids for the treatment of acute, noncancer pain in hospitalized adults. The guidance is intended for clinicians practicing medicine in the inpatient setting (eg, hospitalists, primary care physicians, family physicians, nurse practitioners, and physician assistants) and is intended to apply to hospitalized adults with acute, noncancer pain (ie, pain that typically lasts <3 months or during the period of normal tissue healing) outside of the palliative, end-of-life, and intensive care settings.
Consensus Statement Development
Our working group included experts in opioid use in the hospital setting, defined by 1) engagement in the clinical practice of hospital medicine and 2) involvement in clinical research related to usage patterns and clinical outcomes of opioid use in hospitalized patients (see Appendix Table 1). The SHM provided administrative assistance with the project and funded the in-person working group meeting, but it had no role in formulating the recommendations. The SHM Board of Directors provided approval of the Consensus Statement without modification.
An overview of the sequential steps in the Consensus Statement development process is described below; details of the methods and results can be found in the Appendix (eMethods).
Performing the Systematic Review
Drafting the Consensus Statement
After performing the systematic review, the working group drafted and iteratively revised a set of recommendations using a variation of the Delphi Method8 to identify consensus among group members.
External Review
Following agreement on a draft set of recommendations, we obtained feedback from external groups, including 1) individuals involved in the SHM’s Reducing Adverse Drug Events Related to Opioids (RADEO) initiative, including those involved in the development of the implementation guide and site leads for the Mentored Implementation program, 2) SHM members, SHM Patient-Family Advisory Council (PFAC) members, and leaders of other relevant professional societies, and 3) peer-reviewers at the Journal of Hospital Medicine.
RESULTS
Deciding Whether to Use Opioids During Hospitalization
1. SHM recommends that clinicians limit the use of opioids to patients with 1) severe pain or 2) moderate pain that has not responded to nonopioid therapy, or where nonopioid therapy is contraindicated or anticipated to be ineffective.
Opioids are associated with several well-recognized risks ranging from mild to severe, including nausea, constipation, urinary retention, falls, delirium, sedation, physical dependence, addiction, respiratory depression, and death. Given these risks, the risk-to-benefit ratio is generally not favorable at lower levels of pain severity. Furthermore, for most painful conditions, including those causing severe pain, nonopioid analgesics, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), have been demonstrated to be equally or more effective with less risk of harm than opioids.9-13 Clinicians should consider drug–drug and drug–disease associations when deciding between these different therapies and make a determination in each patient regarding whether the benefits outweigh the risks. Often, drug–disease interactions do not represent absolute contraindications, and risks can be mitigated by adhering to dosage limits and, with respect to NSAIDs, 1) monitoring renal function, 2) monitoring volume status in patients with congestive heart failure, and 3) considering a selective cyclooxygenase-2 (COX-2) inhibitor rather than a nonselective NSAID or pairing the NSAID with an acid-suppressive medication in patients with a history of peptic ulcer disease or at elevated risk for gastroduodenal disease. For these reasons, a trial of nonopioid therapy (including pharmacologic and nonpharmacologic modalities) should always be considered before using opioids for pain of any severity. This does not imply that a trial of nonopioid therapy must be performed in all patients, but rather, that the likelihood of benefit and associated risks of opioid and nonopioid therapy should be considered for all patients in determining the best initial management strategy.
2. SHM recommends that clinicians use extra caution when administering opioids to patients with risk factors for opioid-related adverse events.
Several factors have been consistently demonstrated to increase the risk of opioid-related adverse events–most importantly, respiratory depression and overdose–in varied patient populations and settings, including age 65 years and older,1,14-17 renal insufficiency,1,14,18 hepatic insufficiency,1,14 chronic respiratory failure (including chronic obstructive pulmonary disease, sleep apnea, etc.), and receipt of other central nervous system (CNS) depressant medications (including, but not limited to, benzodiazepines).1,18-20 History of any substance use disorder and psychiatric disorders have been associated with an increased risk for the development of opioid use disorder.21-24 These factors should be weighed against the benefits when deciding on opioid appropriateness in a given patient. However, identification of these risks should not preclude opioids as part of pain management. When a decision is made to use opioids in patients with these risk factors, clinicians should 1) use a reduced starting dose (generally, at least a 50% reduction in the usual starting dose) and 2) consider closer monitoring for adverse effects (eg, more frequent nursing assessments, capnography, or more frequent outpatient visits).
3. SHM recommends that clinicians review the information contained in the prescription drug monitoring program (PDMP) database to inform decision-making around opioid therapy.
Although data on the impact of use of the state PDMP database on prescribing practices or patient outcomes are limited, PDMP use has been advocated by multiple guidelines on acute pain management.25-27 The PDMP provides information that can be useful in several ways, including 1) confirmation of prior opioid exposure and dosage, which should be used to guide appropriate dosage selection in the hospital, 2) identification of existing controlled substance prescriptions, which should be considered in prescribing decisions in the hospital and on discharge, and 3) identification of signs of aberrant behavior. For example, the identification of controlled substance prescriptions written by multiple different clinicians can facilitate early identification of potential diversion or evolving or existing opioid use disorder and the opportunity for intervention,28 which may include referral to support services, initiation of medication-assisted treatment, and/or pain specialist consultation when available. Concerns regarding evolving or existing opioid use disorder should prompt further discussion between the clinician and the patient, both to clarify their understanding of their recent prescription history and to discuss concerns for patient safety related to the increased risk of opioid-related adverse effects (including respiratory depression and overdose) among patients with controlled substance prescriptions written by multiple providers. Although such concerns should not automatically preclude the use of opioids for acute pain in the hospital setting, they should be considered in the assessment of whether the benefits of opioid therapy outweigh the risks for a given patient.
4. SHM recommends that clinicians educate patients and families or caregivers about the potential risks and side effects of opioid therapy as well as alternative pharmacologic and nonpharmacologic therapies for managing pain.
Patients are often unaware of the risks of opioid therapy (see Consensus Statement 1 for key risks),29 or that there are often equally effective alternative therapies. As with any therapy associated with substantial risk, clinicians should discuss these risks with patients and/or caregivers at the outset of therapy, as well as the potential benefits of nonopioid pharmacologic and nonpharmacologic therapies for managing pain. Patients should be informed that they may request nonopioid therapy in lieu of opioids, even for severe pain.
Once a Decision Has Been Made to Use Opioids During Hospitalization
5. SHM recommends that clinicians use the lowest effective opioid dose for the shortest duration possible.
Higher opioid doses are associated with an increased incidence of opioid-related adverse events, particularly overdose, in studies of both inpatient and outpatient populations.1,17,19,30,31 Studies in the inpatient and outpatient settings consistently demonstrate that risk increases with dosage.19,30,31 Clinicians should reduce the usual starting dose by at least 50% among patients with conditions that increase susceptibility to opioid-related adverse events (see Consensus Statement 2). The ongoing need for opioids should be re-assessed regularly-at least daily-during the hospitalization, with attempts at tapering as healing occurs and/or pain and function improve.
6. SHM recommends that clinicians use immediate-release opioid formulations and avoid initiation of long-acting or extended-release formulations (including transdermal fentanyl) for treatment of acute pain.
Studies in outpatient settings demonstrate that the use of long-acting opioids is associated with greater risk for overdose–especially in opioid-naïve patients–and long-term use.32,33 Further, hospitalized patients frequently have fluctuating renal function and rapidly changing pain levels. We therefore recommend that initiation of long-acting opioids be avoided for the treatment of acute, noncancer pain in hospitalized medical patients. It is important to note that although we recommend avoiding initiation of long-acting opioids for the treatment of acute, noncancer pain, there are circumstances outside of the scope of this Consensus Statement for which initiation of long-acting opioids may be indicated, including the treatment of opioid withdrawal. We also do not recommend discontinuation of long-acting or extended-release opioids in patients who are taking these medications for chronic pain at the time of hospital admission (unless there are concerns regarding adverse effects or drug–disease interactions).
7. SHM recommends that clinicians use the oral route of administration whenever possible. Intravenous opioids should be reserved for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal malabsorption, or when immediate pain control and/or rapid dose titration is necessary.
Intravenous opioid administration is associated with an increased risk of side effects, adverse events, and medication errors.34-36 Additionally, studies demonstrate that in general, the addiction potential of medications is greater the more rapid the onset of action (the onset of action is 5–10 min for intravenous and 15–30 minutes for oral administration).37,38 Furthermore, the duration of action is greater for oral compared to that of intravenous administration, potentially allowing for more consistent pain relief and less frequent administrations. As such, intravenous administration should be reserved for situations when oral administration is not possible or likely to be ineffective, or when immediate pain control and/or rapid titration is necessary.
8. SHM recommends that clinicians use an opioid equivalency table or calculator to understand the relative potency of different opioids 1) when initiating opioid therapy, 2) when changing from one route of administration to another, and 3) when changing from one opioid to another. When changing from one opioid to another, clinicians should generally reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids as well as possible incomplete cross-tolerance.
Most errors causing preventable adverse drug events in hospitals occur at the ordering stage.39,40 Clinicians are often unaware of the potency of different types of opioids relative to each other or to morphine (ie, morphine equivalent dose), which can lead to inadvertent overdose when initiating therapy with nonmorphine opioids and when converting from one opioid to another. To facilitate safe opioid use, we recommend that clinicians use one of several available opioid equivalency tables or calculators to better understand the relative potencies of opioids and to inform both starting dose calculations and conversions between opioids and routes of administration. When converting from one opioid to another, we caution clinicians to reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids and the potential for incomplete cross-tolerance, wherein tolerance to a currently administered opioid does not extend completely to other opioids. Clinicians should use extreme caution when performing conversions to and from methadone and consider consultation with a hospital pharmacist or a pain management specialist, when available, to assist with conversion decisions and calculations.
9. SHM recommends that clinicians pair opioids with scheduled nonopioid analgesic medications, unless contraindicated, and always consider pairing with nonpharmacologic pain management strategies (ie, multimodal analgesia).
Concurrent receipt of opioids and nonopioid analgesic medications (including acetaminophen, NSAIDs, and gabapentin or pregabalin, depending on the underlying pathophysiology of the pain) has been demonstrated to reduce total opioid requirements and improve pain management.41,42 Clinicians should be familiar with contraindications and maximum dosage recommendations for each of these adjunctive nonopioid medications. We recommend separate orders for each, rather than using drug formulations that combine opioids and nonopioid analgesics in the same pill, due to the risk of inadvertently exceeding the maximum recommended doses of the nonopioid analgesic (particularly acetaminophen) with combination products. We recommend that nonopioid analgesics be ordered at a scheduled frequency, rather than as needed, to facilitate consistent administration that is not dependent on opioid administration. Topical agents, including lidocaine and capsaicin, should also be considered. Nonpharmacologic pain management strategies can include procedure-based (eg, regional and local anesthesia) and nonprocedure-based therapies depending on the underlying condition and institutional availability. Although few studies have assessed the benefit of nonpharmacologic, nonprocedure-based therapies for the treatment of acute pain in hospitalized patients, the lack of harm associated with their use argues for their adoption. Simple nonpharmacologic therapies that can usually be provided to patients in any hospital setting include music therapy, cold or hot packs, chaplain or social work visits (possibly including mindfulness training),43 and physical therapy, among others.
10. SHM recommends that, unless contraindicated, clinicians order a bowel regimen to prevent opioid-induced constipation in patients receiving opioids.
Constipation is a common adverse effect of opioid therapy and results from the activation of mu opioid receptors in the colon, resulting in decreased peristalsis. Hospitalized patients are already prone to constipation due to their often-limited physical mobility. To mitigate this complication, we recommend the administration of a bowel regimen to all hospitalized medical patients receiving opioid therapy, provided the patient is not having diarrhea. Given the mechanism of opioid-induced constipation, stimulant laxatives (eg, senna, bisacodyl) have been recommended for this purpose.44 Osmotic laxatives (eg, polyethylene glycol, lactulose) have demonstrated efficacy for the treatment of constipation more generally (ie, not necessarily opioid-induced constipation). Stool softeners, although frequently used in the inpatient setting, are not recommended due to limited and conflicting evidence for efficacy in prevention or treatment of constipation.45 Bowel movements should be tracked during hospitalization, and the bowel regimen modified accordingly.
11. SHM recommends that clinicians limit co-administration of opioids with other central nervous system depressant medications to the extent possible.
This combination has been demonstrated to increase the risk of opioid-related adverse events in multiple settings of care, including during hospitalization.1,18,19 Although benzodiazepines have received the most attention in this respect, other medications with CNS depressant properties may also increase the risk, including, but not limited to, nonbenzodiazepine sedative-hypnotics (eg, zolpidem, zaleplon, zopiclone), muscle relaxants, sedating antidepressants, antipsychotics, and antihistamines.18,19,46 For some patients, the combination will be unavoidable, and we do not suggest routine discontinuation of longstanding medications that preexisted hospitalization, given the risks of withdrawal and/or worsening of the underlying condition for which these medications are prescribed. Rather, clinicians should carefully consider the necessity of each medication class with input from the patient’s outpatient providers, taper the frequency and/or the dose of CNS depressants when appropriate and feasible, and avoid new coprescriptions to the extent possible, both during hospitalization and on hospital discharge.
12. SHM recommends that clinicians work with patients and families or caregivers to establish realistic goals and expectations of opioid therapy and the expected course of recovery.
Discussing expectations at the start of therapy is important to facilitate a clear understanding of how meaningful improvement will be defined and measured during the hospitalization and how long the patient is anticipated to require opioid therapy. Meaningful improvement should be defined to include improvement in both pain and function. Clinicians should discuss with patients 1) that the goal of opioid therapy is tolerability of pain such that meaningful improvement in function can be achieved and 2) that a decrease in pain intensity in the absence of improved function is not considered meaningful improvement in most situations and should prompt reevaluation of the appropriateness of continued opioid therapy as well as close follow-up with a clinician following hospital discharge. Discussions regarding the expected course of recovery should include that acute pain is expected to resolve as the underlying medical condition improves and that although pain may persist beyond the hospitalization, pain that is severe enough to require opioids will often be resolved or almost resolved by the time of hospital discharge.
13. SHM recommends that clinicians monitor the response to opioid therapy, including assessment for functional improvement and development of adverse effects.
Pain severity and function should be assessed at least daily, and improvement in reported pain severity without improvement in function over several days should, in most circumstances, prompt reconsideration of ongoing opioid therapy and reconsideration of the underlying etiology of pain. Although hospital-specific functional measures in the setting of acute pain have not yet been validated, we suggest that such measures and goals should be individualized based on preexisting function and may include the ability to sit up or move in bed, move to a chair, work with physical therapy, or ambulate in the hallway. Protocols for the assessment for adverse effects are not well established. Because sedation typically precedes respiratory depression, it is generally recommended that patients are evaluated (eg, by nursing staff) for sedation after each opioid administration (10–20 minutes for intravenous and 30–60 minutes for oral administration based on the time-to-peak effect). Whether certain patients may benefit from more intensive respiratory monitoring, such as pulse oximetry or capnography, is an area of active investigation and not yet established.
Prescribing at the Time of Hospital Discharge
14. SHM recommends that clinicians ask patients about any existing opioid supply at home and account for any such supply when issuing an opioid prescription on discharge.
Even in the setting of acute pain, patients may have previously received an opioid prescription from an outpatient clinician prior to hospitalization. Unused prescription opioids create the possibility of both overdose (when patients take multiple opioids concurrently, intentionally or inadvertently) and diversion (many adults with prescription opioid misuse obtained their opioids from a friend or a relative who may or may not have known that this occurred47). The PDMP database can provide information related to the potential existence of any prior opioid supplies, which should be confirmed with the patient and considered when providing a new prescription on hospital discharge. Information on proper disposal should be provided if use of the preexisting opioid is no longer intended.
15. SHM recommends that clinicians prescribe the minimum quantity of opioids anticipated to be necessary based on the expected course and duration of pain that is severe enough to require opioid therapy after hospital discharge.
16. SHM recommends that clinicians ensure that patients and families or caregivers receive information regarding how to minimize the risks of opioid therapy for themselves, their families, and their communities. This includes but is not limited to 1) how to take their opioids correctly (the planned medications, doses, schedule); 2) that they should take the minimum quantity necessary to achieve tolerable levels of pain and meaningful functional improvement, reducing the dose and/or frequency as pain and function improve; 3) how to safeguard their supply and dispose of any unused supply; 4) that they should avoid agents that may potentiate the sedative effect of opioids, including sleeping medication and alcohol; 5) that they should avoid driving or operating heavy machinery while taking opioids; and 6) that they should seek help if they begin to experience any potential adverse effects, with inclusion of information on early warning signs.
Clear and concise patient instructions on home opioid dosing and administration will limit opioid-related adverse events and dosing errors upon hospital discharge. Each of these recommendations derive from one or more of the existing guidelines and reflect the transfer of responsibility for safe opioid use practices that occurs as patients transition from a closely monitored inpatient setting to the more self-regulated home environment.
DISCUSSION AND AREAS FOR FUTURE RESEARCH
This Consensus Statement reflects a synthesis of the key recommendations from a systematic review of existing guidelines on acute pain management, adapted for a hospital-specific scope of practice. Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.
Although these recommendations are not intended to apply to the immediate perioperative setting (ie, care in the postanesthesia care unit), many of the recommendations in the existing guidelines upon which this Consensus Statement was based were intended for the postoperative setting, and, as others have noted, recommendations in this setting are mostly comparable to those for treating acute pain more generally.27 Those interested in pain management in the postoperative setting specifically may wish to review the recent guidelines released by the American Pain Society,50 the content of which is in close alignment with our Consensus Statement.
Several important issues were raised during the extensive external feedback process undertaken as part of the development of this Consensus Statement. Although many issues were incorporated into the recommendations, there were several suggestions for which we felt the evidence base was not sufficient to allow a clear or valid recommendation to be made. For example, several reviewers requested endorsement of specific patient education tools and opioid equivalency calculators. In the absence of tools specifically validated for this purpose, we felt that the evidence was insufficient to make specific recommendations. Validating such tools for use in the inpatient setting should be an area of future investigation. In the meantime, we note that there are several existing and widely available resources for both patient education (ie, opioid information sheets, including opioid risks, safe containment and disposal, and safe use practices) and opioid equivalency calculations that clinicians and hospitals can adapt for their purposes.
Several individuals suggested recommendations on communication with outpatient continuity providers around opioid management decisions during hospitalization and on discharge. Although we believe that it is of paramount importance for outpatient providers to be aware of and have input into these decisions, the optimal timing and the method for such communication are unclear and likely to be institution-specific depending on the availability and integration of electronic records across care settings. We recommend that clinicians use their judgment as to the best format and timing for assuring that outpatient physicians are aware of and have input into these important management decisions with downstream consequences.
Concerns were also raised about the time required to complete the recommended practices and the importance of emphasizing the need for a team-based approach in this realm. We agree wholeheartedly with this sentiment and believe that many of the recommended practices can and should be automated and/or shared across the care team. For example, PDMPs allow prescribers to appoint delegates to check the PDMP on their behalf. Additionally, we suggest that hospitals work to develop systems to assist care teams with performance of these tasks in a standardized and streamlined manner (eg, integrating access to the PDMP and opioid equivalency tables within the electronic health record and developing standard patient educational handouts). Provision of written materials on opioid risks, side effects, and safety practices may be helpful in facilitating consistent messaging and efficient workflow for members of the care team.
Finally, the working group carefully considered whether to include a recommendation regarding naloxone prescribing at the time of hospital discharge. The provision of naloxone kits to laypersons through Overdose Education and Naloxone Distribution Programs has been shown to reduce opioid overdose deaths51,52 and hospitalizations53,54 and is both safe and cost-effective.55 The Centers for Disease Control and Preventionrecommend that clinicians “consider offering naloxone to patients with a history of overdose, a current or past substance use disorder, receipt of ≥50 mg of morphine equivalents per day or concurrent benzodiazepine use.”1 However, these recommendations are intended for patients on chronic opioid therapy; presently, no clear evidence exists to guide decisions about the benefits and costs associated with prescribing naloxone in the setting of short-term opioid therapy for acute pain. Further research in this area is warranted.
The greatest limitation of this Consensus Statement is the lack of high-quality studies informing most of the recommendations in the guidelines upon which our Consensus Statement was based. The majority of recommendations in the existing guidelines were based on expert opinion alone. Additional research is necessary before evidence-based recommendations can be formulated.
Accordingly, the working group identified several key areas for future research, in addition to those noted above. First, ongoing efforts to develop and evaluate the effectiveness of nonopioid and nonpharmacologic management strategies for acute pain in hospitalized patients are necessary. Second, studies identifying the risk factors for opioid-related adverse events in hospitalized patients would help inform management decisions and allow deployment of resources and specialized monitoring strategies to patients at heightened risk. The working group also noted the need for research investigating the impact of PDMP use on management decisions and downstream outcomes among hospitalized patients. Finally, conversations around pain management and concerns related to aberrant behaviors are often challenging in the hospital setting owing to the brief, high-intensity nature of the care and the lack of a longstanding therapeutic alliance. There is a great need to develop strategies and language to facilitate these conversations.
In conclusion, until more high-quality evidence becomes available, clinicians can use the recommendations contained in this Consensus Statement along with their clinical judgment and consultation with pharmacists, interventional pain specialists, and other staff (eg, social work, nursing) to help facilitate consistent, high-quality care across providers and hospitals. We believe that doing so will help increase the appropriateness of opioid therapy, minimize adverse events, facilitate shared decision-making, and foster stronger therapeutic alliances at the outset of the hospitalization for patients suffering from acute pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from the SHM for their facilitation of this project and dedication to this purpose.
The authors would also like to thank the many individuals who provided comments on the draft recommendations, including the participants in the SHM RADEO program; the SHM members; the representatives of specialty societies, including the American Academy of Family Physicians, the American College of Physicians, the American Hospital Association, the American Society of Addiction Medicine, the American Society of Anesthesiologists, the American Society of Health-System Pharmacists, the Society of Critical Care Medicine, and the Society of General Internal Medicine; and the representatives of patient advocacy groups, including SHM PFAC, Regions Hospital Patient and Family Advisory Committee, Patient and Family Centered Care Council of St. Louis Children’s Hospital, Missouri Family Partnership, and Parent and Family Care.
Disclosures: Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena reports receiving consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics, a consultancy to the life sciences industry (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance with the project and funded the in-person working group meeting but had no role in or influence on developing the content of the recommendations themselves. The SHM Board of Directors provided approval to submit the manuscript for publication without modification. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported in part by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, and reporting of the study
1. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States. JAMA. 2016;315(15):1624-1645. PubMed
55. Coffin PO, Sullivan SD. COst-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. PubMed
54. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. 2015;64(23):631-635. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
52. Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance abuse 2015;36(2):240-253. PubMed
51. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. PubMed
50. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. PubMed
49. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127-2132. PubMed
48. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine 2008;33(2):199-204. PubMed
47. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. PubMed
46. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. PubMed
45. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. PubMed
44. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. PubMed
43. Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. PubMed
42. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial [published online ahead of print December 13, 2017]. JAMA Surg. doi: 10.1001/jamasurg.2017.4915 PubMed
41. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-273. PubMed
40. Davies ED, Schneider F, Childs S, et al. A prevalence study of errors in opioid prescribing in a large teaching hospital. Int J Clin Pract. 2011;65(9):923-929. PubMed
39. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29-34. PubMed
38. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. PubMed
37. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. PubMed
36. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. PubMed
35. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. PubMed
34. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. PubMed
33. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21-27. PubMed
Since the initial reports of an emerging opioid epidemic in the early 2000s, intense focus on improving opioid prescribing in outpatient settings has culminated in new guidelines for chronic pain.1,2 Although opioid stewardship in the setting of chronic pain is of paramount importance in curbing the ongoing epidemic, long-term prescription opioid use often begins with treatment of acute pain.1 In addition to differences in recommended management strategies for acute and chronic pain, there are unique aspects and challenges to pain management in the acute-care setting.
Opioids are commonly used for the treatment of acute pain in hospitalized patients, often at high doses.3 Recent reports highlight that hospital use of opioids impacts downstream use.4-6 Additionally, opioid prescribing practices vary between hospital-based providers and hospitals,3,7 highlighting the need for prescribing standards and guidance. To our knowledge, there are no existing guidelines for improving the safety of opioid use in hospitalized patients outside of the intensive care or immediate perioperative settings.
The Society of Hospital Medicine (SHM) convened a working group to systematically review existing guidelines and develop a consensus statement to assist clinicians in safe opioid use for acute, noncancer pain in hospitalized adults.
Consensus Statement Purpose and Scope
The purpose of this Consensus Statement is to present clinical recommendations on the safe use of opioids for the treatment of acute, noncancer pain in hospitalized adults. The guidance is intended for clinicians practicing medicine in the inpatient setting (eg, hospitalists, primary care physicians, family physicians, nurse practitioners, and physician assistants) and is intended to apply to hospitalized adults with acute, noncancer pain (ie, pain that typically lasts <3 months or during the period of normal tissue healing) outside of the palliative, end-of-life, and intensive care settings.
Consensus Statement Development
Our working group included experts in opioid use in the hospital setting, defined by 1) engagement in the clinical practice of hospital medicine and 2) involvement in clinical research related to usage patterns and clinical outcomes of opioid use in hospitalized patients (see Appendix Table 1). The SHM provided administrative assistance with the project and funded the in-person working group meeting, but it had no role in formulating the recommendations. The SHM Board of Directors provided approval of the Consensus Statement without modification.
An overview of the sequential steps in the Consensus Statement development process is described below; details of the methods and results can be found in the Appendix (eMethods).
Performing the Systematic Review
Drafting the Consensus Statement
After performing the systematic review, the working group drafted and iteratively revised a set of recommendations using a variation of the Delphi Method8 to identify consensus among group members.
External Review
Following agreement on a draft set of recommendations, we obtained feedback from external groups, including 1) individuals involved in the SHM’s Reducing Adverse Drug Events Related to Opioids (RADEO) initiative, including those involved in the development of the implementation guide and site leads for the Mentored Implementation program, 2) SHM members, SHM Patient-Family Advisory Council (PFAC) members, and leaders of other relevant professional societies, and 3) peer-reviewers at the Journal of Hospital Medicine.
RESULTS
Deciding Whether to Use Opioids During Hospitalization
1. SHM recommends that clinicians limit the use of opioids to patients with 1) severe pain or 2) moderate pain that has not responded to nonopioid therapy, or where nonopioid therapy is contraindicated or anticipated to be ineffective.
Opioids are associated with several well-recognized risks ranging from mild to severe, including nausea, constipation, urinary retention, falls, delirium, sedation, physical dependence, addiction, respiratory depression, and death. Given these risks, the risk-to-benefit ratio is generally not favorable at lower levels of pain severity. Furthermore, for most painful conditions, including those causing severe pain, nonopioid analgesics, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), have been demonstrated to be equally or more effective with less risk of harm than opioids.9-13 Clinicians should consider drug–drug and drug–disease associations when deciding between these different therapies and make a determination in each patient regarding whether the benefits outweigh the risks. Often, drug–disease interactions do not represent absolute contraindications, and risks can be mitigated by adhering to dosage limits and, with respect to NSAIDs, 1) monitoring renal function, 2) monitoring volume status in patients with congestive heart failure, and 3) considering a selective cyclooxygenase-2 (COX-2) inhibitor rather than a nonselective NSAID or pairing the NSAID with an acid-suppressive medication in patients with a history of peptic ulcer disease or at elevated risk for gastroduodenal disease. For these reasons, a trial of nonopioid therapy (including pharmacologic and nonpharmacologic modalities) should always be considered before using opioids for pain of any severity. This does not imply that a trial of nonopioid therapy must be performed in all patients, but rather, that the likelihood of benefit and associated risks of opioid and nonopioid therapy should be considered for all patients in determining the best initial management strategy.
2. SHM recommends that clinicians use extra caution when administering opioids to patients with risk factors for opioid-related adverse events.
Several factors have been consistently demonstrated to increase the risk of opioid-related adverse events–most importantly, respiratory depression and overdose–in varied patient populations and settings, including age 65 years and older,1,14-17 renal insufficiency,1,14,18 hepatic insufficiency,1,14 chronic respiratory failure (including chronic obstructive pulmonary disease, sleep apnea, etc.), and receipt of other central nervous system (CNS) depressant medications (including, but not limited to, benzodiazepines).1,18-20 History of any substance use disorder and psychiatric disorders have been associated with an increased risk for the development of opioid use disorder.21-24 These factors should be weighed against the benefits when deciding on opioid appropriateness in a given patient. However, identification of these risks should not preclude opioids as part of pain management. When a decision is made to use opioids in patients with these risk factors, clinicians should 1) use a reduced starting dose (generally, at least a 50% reduction in the usual starting dose) and 2) consider closer monitoring for adverse effects (eg, more frequent nursing assessments, capnography, or more frequent outpatient visits).
3. SHM recommends that clinicians review the information contained in the prescription drug monitoring program (PDMP) database to inform decision-making around opioid therapy.
Although data on the impact of use of the state PDMP database on prescribing practices or patient outcomes are limited, PDMP use has been advocated by multiple guidelines on acute pain management.25-27 The PDMP provides information that can be useful in several ways, including 1) confirmation of prior opioid exposure and dosage, which should be used to guide appropriate dosage selection in the hospital, 2) identification of existing controlled substance prescriptions, which should be considered in prescribing decisions in the hospital and on discharge, and 3) identification of signs of aberrant behavior. For example, the identification of controlled substance prescriptions written by multiple different clinicians can facilitate early identification of potential diversion or evolving or existing opioid use disorder and the opportunity for intervention,28 which may include referral to support services, initiation of medication-assisted treatment, and/or pain specialist consultation when available. Concerns regarding evolving or existing opioid use disorder should prompt further discussion between the clinician and the patient, both to clarify their understanding of their recent prescription history and to discuss concerns for patient safety related to the increased risk of opioid-related adverse effects (including respiratory depression and overdose) among patients with controlled substance prescriptions written by multiple providers. Although such concerns should not automatically preclude the use of opioids for acute pain in the hospital setting, they should be considered in the assessment of whether the benefits of opioid therapy outweigh the risks for a given patient.
4. SHM recommends that clinicians educate patients and families or caregivers about the potential risks and side effects of opioid therapy as well as alternative pharmacologic and nonpharmacologic therapies for managing pain.
Patients are often unaware of the risks of opioid therapy (see Consensus Statement 1 for key risks),29 or that there are often equally effective alternative therapies. As with any therapy associated with substantial risk, clinicians should discuss these risks with patients and/or caregivers at the outset of therapy, as well as the potential benefits of nonopioid pharmacologic and nonpharmacologic therapies for managing pain. Patients should be informed that they may request nonopioid therapy in lieu of opioids, even for severe pain.
Once a Decision Has Been Made to Use Opioids During Hospitalization
5. SHM recommends that clinicians use the lowest effective opioid dose for the shortest duration possible.
Higher opioid doses are associated with an increased incidence of opioid-related adverse events, particularly overdose, in studies of both inpatient and outpatient populations.1,17,19,30,31 Studies in the inpatient and outpatient settings consistently demonstrate that risk increases with dosage.19,30,31 Clinicians should reduce the usual starting dose by at least 50% among patients with conditions that increase susceptibility to opioid-related adverse events (see Consensus Statement 2). The ongoing need for opioids should be re-assessed regularly-at least daily-during the hospitalization, with attempts at tapering as healing occurs and/or pain and function improve.
6. SHM recommends that clinicians use immediate-release opioid formulations and avoid initiation of long-acting or extended-release formulations (including transdermal fentanyl) for treatment of acute pain.
Studies in outpatient settings demonstrate that the use of long-acting opioids is associated with greater risk for overdose–especially in opioid-naïve patients–and long-term use.32,33 Further, hospitalized patients frequently have fluctuating renal function and rapidly changing pain levels. We therefore recommend that initiation of long-acting opioids be avoided for the treatment of acute, noncancer pain in hospitalized medical patients. It is important to note that although we recommend avoiding initiation of long-acting opioids for the treatment of acute, noncancer pain, there are circumstances outside of the scope of this Consensus Statement for which initiation of long-acting opioids may be indicated, including the treatment of opioid withdrawal. We also do not recommend discontinuation of long-acting or extended-release opioids in patients who are taking these medications for chronic pain at the time of hospital admission (unless there are concerns regarding adverse effects or drug–disease interactions).
7. SHM recommends that clinicians use the oral route of administration whenever possible. Intravenous opioids should be reserved for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal malabsorption, or when immediate pain control and/or rapid dose titration is necessary.
Intravenous opioid administration is associated with an increased risk of side effects, adverse events, and medication errors.34-36 Additionally, studies demonstrate that in general, the addiction potential of medications is greater the more rapid the onset of action (the onset of action is 5–10 min for intravenous and 15–30 minutes for oral administration).37,38 Furthermore, the duration of action is greater for oral compared to that of intravenous administration, potentially allowing for more consistent pain relief and less frequent administrations. As such, intravenous administration should be reserved for situations when oral administration is not possible or likely to be ineffective, or when immediate pain control and/or rapid titration is necessary.
8. SHM recommends that clinicians use an opioid equivalency table or calculator to understand the relative potency of different opioids 1) when initiating opioid therapy, 2) when changing from one route of administration to another, and 3) when changing from one opioid to another. When changing from one opioid to another, clinicians should generally reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids as well as possible incomplete cross-tolerance.
Most errors causing preventable adverse drug events in hospitals occur at the ordering stage.39,40 Clinicians are often unaware of the potency of different types of opioids relative to each other or to morphine (ie, morphine equivalent dose), which can lead to inadvertent overdose when initiating therapy with nonmorphine opioids and when converting from one opioid to another. To facilitate safe opioid use, we recommend that clinicians use one of several available opioid equivalency tables or calculators to better understand the relative potencies of opioids and to inform both starting dose calculations and conversions between opioids and routes of administration. When converting from one opioid to another, we caution clinicians to reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids and the potential for incomplete cross-tolerance, wherein tolerance to a currently administered opioid does not extend completely to other opioids. Clinicians should use extreme caution when performing conversions to and from methadone and consider consultation with a hospital pharmacist or a pain management specialist, when available, to assist with conversion decisions and calculations.
9. SHM recommends that clinicians pair opioids with scheduled nonopioid analgesic medications, unless contraindicated, and always consider pairing with nonpharmacologic pain management strategies (ie, multimodal analgesia).
Concurrent receipt of opioids and nonopioid analgesic medications (including acetaminophen, NSAIDs, and gabapentin or pregabalin, depending on the underlying pathophysiology of the pain) has been demonstrated to reduce total opioid requirements and improve pain management.41,42 Clinicians should be familiar with contraindications and maximum dosage recommendations for each of these adjunctive nonopioid medications. We recommend separate orders for each, rather than using drug formulations that combine opioids and nonopioid analgesics in the same pill, due to the risk of inadvertently exceeding the maximum recommended doses of the nonopioid analgesic (particularly acetaminophen) with combination products. We recommend that nonopioid analgesics be ordered at a scheduled frequency, rather than as needed, to facilitate consistent administration that is not dependent on opioid administration. Topical agents, including lidocaine and capsaicin, should also be considered. Nonpharmacologic pain management strategies can include procedure-based (eg, regional and local anesthesia) and nonprocedure-based therapies depending on the underlying condition and institutional availability. Although few studies have assessed the benefit of nonpharmacologic, nonprocedure-based therapies for the treatment of acute pain in hospitalized patients, the lack of harm associated with their use argues for their adoption. Simple nonpharmacologic therapies that can usually be provided to patients in any hospital setting include music therapy, cold or hot packs, chaplain or social work visits (possibly including mindfulness training),43 and physical therapy, among others.
10. SHM recommends that, unless contraindicated, clinicians order a bowel regimen to prevent opioid-induced constipation in patients receiving opioids.
Constipation is a common adverse effect of opioid therapy and results from the activation of mu opioid receptors in the colon, resulting in decreased peristalsis. Hospitalized patients are already prone to constipation due to their often-limited physical mobility. To mitigate this complication, we recommend the administration of a bowel regimen to all hospitalized medical patients receiving opioid therapy, provided the patient is not having diarrhea. Given the mechanism of opioid-induced constipation, stimulant laxatives (eg, senna, bisacodyl) have been recommended for this purpose.44 Osmotic laxatives (eg, polyethylene glycol, lactulose) have demonstrated efficacy for the treatment of constipation more generally (ie, not necessarily opioid-induced constipation). Stool softeners, although frequently used in the inpatient setting, are not recommended due to limited and conflicting evidence for efficacy in prevention or treatment of constipation.45 Bowel movements should be tracked during hospitalization, and the bowel regimen modified accordingly.
11. SHM recommends that clinicians limit co-administration of opioids with other central nervous system depressant medications to the extent possible.
This combination has been demonstrated to increase the risk of opioid-related adverse events in multiple settings of care, including during hospitalization.1,18,19 Although benzodiazepines have received the most attention in this respect, other medications with CNS depressant properties may also increase the risk, including, but not limited to, nonbenzodiazepine sedative-hypnotics (eg, zolpidem, zaleplon, zopiclone), muscle relaxants, sedating antidepressants, antipsychotics, and antihistamines.18,19,46 For some patients, the combination will be unavoidable, and we do not suggest routine discontinuation of longstanding medications that preexisted hospitalization, given the risks of withdrawal and/or worsening of the underlying condition for which these medications are prescribed. Rather, clinicians should carefully consider the necessity of each medication class with input from the patient’s outpatient providers, taper the frequency and/or the dose of CNS depressants when appropriate and feasible, and avoid new coprescriptions to the extent possible, both during hospitalization and on hospital discharge.
12. SHM recommends that clinicians work with patients and families or caregivers to establish realistic goals and expectations of opioid therapy and the expected course of recovery.
Discussing expectations at the start of therapy is important to facilitate a clear understanding of how meaningful improvement will be defined and measured during the hospitalization and how long the patient is anticipated to require opioid therapy. Meaningful improvement should be defined to include improvement in both pain and function. Clinicians should discuss with patients 1) that the goal of opioid therapy is tolerability of pain such that meaningful improvement in function can be achieved and 2) that a decrease in pain intensity in the absence of improved function is not considered meaningful improvement in most situations and should prompt reevaluation of the appropriateness of continued opioid therapy as well as close follow-up with a clinician following hospital discharge. Discussions regarding the expected course of recovery should include that acute pain is expected to resolve as the underlying medical condition improves and that although pain may persist beyond the hospitalization, pain that is severe enough to require opioids will often be resolved or almost resolved by the time of hospital discharge.
13. SHM recommends that clinicians monitor the response to opioid therapy, including assessment for functional improvement and development of adverse effects.
Pain severity and function should be assessed at least daily, and improvement in reported pain severity without improvement in function over several days should, in most circumstances, prompt reconsideration of ongoing opioid therapy and reconsideration of the underlying etiology of pain. Although hospital-specific functional measures in the setting of acute pain have not yet been validated, we suggest that such measures and goals should be individualized based on preexisting function and may include the ability to sit up or move in bed, move to a chair, work with physical therapy, or ambulate in the hallway. Protocols for the assessment for adverse effects are not well established. Because sedation typically precedes respiratory depression, it is generally recommended that patients are evaluated (eg, by nursing staff) for sedation after each opioid administration (10–20 minutes for intravenous and 30–60 minutes for oral administration based on the time-to-peak effect). Whether certain patients may benefit from more intensive respiratory monitoring, such as pulse oximetry or capnography, is an area of active investigation and not yet established.
Prescribing at the Time of Hospital Discharge
14. SHM recommends that clinicians ask patients about any existing opioid supply at home and account for any such supply when issuing an opioid prescription on discharge.
Even in the setting of acute pain, patients may have previously received an opioid prescription from an outpatient clinician prior to hospitalization. Unused prescription opioids create the possibility of both overdose (when patients take multiple opioids concurrently, intentionally or inadvertently) and diversion (many adults with prescription opioid misuse obtained their opioids from a friend or a relative who may or may not have known that this occurred47). The PDMP database can provide information related to the potential existence of any prior opioid supplies, which should be confirmed with the patient and considered when providing a new prescription on hospital discharge. Information on proper disposal should be provided if use of the preexisting opioid is no longer intended.
15. SHM recommends that clinicians prescribe the minimum quantity of opioids anticipated to be necessary based on the expected course and duration of pain that is severe enough to require opioid therapy after hospital discharge.
16. SHM recommends that clinicians ensure that patients and families or caregivers receive information regarding how to minimize the risks of opioid therapy for themselves, their families, and their communities. This includes but is not limited to 1) how to take their opioids correctly (the planned medications, doses, schedule); 2) that they should take the minimum quantity necessary to achieve tolerable levels of pain and meaningful functional improvement, reducing the dose and/or frequency as pain and function improve; 3) how to safeguard their supply and dispose of any unused supply; 4) that they should avoid agents that may potentiate the sedative effect of opioids, including sleeping medication and alcohol; 5) that they should avoid driving or operating heavy machinery while taking opioids; and 6) that they should seek help if they begin to experience any potential adverse effects, with inclusion of information on early warning signs.
Clear and concise patient instructions on home opioid dosing and administration will limit opioid-related adverse events and dosing errors upon hospital discharge. Each of these recommendations derive from one or more of the existing guidelines and reflect the transfer of responsibility for safe opioid use practices that occurs as patients transition from a closely monitored inpatient setting to the more self-regulated home environment.
DISCUSSION AND AREAS FOR FUTURE RESEARCH
This Consensus Statement reflects a synthesis of the key recommendations from a systematic review of existing guidelines on acute pain management, adapted for a hospital-specific scope of practice. Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.
Although these recommendations are not intended to apply to the immediate perioperative setting (ie, care in the postanesthesia care unit), many of the recommendations in the existing guidelines upon which this Consensus Statement was based were intended for the postoperative setting, and, as others have noted, recommendations in this setting are mostly comparable to those for treating acute pain more generally.27 Those interested in pain management in the postoperative setting specifically may wish to review the recent guidelines released by the American Pain Society,50 the content of which is in close alignment with our Consensus Statement.
Several important issues were raised during the extensive external feedback process undertaken as part of the development of this Consensus Statement. Although many issues were incorporated into the recommendations, there were several suggestions for which we felt the evidence base was not sufficient to allow a clear or valid recommendation to be made. For example, several reviewers requested endorsement of specific patient education tools and opioid equivalency calculators. In the absence of tools specifically validated for this purpose, we felt that the evidence was insufficient to make specific recommendations. Validating such tools for use in the inpatient setting should be an area of future investigation. In the meantime, we note that there are several existing and widely available resources for both patient education (ie, opioid information sheets, including opioid risks, safe containment and disposal, and safe use practices) and opioid equivalency calculations that clinicians and hospitals can adapt for their purposes.
Several individuals suggested recommendations on communication with outpatient continuity providers around opioid management decisions during hospitalization and on discharge. Although we believe that it is of paramount importance for outpatient providers to be aware of and have input into these decisions, the optimal timing and the method for such communication are unclear and likely to be institution-specific depending on the availability and integration of electronic records across care settings. We recommend that clinicians use their judgment as to the best format and timing for assuring that outpatient physicians are aware of and have input into these important management decisions with downstream consequences.
Concerns were also raised about the time required to complete the recommended practices and the importance of emphasizing the need for a team-based approach in this realm. We agree wholeheartedly with this sentiment and believe that many of the recommended practices can and should be automated and/or shared across the care team. For example, PDMPs allow prescribers to appoint delegates to check the PDMP on their behalf. Additionally, we suggest that hospitals work to develop systems to assist care teams with performance of these tasks in a standardized and streamlined manner (eg, integrating access to the PDMP and opioid equivalency tables within the electronic health record and developing standard patient educational handouts). Provision of written materials on opioid risks, side effects, and safety practices may be helpful in facilitating consistent messaging and efficient workflow for members of the care team.
Finally, the working group carefully considered whether to include a recommendation regarding naloxone prescribing at the time of hospital discharge. The provision of naloxone kits to laypersons through Overdose Education and Naloxone Distribution Programs has been shown to reduce opioid overdose deaths51,52 and hospitalizations53,54 and is both safe and cost-effective.55 The Centers for Disease Control and Preventionrecommend that clinicians “consider offering naloxone to patients with a history of overdose, a current or past substance use disorder, receipt of ≥50 mg of morphine equivalents per day or concurrent benzodiazepine use.”1 However, these recommendations are intended for patients on chronic opioid therapy; presently, no clear evidence exists to guide decisions about the benefits and costs associated with prescribing naloxone in the setting of short-term opioid therapy for acute pain. Further research in this area is warranted.
The greatest limitation of this Consensus Statement is the lack of high-quality studies informing most of the recommendations in the guidelines upon which our Consensus Statement was based. The majority of recommendations in the existing guidelines were based on expert opinion alone. Additional research is necessary before evidence-based recommendations can be formulated.
Accordingly, the working group identified several key areas for future research, in addition to those noted above. First, ongoing efforts to develop and evaluate the effectiveness of nonopioid and nonpharmacologic management strategies for acute pain in hospitalized patients are necessary. Second, studies identifying the risk factors for opioid-related adverse events in hospitalized patients would help inform management decisions and allow deployment of resources and specialized monitoring strategies to patients at heightened risk. The working group also noted the need for research investigating the impact of PDMP use on management decisions and downstream outcomes among hospitalized patients. Finally, conversations around pain management and concerns related to aberrant behaviors are often challenging in the hospital setting owing to the brief, high-intensity nature of the care and the lack of a longstanding therapeutic alliance. There is a great need to develop strategies and language to facilitate these conversations.
In conclusion, until more high-quality evidence becomes available, clinicians can use the recommendations contained in this Consensus Statement along with their clinical judgment and consultation with pharmacists, interventional pain specialists, and other staff (eg, social work, nursing) to help facilitate consistent, high-quality care across providers and hospitals. We believe that doing so will help increase the appropriateness of opioid therapy, minimize adverse events, facilitate shared decision-making, and foster stronger therapeutic alliances at the outset of the hospitalization for patients suffering from acute pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from the SHM for their facilitation of this project and dedication to this purpose.
The authors would also like to thank the many individuals who provided comments on the draft recommendations, including the participants in the SHM RADEO program; the SHM members; the representatives of specialty societies, including the American Academy of Family Physicians, the American College of Physicians, the American Hospital Association, the American Society of Addiction Medicine, the American Society of Anesthesiologists, the American Society of Health-System Pharmacists, the Society of Critical Care Medicine, and the Society of General Internal Medicine; and the representatives of patient advocacy groups, including SHM PFAC, Regions Hospital Patient and Family Advisory Committee, Patient and Family Centered Care Council of St. Louis Children’s Hospital, Missouri Family Partnership, and Parent and Family Care.
Disclosures: Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena reports receiving consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics, a consultancy to the life sciences industry (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance with the project and funded the in-person working group meeting but had no role in or influence on developing the content of the recommendations themselves. The SHM Board of Directors provided approval to submit the manuscript for publication without modification. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported in part by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, and reporting of the study
Since the initial reports of an emerging opioid epidemic in the early 2000s, intense focus on improving opioid prescribing in outpatient settings has culminated in new guidelines for chronic pain.1,2 Although opioid stewardship in the setting of chronic pain is of paramount importance in curbing the ongoing epidemic, long-term prescription opioid use often begins with treatment of acute pain.1 In addition to differences in recommended management strategies for acute and chronic pain, there are unique aspects and challenges to pain management in the acute-care setting.
Opioids are commonly used for the treatment of acute pain in hospitalized patients, often at high doses.3 Recent reports highlight that hospital use of opioids impacts downstream use.4-6 Additionally, opioid prescribing practices vary between hospital-based providers and hospitals,3,7 highlighting the need for prescribing standards and guidance. To our knowledge, there are no existing guidelines for improving the safety of opioid use in hospitalized patients outside of the intensive care or immediate perioperative settings.
The Society of Hospital Medicine (SHM) convened a working group to systematically review existing guidelines and develop a consensus statement to assist clinicians in safe opioid use for acute, noncancer pain in hospitalized adults.
Consensus Statement Purpose and Scope
The purpose of this Consensus Statement is to present clinical recommendations on the safe use of opioids for the treatment of acute, noncancer pain in hospitalized adults. The guidance is intended for clinicians practicing medicine in the inpatient setting (eg, hospitalists, primary care physicians, family physicians, nurse practitioners, and physician assistants) and is intended to apply to hospitalized adults with acute, noncancer pain (ie, pain that typically lasts <3 months or during the period of normal tissue healing) outside of the palliative, end-of-life, and intensive care settings.
Consensus Statement Development
Our working group included experts in opioid use in the hospital setting, defined by 1) engagement in the clinical practice of hospital medicine and 2) involvement in clinical research related to usage patterns and clinical outcomes of opioid use in hospitalized patients (see Appendix Table 1). The SHM provided administrative assistance with the project and funded the in-person working group meeting, but it had no role in formulating the recommendations. The SHM Board of Directors provided approval of the Consensus Statement without modification.
An overview of the sequential steps in the Consensus Statement development process is described below; details of the methods and results can be found in the Appendix (eMethods).
Performing the Systematic Review
Drafting the Consensus Statement
After performing the systematic review, the working group drafted and iteratively revised a set of recommendations using a variation of the Delphi Method8 to identify consensus among group members.
External Review
Following agreement on a draft set of recommendations, we obtained feedback from external groups, including 1) individuals involved in the SHM’s Reducing Adverse Drug Events Related to Opioids (RADEO) initiative, including those involved in the development of the implementation guide and site leads for the Mentored Implementation program, 2) SHM members, SHM Patient-Family Advisory Council (PFAC) members, and leaders of other relevant professional societies, and 3) peer-reviewers at the Journal of Hospital Medicine.
RESULTS
Deciding Whether to Use Opioids During Hospitalization
1. SHM recommends that clinicians limit the use of opioids to patients with 1) severe pain or 2) moderate pain that has not responded to nonopioid therapy, or where nonopioid therapy is contraindicated or anticipated to be ineffective.
Opioids are associated with several well-recognized risks ranging from mild to severe, including nausea, constipation, urinary retention, falls, delirium, sedation, physical dependence, addiction, respiratory depression, and death. Given these risks, the risk-to-benefit ratio is generally not favorable at lower levels of pain severity. Furthermore, for most painful conditions, including those causing severe pain, nonopioid analgesics, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), have been demonstrated to be equally or more effective with less risk of harm than opioids.9-13 Clinicians should consider drug–drug and drug–disease associations when deciding between these different therapies and make a determination in each patient regarding whether the benefits outweigh the risks. Often, drug–disease interactions do not represent absolute contraindications, and risks can be mitigated by adhering to dosage limits and, with respect to NSAIDs, 1) monitoring renal function, 2) monitoring volume status in patients with congestive heart failure, and 3) considering a selective cyclooxygenase-2 (COX-2) inhibitor rather than a nonselective NSAID or pairing the NSAID with an acid-suppressive medication in patients with a history of peptic ulcer disease or at elevated risk for gastroduodenal disease. For these reasons, a trial of nonopioid therapy (including pharmacologic and nonpharmacologic modalities) should always be considered before using opioids for pain of any severity. This does not imply that a trial of nonopioid therapy must be performed in all patients, but rather, that the likelihood of benefit and associated risks of opioid and nonopioid therapy should be considered for all patients in determining the best initial management strategy.
2. SHM recommends that clinicians use extra caution when administering opioids to patients with risk factors for opioid-related adverse events.
Several factors have been consistently demonstrated to increase the risk of opioid-related adverse events–most importantly, respiratory depression and overdose–in varied patient populations and settings, including age 65 years and older,1,14-17 renal insufficiency,1,14,18 hepatic insufficiency,1,14 chronic respiratory failure (including chronic obstructive pulmonary disease, sleep apnea, etc.), and receipt of other central nervous system (CNS) depressant medications (including, but not limited to, benzodiazepines).1,18-20 History of any substance use disorder and psychiatric disorders have been associated with an increased risk for the development of opioid use disorder.21-24 These factors should be weighed against the benefits when deciding on opioid appropriateness in a given patient. However, identification of these risks should not preclude opioids as part of pain management. When a decision is made to use opioids in patients with these risk factors, clinicians should 1) use a reduced starting dose (generally, at least a 50% reduction in the usual starting dose) and 2) consider closer monitoring for adverse effects (eg, more frequent nursing assessments, capnography, or more frequent outpatient visits).
3. SHM recommends that clinicians review the information contained in the prescription drug monitoring program (PDMP) database to inform decision-making around opioid therapy.
Although data on the impact of use of the state PDMP database on prescribing practices or patient outcomes are limited, PDMP use has been advocated by multiple guidelines on acute pain management.25-27 The PDMP provides information that can be useful in several ways, including 1) confirmation of prior opioid exposure and dosage, which should be used to guide appropriate dosage selection in the hospital, 2) identification of existing controlled substance prescriptions, which should be considered in prescribing decisions in the hospital and on discharge, and 3) identification of signs of aberrant behavior. For example, the identification of controlled substance prescriptions written by multiple different clinicians can facilitate early identification of potential diversion or evolving or existing opioid use disorder and the opportunity for intervention,28 which may include referral to support services, initiation of medication-assisted treatment, and/or pain specialist consultation when available. Concerns regarding evolving or existing opioid use disorder should prompt further discussion between the clinician and the patient, both to clarify their understanding of their recent prescription history and to discuss concerns for patient safety related to the increased risk of opioid-related adverse effects (including respiratory depression and overdose) among patients with controlled substance prescriptions written by multiple providers. Although such concerns should not automatically preclude the use of opioids for acute pain in the hospital setting, they should be considered in the assessment of whether the benefits of opioid therapy outweigh the risks for a given patient.
4. SHM recommends that clinicians educate patients and families or caregivers about the potential risks and side effects of opioid therapy as well as alternative pharmacologic and nonpharmacologic therapies for managing pain.
Patients are often unaware of the risks of opioid therapy (see Consensus Statement 1 for key risks),29 or that there are often equally effective alternative therapies. As with any therapy associated with substantial risk, clinicians should discuss these risks with patients and/or caregivers at the outset of therapy, as well as the potential benefits of nonopioid pharmacologic and nonpharmacologic therapies for managing pain. Patients should be informed that they may request nonopioid therapy in lieu of opioids, even for severe pain.
Once a Decision Has Been Made to Use Opioids During Hospitalization
5. SHM recommends that clinicians use the lowest effective opioid dose for the shortest duration possible.
Higher opioid doses are associated with an increased incidence of opioid-related adverse events, particularly overdose, in studies of both inpatient and outpatient populations.1,17,19,30,31 Studies in the inpatient and outpatient settings consistently demonstrate that risk increases with dosage.19,30,31 Clinicians should reduce the usual starting dose by at least 50% among patients with conditions that increase susceptibility to opioid-related adverse events (see Consensus Statement 2). The ongoing need for opioids should be re-assessed regularly-at least daily-during the hospitalization, with attempts at tapering as healing occurs and/or pain and function improve.
6. SHM recommends that clinicians use immediate-release opioid formulations and avoid initiation of long-acting or extended-release formulations (including transdermal fentanyl) for treatment of acute pain.
Studies in outpatient settings demonstrate that the use of long-acting opioids is associated with greater risk for overdose–especially in opioid-naïve patients–and long-term use.32,33 Further, hospitalized patients frequently have fluctuating renal function and rapidly changing pain levels. We therefore recommend that initiation of long-acting opioids be avoided for the treatment of acute, noncancer pain in hospitalized medical patients. It is important to note that although we recommend avoiding initiation of long-acting opioids for the treatment of acute, noncancer pain, there are circumstances outside of the scope of this Consensus Statement for which initiation of long-acting opioids may be indicated, including the treatment of opioid withdrawal. We also do not recommend discontinuation of long-acting or extended-release opioids in patients who are taking these medications for chronic pain at the time of hospital admission (unless there are concerns regarding adverse effects or drug–disease interactions).
7. SHM recommends that clinicians use the oral route of administration whenever possible. Intravenous opioids should be reserved for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal malabsorption, or when immediate pain control and/or rapid dose titration is necessary.
Intravenous opioid administration is associated with an increased risk of side effects, adverse events, and medication errors.34-36 Additionally, studies demonstrate that in general, the addiction potential of medications is greater the more rapid the onset of action (the onset of action is 5–10 min for intravenous and 15–30 minutes for oral administration).37,38 Furthermore, the duration of action is greater for oral compared to that of intravenous administration, potentially allowing for more consistent pain relief and less frequent administrations. As such, intravenous administration should be reserved for situations when oral administration is not possible or likely to be ineffective, or when immediate pain control and/or rapid titration is necessary.
8. SHM recommends that clinicians use an opioid equivalency table or calculator to understand the relative potency of different opioids 1) when initiating opioid therapy, 2) when changing from one route of administration to another, and 3) when changing from one opioid to another. When changing from one opioid to another, clinicians should generally reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids as well as possible incomplete cross-tolerance.
Most errors causing preventable adverse drug events in hospitals occur at the ordering stage.39,40 Clinicians are often unaware of the potency of different types of opioids relative to each other or to morphine (ie, morphine equivalent dose), which can lead to inadvertent overdose when initiating therapy with nonmorphine opioids and when converting from one opioid to another. To facilitate safe opioid use, we recommend that clinicians use one of several available opioid equivalency tables or calculators to better understand the relative potencies of opioids and to inform both starting dose calculations and conversions between opioids and routes of administration. When converting from one opioid to another, we caution clinicians to reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids and the potential for incomplete cross-tolerance, wherein tolerance to a currently administered opioid does not extend completely to other opioids. Clinicians should use extreme caution when performing conversions to and from methadone and consider consultation with a hospital pharmacist or a pain management specialist, when available, to assist with conversion decisions and calculations.
9. SHM recommends that clinicians pair opioids with scheduled nonopioid analgesic medications, unless contraindicated, and always consider pairing with nonpharmacologic pain management strategies (ie, multimodal analgesia).
Concurrent receipt of opioids and nonopioid analgesic medications (including acetaminophen, NSAIDs, and gabapentin or pregabalin, depending on the underlying pathophysiology of the pain) has been demonstrated to reduce total opioid requirements and improve pain management.41,42 Clinicians should be familiar with contraindications and maximum dosage recommendations for each of these adjunctive nonopioid medications. We recommend separate orders for each, rather than using drug formulations that combine opioids and nonopioid analgesics in the same pill, due to the risk of inadvertently exceeding the maximum recommended doses of the nonopioid analgesic (particularly acetaminophen) with combination products. We recommend that nonopioid analgesics be ordered at a scheduled frequency, rather than as needed, to facilitate consistent administration that is not dependent on opioid administration. Topical agents, including lidocaine and capsaicin, should also be considered. Nonpharmacologic pain management strategies can include procedure-based (eg, regional and local anesthesia) and nonprocedure-based therapies depending on the underlying condition and institutional availability. Although few studies have assessed the benefit of nonpharmacologic, nonprocedure-based therapies for the treatment of acute pain in hospitalized patients, the lack of harm associated with their use argues for their adoption. Simple nonpharmacologic therapies that can usually be provided to patients in any hospital setting include music therapy, cold or hot packs, chaplain or social work visits (possibly including mindfulness training),43 and physical therapy, among others.
10. SHM recommends that, unless contraindicated, clinicians order a bowel regimen to prevent opioid-induced constipation in patients receiving opioids.
Constipation is a common adverse effect of opioid therapy and results from the activation of mu opioid receptors in the colon, resulting in decreased peristalsis. Hospitalized patients are already prone to constipation due to their often-limited physical mobility. To mitigate this complication, we recommend the administration of a bowel regimen to all hospitalized medical patients receiving opioid therapy, provided the patient is not having diarrhea. Given the mechanism of opioid-induced constipation, stimulant laxatives (eg, senna, bisacodyl) have been recommended for this purpose.44 Osmotic laxatives (eg, polyethylene glycol, lactulose) have demonstrated efficacy for the treatment of constipation more generally (ie, not necessarily opioid-induced constipation). Stool softeners, although frequently used in the inpatient setting, are not recommended due to limited and conflicting evidence for efficacy in prevention or treatment of constipation.45 Bowel movements should be tracked during hospitalization, and the bowel regimen modified accordingly.
11. SHM recommends that clinicians limit co-administration of opioids with other central nervous system depressant medications to the extent possible.
This combination has been demonstrated to increase the risk of opioid-related adverse events in multiple settings of care, including during hospitalization.1,18,19 Although benzodiazepines have received the most attention in this respect, other medications with CNS depressant properties may also increase the risk, including, but not limited to, nonbenzodiazepine sedative-hypnotics (eg, zolpidem, zaleplon, zopiclone), muscle relaxants, sedating antidepressants, antipsychotics, and antihistamines.18,19,46 For some patients, the combination will be unavoidable, and we do not suggest routine discontinuation of longstanding medications that preexisted hospitalization, given the risks of withdrawal and/or worsening of the underlying condition for which these medications are prescribed. Rather, clinicians should carefully consider the necessity of each medication class with input from the patient’s outpatient providers, taper the frequency and/or the dose of CNS depressants when appropriate and feasible, and avoid new coprescriptions to the extent possible, both during hospitalization and on hospital discharge.
12. SHM recommends that clinicians work with patients and families or caregivers to establish realistic goals and expectations of opioid therapy and the expected course of recovery.
Discussing expectations at the start of therapy is important to facilitate a clear understanding of how meaningful improvement will be defined and measured during the hospitalization and how long the patient is anticipated to require opioid therapy. Meaningful improvement should be defined to include improvement in both pain and function. Clinicians should discuss with patients 1) that the goal of opioid therapy is tolerability of pain such that meaningful improvement in function can be achieved and 2) that a decrease in pain intensity in the absence of improved function is not considered meaningful improvement in most situations and should prompt reevaluation of the appropriateness of continued opioid therapy as well as close follow-up with a clinician following hospital discharge. Discussions regarding the expected course of recovery should include that acute pain is expected to resolve as the underlying medical condition improves and that although pain may persist beyond the hospitalization, pain that is severe enough to require opioids will often be resolved or almost resolved by the time of hospital discharge.
13. SHM recommends that clinicians monitor the response to opioid therapy, including assessment for functional improvement and development of adverse effects.
Pain severity and function should be assessed at least daily, and improvement in reported pain severity without improvement in function over several days should, in most circumstances, prompt reconsideration of ongoing opioid therapy and reconsideration of the underlying etiology of pain. Although hospital-specific functional measures in the setting of acute pain have not yet been validated, we suggest that such measures and goals should be individualized based on preexisting function and may include the ability to sit up or move in bed, move to a chair, work with physical therapy, or ambulate in the hallway. Protocols for the assessment for adverse effects are not well established. Because sedation typically precedes respiratory depression, it is generally recommended that patients are evaluated (eg, by nursing staff) for sedation after each opioid administration (10–20 minutes for intravenous and 30–60 minutes for oral administration based on the time-to-peak effect). Whether certain patients may benefit from more intensive respiratory monitoring, such as pulse oximetry or capnography, is an area of active investigation and not yet established.
Prescribing at the Time of Hospital Discharge
14. SHM recommends that clinicians ask patients about any existing opioid supply at home and account for any such supply when issuing an opioid prescription on discharge.
Even in the setting of acute pain, patients may have previously received an opioid prescription from an outpatient clinician prior to hospitalization. Unused prescription opioids create the possibility of both overdose (when patients take multiple opioids concurrently, intentionally or inadvertently) and diversion (many adults with prescription opioid misuse obtained their opioids from a friend or a relative who may or may not have known that this occurred47). The PDMP database can provide information related to the potential existence of any prior opioid supplies, which should be confirmed with the patient and considered when providing a new prescription on hospital discharge. Information on proper disposal should be provided if use of the preexisting opioid is no longer intended.
15. SHM recommends that clinicians prescribe the minimum quantity of opioids anticipated to be necessary based on the expected course and duration of pain that is severe enough to require opioid therapy after hospital discharge.
16. SHM recommends that clinicians ensure that patients and families or caregivers receive information regarding how to minimize the risks of opioid therapy for themselves, their families, and their communities. This includes but is not limited to 1) how to take their opioids correctly (the planned medications, doses, schedule); 2) that they should take the minimum quantity necessary to achieve tolerable levels of pain and meaningful functional improvement, reducing the dose and/or frequency as pain and function improve; 3) how to safeguard their supply and dispose of any unused supply; 4) that they should avoid agents that may potentiate the sedative effect of opioids, including sleeping medication and alcohol; 5) that they should avoid driving or operating heavy machinery while taking opioids; and 6) that they should seek help if they begin to experience any potential adverse effects, with inclusion of information on early warning signs.
Clear and concise patient instructions on home opioid dosing and administration will limit opioid-related adverse events and dosing errors upon hospital discharge. Each of these recommendations derive from one or more of the existing guidelines and reflect the transfer of responsibility for safe opioid use practices that occurs as patients transition from a closely monitored inpatient setting to the more self-regulated home environment.
DISCUSSION AND AREAS FOR FUTURE RESEARCH
This Consensus Statement reflects a synthesis of the key recommendations from a systematic review of existing guidelines on acute pain management, adapted for a hospital-specific scope of practice. Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.
Although these recommendations are not intended to apply to the immediate perioperative setting (ie, care in the postanesthesia care unit), many of the recommendations in the existing guidelines upon which this Consensus Statement was based were intended for the postoperative setting, and, as others have noted, recommendations in this setting are mostly comparable to those for treating acute pain more generally.27 Those interested in pain management in the postoperative setting specifically may wish to review the recent guidelines released by the American Pain Society,50 the content of which is in close alignment with our Consensus Statement.
Several important issues were raised during the extensive external feedback process undertaken as part of the development of this Consensus Statement. Although many issues were incorporated into the recommendations, there were several suggestions for which we felt the evidence base was not sufficient to allow a clear or valid recommendation to be made. For example, several reviewers requested endorsement of specific patient education tools and opioid equivalency calculators. In the absence of tools specifically validated for this purpose, we felt that the evidence was insufficient to make specific recommendations. Validating such tools for use in the inpatient setting should be an area of future investigation. In the meantime, we note that there are several existing and widely available resources for both patient education (ie, opioid information sheets, including opioid risks, safe containment and disposal, and safe use practices) and opioid equivalency calculations that clinicians and hospitals can adapt for their purposes.
Several individuals suggested recommendations on communication with outpatient continuity providers around opioid management decisions during hospitalization and on discharge. Although we believe that it is of paramount importance for outpatient providers to be aware of and have input into these decisions, the optimal timing and the method for such communication are unclear and likely to be institution-specific depending on the availability and integration of electronic records across care settings. We recommend that clinicians use their judgment as to the best format and timing for assuring that outpatient physicians are aware of and have input into these important management decisions with downstream consequences.
Concerns were also raised about the time required to complete the recommended practices and the importance of emphasizing the need for a team-based approach in this realm. We agree wholeheartedly with this sentiment and believe that many of the recommended practices can and should be automated and/or shared across the care team. For example, PDMPs allow prescribers to appoint delegates to check the PDMP on their behalf. Additionally, we suggest that hospitals work to develop systems to assist care teams with performance of these tasks in a standardized and streamlined manner (eg, integrating access to the PDMP and opioid equivalency tables within the electronic health record and developing standard patient educational handouts). Provision of written materials on opioid risks, side effects, and safety practices may be helpful in facilitating consistent messaging and efficient workflow for members of the care team.
Finally, the working group carefully considered whether to include a recommendation regarding naloxone prescribing at the time of hospital discharge. The provision of naloxone kits to laypersons through Overdose Education and Naloxone Distribution Programs has been shown to reduce opioid overdose deaths51,52 and hospitalizations53,54 and is both safe and cost-effective.55 The Centers for Disease Control and Preventionrecommend that clinicians “consider offering naloxone to patients with a history of overdose, a current or past substance use disorder, receipt of ≥50 mg of morphine equivalents per day or concurrent benzodiazepine use.”1 However, these recommendations are intended for patients on chronic opioid therapy; presently, no clear evidence exists to guide decisions about the benefits and costs associated with prescribing naloxone in the setting of short-term opioid therapy for acute pain. Further research in this area is warranted.
The greatest limitation of this Consensus Statement is the lack of high-quality studies informing most of the recommendations in the guidelines upon which our Consensus Statement was based. The majority of recommendations in the existing guidelines were based on expert opinion alone. Additional research is necessary before evidence-based recommendations can be formulated.
Accordingly, the working group identified several key areas for future research, in addition to those noted above. First, ongoing efforts to develop and evaluate the effectiveness of nonopioid and nonpharmacologic management strategies for acute pain in hospitalized patients are necessary. Second, studies identifying the risk factors for opioid-related adverse events in hospitalized patients would help inform management decisions and allow deployment of resources and specialized monitoring strategies to patients at heightened risk. The working group also noted the need for research investigating the impact of PDMP use on management decisions and downstream outcomes among hospitalized patients. Finally, conversations around pain management and concerns related to aberrant behaviors are often challenging in the hospital setting owing to the brief, high-intensity nature of the care and the lack of a longstanding therapeutic alliance. There is a great need to develop strategies and language to facilitate these conversations.
In conclusion, until more high-quality evidence becomes available, clinicians can use the recommendations contained in this Consensus Statement along with their clinical judgment and consultation with pharmacists, interventional pain specialists, and other staff (eg, social work, nursing) to help facilitate consistent, high-quality care across providers and hospitals. We believe that doing so will help increase the appropriateness of opioid therapy, minimize adverse events, facilitate shared decision-making, and foster stronger therapeutic alliances at the outset of the hospitalization for patients suffering from acute pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from the SHM for their facilitation of this project and dedication to this purpose.
The authors would also like to thank the many individuals who provided comments on the draft recommendations, including the participants in the SHM RADEO program; the SHM members; the representatives of specialty societies, including the American Academy of Family Physicians, the American College of Physicians, the American Hospital Association, the American Society of Addiction Medicine, the American Society of Anesthesiologists, the American Society of Health-System Pharmacists, the Society of Critical Care Medicine, and the Society of General Internal Medicine; and the representatives of patient advocacy groups, including SHM PFAC, Regions Hospital Patient and Family Advisory Committee, Patient and Family Centered Care Council of St. Louis Children’s Hospital, Missouri Family Partnership, and Parent and Family Care.
Disclosures: Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena reports receiving consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics, a consultancy to the life sciences industry (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance with the project and funded the in-person working group meeting but had no role in or influence on developing the content of the recommendations themselves. The SHM Board of Directors provided approval to submit the manuscript for publication without modification. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported in part by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, and reporting of the study
1. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States. JAMA. 2016;315(15):1624-1645. PubMed
55. Coffin PO, Sullivan SD. COst-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. PubMed
54. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. 2015;64(23):631-635. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
52. Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance abuse 2015;36(2):240-253. PubMed
51. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. PubMed
50. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. PubMed
49. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127-2132. PubMed
48. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine 2008;33(2):199-204. PubMed
47. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. PubMed
46. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. PubMed
45. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. PubMed
44. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. PubMed
43. Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. PubMed
42. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial [published online ahead of print December 13, 2017]. JAMA Surg. doi: 10.1001/jamasurg.2017.4915 PubMed
41. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-273. PubMed
40. Davies ED, Schneider F, Childs S, et al. A prevalence study of errors in opioid prescribing in a large teaching hospital. Int J Clin Pract. 2011;65(9):923-929. PubMed
39. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29-34. PubMed
38. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. PubMed
37. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. PubMed
36. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. PubMed
35. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. PubMed
34. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. PubMed
33. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21-27. PubMed
1. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States. JAMA. 2016;315(15):1624-1645. PubMed
55. Coffin PO, Sullivan SD. COst-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. PubMed
54. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. 2015;64(23):631-635. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
52. Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance abuse 2015;36(2):240-253. PubMed
51. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. PubMed
50. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. PubMed
49. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127-2132. PubMed
48. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine 2008;33(2):199-204. PubMed
47. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. PubMed
46. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. PubMed
45. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. PubMed
44. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. PubMed
43. Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. PubMed
42. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial [published online ahead of print December 13, 2017]. JAMA Surg. doi: 10.1001/jamasurg.2017.4915 PubMed
41. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-273. PubMed
40. Davies ED, Schneider F, Childs S, et al. A prevalence study of errors in opioid prescribing in a large teaching hospital. Int J Clin Pract. 2011;65(9):923-929. PubMed
39. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29-34. PubMed
38. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. PubMed
37. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. PubMed
36. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. PubMed
35. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. PubMed
34. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. PubMed
33. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21-27. PubMed
© 2018 Society of Hospital Medicine
Hospital Value‐Based Purchasing
The Centers for Medicaid and Medicare Services' (CMS) Hospital Inpatient Value‐Based Purchasing (VBP) Program, which was signed into law as part of the Patient Protection and Affordable Care Act of 2010, aims to incentivize inpatient providers to deliver high‐value, as opposed to high‐volume, healthcare.[1] Beginning on October 1, 2012, the start of the 2013 fiscal year (FY), hospitals participating in the VBP program became eligible for a variety of performance‐based incentive payments from CMS. These payments are based on an acute care hospital's ability to meet performance measurements in 6 care domains: (1) patient safety, (2) care coordination, (3) clinical processes and outcomes, (4) population or community health, (5) efficiency and cost reduction, and (6) patient‐ and caregiver‐centered experience.[2] The VBP program's ultimate purpose is to enable CMS to improve the health of Medicare beneficiaries by purchasing better care for them at a lower cost. These 3 characteristics of careimproved health, improved care, and lower costsare the foundation of CMS' conception of value.[1, 2] They are closely related to an economic conception of value, which is the difference between an intervention's benefit and its cost.
Although in principle not a new idea, the formal mandate of hospitals to provide high‐value healthcare through financial incentives marks an important change in Medicare and Medicaid policy. In this opportune review of VBP, we first discuss the relevant historical changes in the reimbursement environment of US hospitals that have set the stage for VBP. We then describe the structure of CMS' VBP program, with a focus on which facilities are eligible to participate in the program, the specific outcomes measured and incentivized, how rewards and penalties are allocated, and how the program will be funded. In an effort to anticipate some of the issues that lie ahead, we then highlight a number of potential challenges to the success of VBP, and discuss how VBP will impact the delivery and reimbursement of inpatient care services. We conclude by examining how the VBP program is likely to evolve over time.
HISTORICAL CONTEXT FOR VBP
Over the last decade, CMS has embarked on a number of initiatives to incentivize the provision of higher‐quality and more cost‐effective care. For example, in 2003, CMS implemented a national pay‐for‐performance (P4P) pilot project called the Premier Hospital Quality Incentive Demonstration (HQID).[3, 4] HQID, which ran for 6 years, tracked and rewarded the performance of 216 hospitals in 6 healthcare service domains: (1) acute myocardial infarction (AMI), (2) congestive heart failure (CHF), (3) pneumonia, (4) coronary artery bypass graft surgery, (5) hip and knee replacement surgery, and (6) perioperative management of surgical patients (including prevention of surgical site infections).[4] CMS then introduced its Hospital Compare Web site in 2005 to facilitate public reporting of hospital‐level quality outcomes.[3, 5] This Web site provides the public with access to data on hospital performance across a wide array of measures of process quality, clinical outcomes, spending, and resource utilization.[5] Next, in October 2008, CMS stopped reimbursing hospitals for a number of costly and common hospital‐acquired complications, including hospital‐acquired bloodstream infections and urinary tract infections, patient falls, and pressure ulcers.[3, 6] VBP is the latest and most comprehensive step that CMS has taken in its decade‐long effort to shift from volume to value‐based compensation for inpatient care.
Although CMS appears fully invested in using performance incentives to increase healthcare value, existing evidence of the effects of P4P on patient outcomes remains quite mixed.[7] On one hand, an analysis of an inpatient P4P program sponsored by the United Kingdom's National Health Service's (NHS) suggests that P4P may improve quality and save lives; indeed, hospitals that participated in the NHS P4P program significantly reduced inpatient mortality from pneumonia, saving an estimated 890 lives.[8] Additional empirical work suggests that the HQID was also associated with early improvements in healthcare quality.[9] However, a subsequent long‐term analysis found that participation in HQID had no discernible effect on 30‐day mortality rates.[10] Moreover, a meta‐analysis of P4P incentives for individual practitioners found few methodologically robust studies of P4P for clinicians and concluded that P4P's effects on individual practice patterns and outcomes remain largely uncertain.[11]
VBP: STRUCTURE AND DESIGN
This section reviews the structure of the VBP program. We describe current VBP eligibility criteria and sources of funding for the program, how hospitals participating in VBP are evaluated, and how VBP incentives for FY 2013 have been calculated.
Hospital Eligibility for VBP
All acute care hospitals in the United States (excluding Maryland) that are not psychiatric hospitals, rehabilitation hospitals, long‐term care facilities, children's hospitals, or cancer hospitals are eligible to participate in VBP in FY 2013 (full eligibility criteria is outlined in Table 1). For FY 2013, CMS chose to incentivize measures in just 2 care domains: (1) clinical processes of care and (2) patient experience of care. To be eligible for VBP in FY 2013, a hospital must report at least 10 cases each in at least 4 of 12 measures included in the clinical processes of care domain (Table 2), and/or must have at least 100 completed Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). Designed and validated by CMS, the HCAHPS survey provides hospitals with a standardized instrument for gathering information about patient satisfaction with, and perspectives on, their hospital care.[12] HCAHPS will be used to assess 8 patient experience of care measures (Table 3).
|
| Inclusion criteria |
| Acute care hospital |
| Located in all 50 US states or District of Columbia (excluding Maryland) |
| Has at least 10 cases in at least 4 of 12 clinical process of care measures and/or at least 100 completed HCAHPS surveys |
| Exclusion criteria |
| Psychiatric, rehabilitation, long‐term care, children's or cancer hospital |
| Does not participate in Hospital Inpatient Quality Reporting Program during the VBP performance period |
| Cited by the Secretary of HHS for significant patient safety violations during performance period |
| Hospital does not meet minimum reporting requirements for number of cases, process measures, and surveys needed to participate in VBP |
| Disease Process | Process of Care Measure |
|---|---|
| |
| Acute myocardial infarction | Fibrinolytic therapy received within 30 minutes of hospital arrival |
| Primary percutaneous coronary intervention received within 90 minutes of hospital arrival | |
| Heart failure | Discharge instructions provided |
| Pneumonia | Blood cultures performed in the emergency department prior to initial antibiotic received in hospital |
| Initial antibiotic selection for community‐acquired pneumonia in immunocompetent patient | |
| Healthcare‐associated infections | Prophylactic antibiotic received within 1 hour prior to surgical incision |
| Prophylactic antibiotic selection for surgical patients | |
| Prophylactic antibiotics discontinued within 24 hours after surgery ends | |
| Cardiac surgery patients with controlled 6:00 am postoperative serum glucose | |
| Surgeries | Surgery patients on ‐blocker prior to arrival that received ‐blocker during perioperative period |
| Surgery patients with recommended venous thromboembolism prophylaxis ordered | |
| Surgery patients who received appropriate venous thromboembolism prophylaxis within 24 hours prior to surgery to 24 hours after surgery | |
| Communication with nurses |
| Communication with doctors |
| Responsiveness of hospital staff |
| Pain management |
| Communication about medicines |
| Cleanliness and quietness of hospital environment |
| Discharge information |
| Overall rating of hospital |
Participation in the program is mandatory for eligible hospitals, and CMS estimates that more than 3000 facilities across the United States will participate in FY 2013. Roughly $850 million dollars in VBP incentives will be paid out to these participating hospitals in FY 2013. The program is being financed through a 1% across‐the‐board reduction in FY 2013 diagnosis‐related group (DRG)‐based inpatient payments to participating hospitals. On December 20, 2012, CMS publically announced FY 2013 VBP incentives for all participating hospitals. Each hospital's incentive is retroactive and based on its performance between July 1, 2011 and March 31, 2012.
All data used for calculating VBP incentives is reported to CMS through its Hospital Inpatient Quality Reporting (Hospital IQR) Program, a national program instituted in 2003 that rewards hospitals for reporting designated quality measures. As of 2007, approximately 95% of eligible US hospitals were using the Hospital IQR program.[1] Measures evaluated via chart abstracts and surveys reflect a hospital's performance for its entire patient population, whereas measures assessed with claims data reflect hospital performance only for Medicare patients.
Evaluation of Hospitals
In FY 2013, hospital VBP incentive payments will be based entirely on performance in 2 domains: (1) clinical processes of care (weighted 70%) and (2) patient experience of care (weighted 30%). For each domain, CMS will evaluate each hospital's improvement over time as well as achievement compared to other hospitals in the VBP program. By assessing and rewarding both achievement and improvement, CMS will ensure that lower‐performing hospitals will still be rewarded for making substantial improvements in quality. To evaluate the first metricimprovement over timeCMS will compare a hospital's performance during a given reporting period with its baseline performance 2 years prior to this block of time. A hospital receives improvement points for improving its performance over time. To assess the second metricachievement compared to other hospitals in the VBP programCMS will compare each hospital's performance during a reporting period with the baseline performance (eg, performance 2 years prior to reporting period) of all other hospitals in the VBP program. A hospital is awarded achievement points if its performance exceeds the 50th percentile of all hospitals during the baseline performance period. Improvement scores range from 0 to 9, whereas achievement scores range from 0 to 10. The greater of a hospital's improvement and achievement scores on each VBP measure are used to calculate each hospital's total earned clinical care domain score and total earned HCAHPS base score. Hospitals that lack baseline performance data, which is required to assess improvement, will be evaluated solely on the basis of achievement points.[1] The total earned clinical care domain score is multiplied by 70% to reach the clinical care domain's contribution to a hospital's total performance score.
Each hospital's total patient experience domain, or HCAHPS performance, score consists of 2 components: a total earned HCAHPS base score as described above and a consistency score. The consistency score evaluates the reliability of a hospital's performance across all 8 patient experience of care measures (Table 3). If a hospital is above the 50th percentile of all hospital scores during the baseline period on all 8 measures, then it receives 100% of its consistency points. If a hospital is at the 0 percentile for a given measure, then it receives 0 consistency points for all measures. This provision promotes consistency by harshly penalizing hospitals with extremely poor performance on any 1 specific measure. If 1 or more measures are between the 0 and 50th percentiles, then it will receive a consistency score that takes into account how many measures were below the 50th percentile and their distance from this threshold. Each hospital's total HCAHPS performance score (the sum of total earned HCAHPS base points and consistency points) is then multiplied by 30% to arrive at the patient experience of care domain's contribution to a hospital's total performance score.
Importantly, CMS excluded from its VBP initiative 10 clinical process measures reported in the Hospital IQR Program because they are topped out; that is, almost all hospitals already perform them at very high rates (Table 4). Examples of these topped out process measures include administration of aspirin to all patients with AMI on arrival at the hospital; counseling of patients with AMI, CHF, and pneumonia about smoking cessation; and prescribing angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers to patients with CHF and left ventricular dysfunction.[1]
| Disease Process | Measure |
|---|---|
| |
| Acute myocardial infarction | Aspirin administered on arrival to the emergency department |
| ACEI or ARB prescribed on discharge | |
| Patient counseled about smoking cessation | |
| ‐Blocker prescribed on discharge | |
| Aspirin prescribed at discharge | |
| Heart failure | Patient counseled about smoking cessation |
| Evaluation of left ventricular systolic function | |
| ACEI or ARB prescribed for left ventricular systolic dysfunction | |
| Pneumonia | Patient counseled about smoking cessation |
| Surgical Care Improvement Project | Surgery patients with appropriate hair removal |
Calculation of VBP Incentives and Public Reporting
A hospital's total performance score for FY 2013 is equal to the sum of 70% of its clinical care domain score and 30% of its total HCAHPS performance score. This total performance score is entered into a linear mathematical formula to calculate each hospital's incentive payment. CMS projects that VBP will lead to a net increase in Medicare payments for one‐half of hospitals and a net decrease in payments for the other half of participating facilities.[1]
In December 2012, CMS publicly disclosed information about the initial performance of each hospital in the VBP program. Reported information included: (1) hospital performance for each applicable performance measure, (2) hospital performance by disease condition or procedure, and (3) hospital's total performance score. Initial analyses of this performance data revealed that 1557 hospitals will receive bonus payments under VBP in FY 2013, whereas 1427 hospitals will lose money under this program. Treasure Valley Hospital, a 10‐bed physician‐owned hospital in Boise, Idaho, will receive a 0.83% increase in Medicare payments, the largest payment increase under VBP in 2013. Conversely, Auburn Community Hospital in upstate New York, will suffer the most severe payment reduction: 0.9% per Medicare admission. The penalty will cost Auburn Hospital about $100,000, which is slightly more than 0.1% of its yearly $85 million operating budget.[13] For almost two‐thirds of participating hospitals, FY 2013 Medicare payments will change by <0.25%.[13] Additional information about VBP payments for FY 2013, including the number of hospitals who received VBP incentives and the size and range of these payments, is now accessible to the public through CMS' Hospital Compare Web site (
CHALLENGES OF VBP
As the Medicare VBP program evolves, and hospitals confront ever‐larger financial incentives to deliver high‐value as opposed to high‐volume care, it will be important to recognize limitations of the VBP program as they arise. Here we briefly discuss several conceptual and implementation challenges that physicians and policymakers should consider when assessing the merits of VBP in promoting high‐quality healthcare.
Rigorous and Continuous Evaluation of VBP Programs
The main premise of using VBP to incentivize hospitals to deliver high‐quality cost‐effective care is that the process measures used to determine hospital quality do impact patient outcomes. However, it is already well established that improvements in measures of process quality are not always associated with improvements in patient outcomes.[14, 15, 16] Moreover, incentivizing specific process measures encourages hospitals to shift resources away from other aspects of care delivery, which may have ambiguous, or even deleterious, effects on patient outcomes. Although incentives ideally push hospitals to shift resources away from low‐quality care toward high‐quality care, in practice this is not always the case. Hospital resources may instead be drawn away from areas that are not yet incented by VBP, but for which improvements in quality of care are desperately needed. The same empirical focus behind using VBP to incentivize hospitals to improve patient outcomes efficiently should be used to evaluate whether VBP is continually meeting its stated goals: reducing overall patient morbidity and mortality and improving patient satisfaction at ideally lower cost. The experience of the US education system with public policies designed to improve student testing performance may serve as a cautionary example here. Such policies, which provide financial rewards to schools whose students perform well on standardized tests, can indeed raise testing performance. However, these policies also lead educators to teach to the test, and to neglect important topics that are not tested on standardized exams.[17]
Prioritization of Process Measures
As payment incentives for VBP currently stand, process measures are weighted equally regardless of the clinical benefits they generate and the resources required to achieve improvements in process quality. For instance, 2 process measures, continuing home ‐blocker medications for patients with coronary artery disease undergoing surgery and early percutaneous coronary intervention for patients with AMI, may be weighted equally as process measures although both their clinical benefits and the costs of implementation are very different. Some hospitals responding to VBP incentives may choose to invest in areas where their ability to earn VBP incentive payments is high and the costs of improvement are low, although those areas may not be where interventions are most needed because clinical outcomes could be most improved. Recognizing that process measures have heterogeneous benefits and costs of implementation is important when prioritizing their reimbursement in VBP.
Measuring Improvements in Hospital Quality
Tying hospital financial compensation to hospital quality implies that measures of hospital quality should be robust. To incentivize hospitals to improve quality not only relative to other hospitals but to themselves in the past, the VBP program has established a baseline performance for each hospital. Each hospital is compared to its baseline performance in subsequent evaluation periods. Thus, properly measuring a hospital's baseline performance is important. During a given baseline period, some hospitals may have better or worse outcomes than their steady state due to random variation alone. Some hospitals deemed to have a low baseline will experience improvements in quality that are not related to active efforts to improve quality but through chance alone. Similarly, some hospitals deemed to have a high baseline will experience reductions in quality through chance. Of course, neither of these changes should be subject to differences in reimbursement because they do not reflect actual organizational changes made by the hospitals. The VBP program has made significant efforts to address this issue by requiring participating hospitals to have a large enough sample of cases such that estimated rates of process quality adherence meet a reliability threshold (ie, are likely to be consistent over time rather than vary substantially through chance alone). However, not all process measures exhibit high reliability, particularly those for which adverse events are rare (eg, foreign objects retained after surgery, air embolisms, and blood incompatibility). Ultimately, CMS's decision to balance the need for statistically reliable data with the goal of including as many hospitals as possible in the VBP program will require ongoing reevaluation of this issue.
Choosing Hospital Comparators Appropriately
In the current VBP program, hospitals will be evaluated in part by how they compare to hospitals nationally. However, studies of regional variation in healthcare have demonstrated large variations in practice patterns across the United States,[18, 19, 20] raising the question of whether hospitals should, at least initially, be compared to hospitals in the same geographic area. Although the ultimate goal of VBP should be to hold hospitals to a national standard, local practice patterns are not easily modified within 1‐ to 2‐year timeframes. Initially comparing hospitals to a national rather than local standard may unfairly penalize hospitals that are relative underperformers nationally but overperformers regionally. Although CMS's policy to reward improvement within hospitals over time mitigates issues arising from a cross‐sectional comparison of hospitals, the issue still remains if many hospitals within a region not only underperform relative to other hospitals nationally but also fail to demonstrate improvement. More broadly, this issue extends to differences across hospitals in factors that impact their ability to meet VBP goals. These factors may include, for example, hospital size, profitability, patient case and insurance mix, and presence of an electronic medical record. Comparing hospitals with vastly different abilities to achieve VBP goals and improve quickly may amount to inequitable policy.
Continual Evaluation of Topped‐Out Measures
Process measures that are met at high rates at nearly all hospitals are not used in evaluations by CMS for VBP. An assumption underlying CMS' decision to not reward hospitals for achieving these topped‐out measures is that once physicians and hospitals make cognitive and system‐level improvements that improve process quality, these gains will persist after the incentive is removed. Thus, CMS hopes and anticipates that although performance incentives will make it easier for well‐meaning physicians to learn to do the right thing, doctors will continue to do the right things for patients after these incentives are removed.[21, 22] Although this assumption may generally be accurate, it is important to continue to evaluate whether measures that are currently topped out continue to remain adequately performed, because rewarding new quality measures will necessarily lead hospitals to reallocate resources away from other clinical activities. Although we hope that the continued public reporting of topped‐out measures will prevent declines in performance on these measures, policy makers and clinicians should be aware that the lack of financial incentives for topped‐out measures may result in declines in quality. To this point, an analysis of 35 Kaiser Permanente facilities from 1997 to 2007 demonstrated that the removal of financial incentives for diabetic retinopathy and cervical cancer screening was associated with subsequent declines in performance of 3% and 1.6% per year, respectively.[23]
Will VBP Incentives Be Large Enough to Change Practice Patterns?
The VBP Program's ability to influence change depends, at least in part, on how the incentives offered under this program compare to the magnitude of the investments that hospitals must make to achieve a given reward. In general, larger incentives are necessary to motivate more significant changes in behavior or to influence organizations to invest the resources needed to achieve change. The incentives offered under VBP in FY 2013 are quite modest. Almost two‐thirds of participating hospitals will see their FY 2013 Medicare revenues change by <0.25%, roughly $125,000 at most.[13, 24] Although these incentives may motivate hospitals that can improve performance and achievement with very modest investments, they may have little impact on organizations that need to make significant upfront investments in care processes to achieve sustainable improvements in care quality. As CMS increases the size of VBP incentives over the next 2 to 4 years, it will also hold hospitals accountable for a broader and increasingly complex set of outcomes. Improving these outcomes may require investments in areas such as information technology and process improvement that far surpass the VBP incentive reward.
Moreover, prior research suggests that financial incentives like those available under VBP may contribute only slightly to performance improvements when public reporting already exists. For example, in a 2‐year study of 613 US hospitals implementing pay‐for‐performance plus public reporting or public reporting only, pay for performance plus public reporting was associated with only a 2.6% to 4.1% increase in a composite measure of quality when compared to hospitals with public reporting only.[9] Similarly, a study of 54 hospitals participating in the CMS pay for performance pilot initiative found no significant improvement in quality of care or outcomes for AMI when compared to 446 control hospitals.[25] A long‐term analysis of pay for performance in the Medicare Premier Hospital Quality Incentive Demonstration found that participation in the program had no discernible effect on 30‐day mortality rates.[10] Finally, a study of physician medical groups contracting with a large network healthcare maintenance organization found that the implementation of pay for performance did not result in major before and after improvements in clinical quality compared to a control group of medical groups.[26]
High‐Value Care Is Not Always Low‐Cost Care
Not surprisingly, the clinical process measures included in CMS' hospital VBP program evaluate a select and relatively small group of high‐value and low‐cost interventions (eg, appropriate administration of antibiotics and tight control of serum glucose in surgical patients). However, an important body of work has demonstrated that high‐cost care (eg, intensive inpatient hospital care for common acute medical conditions) may also be highly valuable in terms of improving survival.[20, 27, 28, 29, 30] As the hospital VBP program evolves, its overseers will need to consider whether to include additional incentives for high‐value high‐cost healthcare services. Such considerations will likely become increasingly salient as healthcare delivery organizations move toward capitated delivery models. In particular, the VBP program's Medicare Spending Per Beneficiary measure, which quantifies inpatient and subsequent outpatient spending per beneficiary after a given hospitalization episode, will need to distinguish between higher‐spending hospitals that provide highly effective care (eg, care that reduces mortality and readmissions) and facilities that provide less‐effective care.
FUTURE OF VBP
Although the future of VBP is unknown, CMS is likely to modify the program in a number of ways over the next 3 to 5 years. First, CMS will likely expand the breadth and focus of incentivized measures in the VBP program. In FY 2014, for example, CMS is adding a set of 3, 30‐day mortality outcome measures to VBP: 30‐day risk‐adjusted mortality for AMI, CHF, and pneumonia.[1] A hospital's performance with respect to these outcomes will represent 25% of its total performance score in 2014, whereas the clinical process of care and patient experience of care domains will account for 45% and 30% of this score, respectively. In 2015, patient experience and outcome measures will account for 30% each in a hospital's performance score, whereas process and efficiency measures will each account for 20% of this score, respectively. The composition of this performance score evidences a shift away from rewarding process‐based measures and toward incentivizing measures of clinical outcomes and patient satisfaction, the latter of which may be highly subjective and more representative of a hospital's catchment population than of a hospital's care itself.[31] Additional measures in the domains of patient safety, care coordination, population and community health, emergency room wait times, and cost control may also be added to the VBP program in FY 2015 to FY 2017. Furthermore, CMS will continue to reevaluate the appropriateness of measures that are already included in VBP and will stop incentivizing measures that have become topped out, or are no longer supported by the National Quality Forum.[1, 13]
Second, CMS has established an annual gradual increase of 0.25% in the percentage of each hospital's inpatient DRG‐based payment that is at stake under VBP. In FY 2014, for example, participating hospitals will be required to contribute 1.25% of inpatient DRG payments to the VBP program. This percentage is likely to increase to 2% or more by 2017.[1, 32]
Third, expansions of the VBP program complement a number of other quality improvement efforts overseen by CMS, including the Hospital Readmissions Reduction Program. Effective for discharges beginning on October 1, 2012, hospitals with excess readmissions for AMI, CHF, and pneumonia are at risk for reimbursement reductions for all Medicare admissions in proportion to the rate of excess rehospitalizations. Some of the same concerns about the hospital VBP program outlined above have also been raised for this program, namely, whether readmission penalties will be large enough to impact hospital behavior, whether readmissions are even preventable,[33, 34] and whether adjustments in hospital‐level policies will reduce admissions that are known to be heavily influenced by patient economic and social factors that are outside of a hospital's control.[35, 36] Despite the limitations of VBP and the challenges that lie ahead, there is optimism that rewarding hospitals that provide high‐value rather than high‐volume care will not only improve outcomes of hospitalized patients in the United States, but will potentially be able to do so at a lower cost. Encouraging hospitals to improve their quality of care may also have important spillover effects on other healthcare domains. For example, hospitals that adopt systems to ensure prompt delivery of antibiotics to patients with pneumonia may also observe positive spillover effects with the prompt antibiotic management of other acute infectious illnesses that are not covered by VBP. VBP may have spillover effects on medical malpractice liability and defensive medicine as well. Indeed, financial incentives to practice higher‐quality evidenced‐based care may reduce medical malpractice liability and defensive medicine.
The government's ultimate goal in implementing VBP is to identify a broad and clinically relevant set of outcome measures that can be used to incentivize hospitals to deliver high‐quality as opposed to high‐volume healthcare. The first wave of outcome measures has already been instituted. It remains to be seen whether the incentive rewards of Medicare's hospital VBP program will be large enough that hospitals feel compelled to improve and compete for them.
- Centers for Medicare and Medicaid Services. Hospital Value‐Based Purchasing Web site. 2013. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/hospital‐value‐based‐purchasing/index.html. Accessed March 4, 2013.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367:292–295.
- , . Hospital value‐based purchasing: will Medicare's new policy exacerbate disparities? Circ Cardiovasc Qual Outcomes. 2012;5:148–149.
- Centers for Medicare and Medicaid Services. CMS/premier hospital quality incentive demonstration (QHID). 2013. Available at: https://www.premierinc.com/quality‐safety/tools‐services/p4p/hqi/faqs.jsp. Accessed March 5, 2013.
- Centers for Medicare and Medicaid Services. Hospital Compare Web site. 2013. Available at: http://www.medicare.gov/hospitalcompare. Accessed March 4, 2013.
- , , . “Never events”: not every hospital‐acquired infection is preventable. Clin Infect Dis. 2009;49:743–746.
- . Will pay for performance improve quality of care? The answer is in the details. N Engl J Med. 2012;367:1852–1853.
- , , , , , . Reduced mortality with hospital pay for performance in England. N Engl J Med. 2012;367:1821–1828.
- , , , et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–496.
- , , , . The long‐term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157:889–899.
- Centers for Medicare and Medicaid Services. Hospital Consumer Assessment Of Healthcare Providers and Systems Web site. 2013. Available at: http://www.hcahpsonline.org. Accessed March 5, 2013.
- . Medicare discloses hospitals' bonuses, penalties based on quality. Kaiser Health News. December 20, 2012. Available at: http://www.kaiserhealthnews.org/stories/2012/december/21/medicare‐hospitals‐value‐based‐purchasing.aspx?referrer=search. Accessed March 26, 2013.
- , , , . Hospital quality and intensity of spending: is there an association? Health Aff (Millwood). 2009;28:w566–w572.
- , , , et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70.
- , , . The advantages and disadvantages of process‐based measures of health care quality. Int J Qual Health Care. 2001;13:469–474.
- . Accountability, incentives and behavior: the impact of high‐stakes testing in the Chicago public schools. J Public Econ. 2005;89:761–796.
- , , , , , . The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287.
- . Medical care—is more always better? N Engl J Med. 2003;349:1665–1667.
- , , . Hospital spending and inpatient mortality: evidence from California: an observational study. Ann Intern Med. 2011;154:160–167.
- . Making it easy to do it right. N Engl J Med. 2001;345:991–993.
- , , , . A high rate of compliance with neonatal intensive care unit transfusion guidelines persists even after a program to improve transfusion guideline compliance ended. Transfusion. 2011;51:2519–2520.
- , , , et al. The impact of removing financial incentives from clinical quality indicators: longitudinal analysis of four Kaiser Permanente indicators. BMJ. 2010;340:c1898.
- , . Medicare's new hospital value‐based purchasing program is likely to have only a small impact on hospital payments. Health Aff (Millwood). 2012;31:1932–1940.
- , , , et al. Pay for performance, quality of care, and outcomes in acute myocardial infarction. JAMA. 2007;297:2373–2380.
- , , . Can you get what you pay for? Pay‐for‐performance and the quality of healthcare providers. Rand J Econ. 2010;41:64–91.
- , , , . Spending and mortality in US acute care hospitals. Am J Manag Care. 2013;19:e46–e54.
- , , , , , . Development and validation of hospital “end‐of‐life” treatment intensity measures. Med Care. 2009;47:1098–1105.
- , , , et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2:548–557.
- , , , et al. Association of hospital spending intensity with mortality and readmission rates in Ontario hospitals. JAMA. 2012;307:1037–1045.
- , , . Patient satisfaction with hospital care: effects of demographic and institutional characteristics. Med Care. 2000;38:325–334.
- , , . Linking performance with payment: implementing the Physician Value‐Based Payment Modifier. JAMA. 2012;308:2089–2090.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391–E402.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183:E1067–E1072.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366:1366–1369.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681.
The Centers for Medicaid and Medicare Services' (CMS) Hospital Inpatient Value‐Based Purchasing (VBP) Program, which was signed into law as part of the Patient Protection and Affordable Care Act of 2010, aims to incentivize inpatient providers to deliver high‐value, as opposed to high‐volume, healthcare.[1] Beginning on October 1, 2012, the start of the 2013 fiscal year (FY), hospitals participating in the VBP program became eligible for a variety of performance‐based incentive payments from CMS. These payments are based on an acute care hospital's ability to meet performance measurements in 6 care domains: (1) patient safety, (2) care coordination, (3) clinical processes and outcomes, (4) population or community health, (5) efficiency and cost reduction, and (6) patient‐ and caregiver‐centered experience.[2] The VBP program's ultimate purpose is to enable CMS to improve the health of Medicare beneficiaries by purchasing better care for them at a lower cost. These 3 characteristics of careimproved health, improved care, and lower costsare the foundation of CMS' conception of value.[1, 2] They are closely related to an economic conception of value, which is the difference between an intervention's benefit and its cost.
Although in principle not a new idea, the formal mandate of hospitals to provide high‐value healthcare through financial incentives marks an important change in Medicare and Medicaid policy. In this opportune review of VBP, we first discuss the relevant historical changes in the reimbursement environment of US hospitals that have set the stage for VBP. We then describe the structure of CMS' VBP program, with a focus on which facilities are eligible to participate in the program, the specific outcomes measured and incentivized, how rewards and penalties are allocated, and how the program will be funded. In an effort to anticipate some of the issues that lie ahead, we then highlight a number of potential challenges to the success of VBP, and discuss how VBP will impact the delivery and reimbursement of inpatient care services. We conclude by examining how the VBP program is likely to evolve over time.
HISTORICAL CONTEXT FOR VBP
Over the last decade, CMS has embarked on a number of initiatives to incentivize the provision of higher‐quality and more cost‐effective care. For example, in 2003, CMS implemented a national pay‐for‐performance (P4P) pilot project called the Premier Hospital Quality Incentive Demonstration (HQID).[3, 4] HQID, which ran for 6 years, tracked and rewarded the performance of 216 hospitals in 6 healthcare service domains: (1) acute myocardial infarction (AMI), (2) congestive heart failure (CHF), (3) pneumonia, (4) coronary artery bypass graft surgery, (5) hip and knee replacement surgery, and (6) perioperative management of surgical patients (including prevention of surgical site infections).[4] CMS then introduced its Hospital Compare Web site in 2005 to facilitate public reporting of hospital‐level quality outcomes.[3, 5] This Web site provides the public with access to data on hospital performance across a wide array of measures of process quality, clinical outcomes, spending, and resource utilization.[5] Next, in October 2008, CMS stopped reimbursing hospitals for a number of costly and common hospital‐acquired complications, including hospital‐acquired bloodstream infections and urinary tract infections, patient falls, and pressure ulcers.[3, 6] VBP is the latest and most comprehensive step that CMS has taken in its decade‐long effort to shift from volume to value‐based compensation for inpatient care.
Although CMS appears fully invested in using performance incentives to increase healthcare value, existing evidence of the effects of P4P on patient outcomes remains quite mixed.[7] On one hand, an analysis of an inpatient P4P program sponsored by the United Kingdom's National Health Service's (NHS) suggests that P4P may improve quality and save lives; indeed, hospitals that participated in the NHS P4P program significantly reduced inpatient mortality from pneumonia, saving an estimated 890 lives.[8] Additional empirical work suggests that the HQID was also associated with early improvements in healthcare quality.[9] However, a subsequent long‐term analysis found that participation in HQID had no discernible effect on 30‐day mortality rates.[10] Moreover, a meta‐analysis of P4P incentives for individual practitioners found few methodologically robust studies of P4P for clinicians and concluded that P4P's effects on individual practice patterns and outcomes remain largely uncertain.[11]
VBP: STRUCTURE AND DESIGN
This section reviews the structure of the VBP program. We describe current VBP eligibility criteria and sources of funding for the program, how hospitals participating in VBP are evaluated, and how VBP incentives for FY 2013 have been calculated.
Hospital Eligibility for VBP
All acute care hospitals in the United States (excluding Maryland) that are not psychiatric hospitals, rehabilitation hospitals, long‐term care facilities, children's hospitals, or cancer hospitals are eligible to participate in VBP in FY 2013 (full eligibility criteria is outlined in Table 1). For FY 2013, CMS chose to incentivize measures in just 2 care domains: (1) clinical processes of care and (2) patient experience of care. To be eligible for VBP in FY 2013, a hospital must report at least 10 cases each in at least 4 of 12 measures included in the clinical processes of care domain (Table 2), and/or must have at least 100 completed Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). Designed and validated by CMS, the HCAHPS survey provides hospitals with a standardized instrument for gathering information about patient satisfaction with, and perspectives on, their hospital care.[12] HCAHPS will be used to assess 8 patient experience of care measures (Table 3).
|
| Inclusion criteria |
| Acute care hospital |
| Located in all 50 US states or District of Columbia (excluding Maryland) |
| Has at least 10 cases in at least 4 of 12 clinical process of care measures and/or at least 100 completed HCAHPS surveys |
| Exclusion criteria |
| Psychiatric, rehabilitation, long‐term care, children's or cancer hospital |
| Does not participate in Hospital Inpatient Quality Reporting Program during the VBP performance period |
| Cited by the Secretary of HHS for significant patient safety violations during performance period |
| Hospital does not meet minimum reporting requirements for number of cases, process measures, and surveys needed to participate in VBP |
| Disease Process | Process of Care Measure |
|---|---|
| |
| Acute myocardial infarction | Fibrinolytic therapy received within 30 minutes of hospital arrival |
| Primary percutaneous coronary intervention received within 90 minutes of hospital arrival | |
| Heart failure | Discharge instructions provided |
| Pneumonia | Blood cultures performed in the emergency department prior to initial antibiotic received in hospital |
| Initial antibiotic selection for community‐acquired pneumonia in immunocompetent patient | |
| Healthcare‐associated infections | Prophylactic antibiotic received within 1 hour prior to surgical incision |
| Prophylactic antibiotic selection for surgical patients | |
| Prophylactic antibiotics discontinued within 24 hours after surgery ends | |
| Cardiac surgery patients with controlled 6:00 am postoperative serum glucose | |
| Surgeries | Surgery patients on ‐blocker prior to arrival that received ‐blocker during perioperative period |
| Surgery patients with recommended venous thromboembolism prophylaxis ordered | |
| Surgery patients who received appropriate venous thromboembolism prophylaxis within 24 hours prior to surgery to 24 hours after surgery | |
| Communication with nurses |
| Communication with doctors |
| Responsiveness of hospital staff |
| Pain management |
| Communication about medicines |
| Cleanliness and quietness of hospital environment |
| Discharge information |
| Overall rating of hospital |
Participation in the program is mandatory for eligible hospitals, and CMS estimates that more than 3000 facilities across the United States will participate in FY 2013. Roughly $850 million dollars in VBP incentives will be paid out to these participating hospitals in FY 2013. The program is being financed through a 1% across‐the‐board reduction in FY 2013 diagnosis‐related group (DRG)‐based inpatient payments to participating hospitals. On December 20, 2012, CMS publically announced FY 2013 VBP incentives for all participating hospitals. Each hospital's incentive is retroactive and based on its performance between July 1, 2011 and March 31, 2012.
All data used for calculating VBP incentives is reported to CMS through its Hospital Inpatient Quality Reporting (Hospital IQR) Program, a national program instituted in 2003 that rewards hospitals for reporting designated quality measures. As of 2007, approximately 95% of eligible US hospitals were using the Hospital IQR program.[1] Measures evaluated via chart abstracts and surveys reflect a hospital's performance for its entire patient population, whereas measures assessed with claims data reflect hospital performance only for Medicare patients.
Evaluation of Hospitals
In FY 2013, hospital VBP incentive payments will be based entirely on performance in 2 domains: (1) clinical processes of care (weighted 70%) and (2) patient experience of care (weighted 30%). For each domain, CMS will evaluate each hospital's improvement over time as well as achievement compared to other hospitals in the VBP program. By assessing and rewarding both achievement and improvement, CMS will ensure that lower‐performing hospitals will still be rewarded for making substantial improvements in quality. To evaluate the first metricimprovement over timeCMS will compare a hospital's performance during a given reporting period with its baseline performance 2 years prior to this block of time. A hospital receives improvement points for improving its performance over time. To assess the second metricachievement compared to other hospitals in the VBP programCMS will compare each hospital's performance during a reporting period with the baseline performance (eg, performance 2 years prior to reporting period) of all other hospitals in the VBP program. A hospital is awarded achievement points if its performance exceeds the 50th percentile of all hospitals during the baseline performance period. Improvement scores range from 0 to 9, whereas achievement scores range from 0 to 10. The greater of a hospital's improvement and achievement scores on each VBP measure are used to calculate each hospital's total earned clinical care domain score and total earned HCAHPS base score. Hospitals that lack baseline performance data, which is required to assess improvement, will be evaluated solely on the basis of achievement points.[1] The total earned clinical care domain score is multiplied by 70% to reach the clinical care domain's contribution to a hospital's total performance score.
Each hospital's total patient experience domain, or HCAHPS performance, score consists of 2 components: a total earned HCAHPS base score as described above and a consistency score. The consistency score evaluates the reliability of a hospital's performance across all 8 patient experience of care measures (Table 3). If a hospital is above the 50th percentile of all hospital scores during the baseline period on all 8 measures, then it receives 100% of its consistency points. If a hospital is at the 0 percentile for a given measure, then it receives 0 consistency points for all measures. This provision promotes consistency by harshly penalizing hospitals with extremely poor performance on any 1 specific measure. If 1 or more measures are between the 0 and 50th percentiles, then it will receive a consistency score that takes into account how many measures were below the 50th percentile and their distance from this threshold. Each hospital's total HCAHPS performance score (the sum of total earned HCAHPS base points and consistency points) is then multiplied by 30% to arrive at the patient experience of care domain's contribution to a hospital's total performance score.
Importantly, CMS excluded from its VBP initiative 10 clinical process measures reported in the Hospital IQR Program because they are topped out; that is, almost all hospitals already perform them at very high rates (Table 4). Examples of these topped out process measures include administration of aspirin to all patients with AMI on arrival at the hospital; counseling of patients with AMI, CHF, and pneumonia about smoking cessation; and prescribing angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers to patients with CHF and left ventricular dysfunction.[1]
| Disease Process | Measure |
|---|---|
| |
| Acute myocardial infarction | Aspirin administered on arrival to the emergency department |
| ACEI or ARB prescribed on discharge | |
| Patient counseled about smoking cessation | |
| ‐Blocker prescribed on discharge | |
| Aspirin prescribed at discharge | |
| Heart failure | Patient counseled about smoking cessation |
| Evaluation of left ventricular systolic function | |
| ACEI or ARB prescribed for left ventricular systolic dysfunction | |
| Pneumonia | Patient counseled about smoking cessation |
| Surgical Care Improvement Project | Surgery patients with appropriate hair removal |
Calculation of VBP Incentives and Public Reporting
A hospital's total performance score for FY 2013 is equal to the sum of 70% of its clinical care domain score and 30% of its total HCAHPS performance score. This total performance score is entered into a linear mathematical formula to calculate each hospital's incentive payment. CMS projects that VBP will lead to a net increase in Medicare payments for one‐half of hospitals and a net decrease in payments for the other half of participating facilities.[1]
In December 2012, CMS publicly disclosed information about the initial performance of each hospital in the VBP program. Reported information included: (1) hospital performance for each applicable performance measure, (2) hospital performance by disease condition or procedure, and (3) hospital's total performance score. Initial analyses of this performance data revealed that 1557 hospitals will receive bonus payments under VBP in FY 2013, whereas 1427 hospitals will lose money under this program. Treasure Valley Hospital, a 10‐bed physician‐owned hospital in Boise, Idaho, will receive a 0.83% increase in Medicare payments, the largest payment increase under VBP in 2013. Conversely, Auburn Community Hospital in upstate New York, will suffer the most severe payment reduction: 0.9% per Medicare admission. The penalty will cost Auburn Hospital about $100,000, which is slightly more than 0.1% of its yearly $85 million operating budget.[13] For almost two‐thirds of participating hospitals, FY 2013 Medicare payments will change by <0.25%.[13] Additional information about VBP payments for FY 2013, including the number of hospitals who received VBP incentives and the size and range of these payments, is now accessible to the public through CMS' Hospital Compare Web site (
CHALLENGES OF VBP
As the Medicare VBP program evolves, and hospitals confront ever‐larger financial incentives to deliver high‐value as opposed to high‐volume care, it will be important to recognize limitations of the VBP program as they arise. Here we briefly discuss several conceptual and implementation challenges that physicians and policymakers should consider when assessing the merits of VBP in promoting high‐quality healthcare.
Rigorous and Continuous Evaluation of VBP Programs
The main premise of using VBP to incentivize hospitals to deliver high‐quality cost‐effective care is that the process measures used to determine hospital quality do impact patient outcomes. However, it is already well established that improvements in measures of process quality are not always associated with improvements in patient outcomes.[14, 15, 16] Moreover, incentivizing specific process measures encourages hospitals to shift resources away from other aspects of care delivery, which may have ambiguous, or even deleterious, effects on patient outcomes. Although incentives ideally push hospitals to shift resources away from low‐quality care toward high‐quality care, in practice this is not always the case. Hospital resources may instead be drawn away from areas that are not yet incented by VBP, but for which improvements in quality of care are desperately needed. The same empirical focus behind using VBP to incentivize hospitals to improve patient outcomes efficiently should be used to evaluate whether VBP is continually meeting its stated goals: reducing overall patient morbidity and mortality and improving patient satisfaction at ideally lower cost. The experience of the US education system with public policies designed to improve student testing performance may serve as a cautionary example here. Such policies, which provide financial rewards to schools whose students perform well on standardized tests, can indeed raise testing performance. However, these policies also lead educators to teach to the test, and to neglect important topics that are not tested on standardized exams.[17]
Prioritization of Process Measures
As payment incentives for VBP currently stand, process measures are weighted equally regardless of the clinical benefits they generate and the resources required to achieve improvements in process quality. For instance, 2 process measures, continuing home ‐blocker medications for patients with coronary artery disease undergoing surgery and early percutaneous coronary intervention for patients with AMI, may be weighted equally as process measures although both their clinical benefits and the costs of implementation are very different. Some hospitals responding to VBP incentives may choose to invest in areas where their ability to earn VBP incentive payments is high and the costs of improvement are low, although those areas may not be where interventions are most needed because clinical outcomes could be most improved. Recognizing that process measures have heterogeneous benefits and costs of implementation is important when prioritizing their reimbursement in VBP.
Measuring Improvements in Hospital Quality
Tying hospital financial compensation to hospital quality implies that measures of hospital quality should be robust. To incentivize hospitals to improve quality not only relative to other hospitals but to themselves in the past, the VBP program has established a baseline performance for each hospital. Each hospital is compared to its baseline performance in subsequent evaluation periods. Thus, properly measuring a hospital's baseline performance is important. During a given baseline period, some hospitals may have better or worse outcomes than their steady state due to random variation alone. Some hospitals deemed to have a low baseline will experience improvements in quality that are not related to active efforts to improve quality but through chance alone. Similarly, some hospitals deemed to have a high baseline will experience reductions in quality through chance. Of course, neither of these changes should be subject to differences in reimbursement because they do not reflect actual organizational changes made by the hospitals. The VBP program has made significant efforts to address this issue by requiring participating hospitals to have a large enough sample of cases such that estimated rates of process quality adherence meet a reliability threshold (ie, are likely to be consistent over time rather than vary substantially through chance alone). However, not all process measures exhibit high reliability, particularly those for which adverse events are rare (eg, foreign objects retained after surgery, air embolisms, and blood incompatibility). Ultimately, CMS's decision to balance the need for statistically reliable data with the goal of including as many hospitals as possible in the VBP program will require ongoing reevaluation of this issue.
Choosing Hospital Comparators Appropriately
In the current VBP program, hospitals will be evaluated in part by how they compare to hospitals nationally. However, studies of regional variation in healthcare have demonstrated large variations in practice patterns across the United States,[18, 19, 20] raising the question of whether hospitals should, at least initially, be compared to hospitals in the same geographic area. Although the ultimate goal of VBP should be to hold hospitals to a national standard, local practice patterns are not easily modified within 1‐ to 2‐year timeframes. Initially comparing hospitals to a national rather than local standard may unfairly penalize hospitals that are relative underperformers nationally but overperformers regionally. Although CMS's policy to reward improvement within hospitals over time mitigates issues arising from a cross‐sectional comparison of hospitals, the issue still remains if many hospitals within a region not only underperform relative to other hospitals nationally but also fail to demonstrate improvement. More broadly, this issue extends to differences across hospitals in factors that impact their ability to meet VBP goals. These factors may include, for example, hospital size, profitability, patient case and insurance mix, and presence of an electronic medical record. Comparing hospitals with vastly different abilities to achieve VBP goals and improve quickly may amount to inequitable policy.
Continual Evaluation of Topped‐Out Measures
Process measures that are met at high rates at nearly all hospitals are not used in evaluations by CMS for VBP. An assumption underlying CMS' decision to not reward hospitals for achieving these topped‐out measures is that once physicians and hospitals make cognitive and system‐level improvements that improve process quality, these gains will persist after the incentive is removed. Thus, CMS hopes and anticipates that although performance incentives will make it easier for well‐meaning physicians to learn to do the right thing, doctors will continue to do the right things for patients after these incentives are removed.[21, 22] Although this assumption may generally be accurate, it is important to continue to evaluate whether measures that are currently topped out continue to remain adequately performed, because rewarding new quality measures will necessarily lead hospitals to reallocate resources away from other clinical activities. Although we hope that the continued public reporting of topped‐out measures will prevent declines in performance on these measures, policy makers and clinicians should be aware that the lack of financial incentives for topped‐out measures may result in declines in quality. To this point, an analysis of 35 Kaiser Permanente facilities from 1997 to 2007 demonstrated that the removal of financial incentives for diabetic retinopathy and cervical cancer screening was associated with subsequent declines in performance of 3% and 1.6% per year, respectively.[23]
Will VBP Incentives Be Large Enough to Change Practice Patterns?
The VBP Program's ability to influence change depends, at least in part, on how the incentives offered under this program compare to the magnitude of the investments that hospitals must make to achieve a given reward. In general, larger incentives are necessary to motivate more significant changes in behavior or to influence organizations to invest the resources needed to achieve change. The incentives offered under VBP in FY 2013 are quite modest. Almost two‐thirds of participating hospitals will see their FY 2013 Medicare revenues change by <0.25%, roughly $125,000 at most.[13, 24] Although these incentives may motivate hospitals that can improve performance and achievement with very modest investments, they may have little impact on organizations that need to make significant upfront investments in care processes to achieve sustainable improvements in care quality. As CMS increases the size of VBP incentives over the next 2 to 4 years, it will also hold hospitals accountable for a broader and increasingly complex set of outcomes. Improving these outcomes may require investments in areas such as information technology and process improvement that far surpass the VBP incentive reward.
Moreover, prior research suggests that financial incentives like those available under VBP may contribute only slightly to performance improvements when public reporting already exists. For example, in a 2‐year study of 613 US hospitals implementing pay‐for‐performance plus public reporting or public reporting only, pay for performance plus public reporting was associated with only a 2.6% to 4.1% increase in a composite measure of quality when compared to hospitals with public reporting only.[9] Similarly, a study of 54 hospitals participating in the CMS pay for performance pilot initiative found no significant improvement in quality of care or outcomes for AMI when compared to 446 control hospitals.[25] A long‐term analysis of pay for performance in the Medicare Premier Hospital Quality Incentive Demonstration found that participation in the program had no discernible effect on 30‐day mortality rates.[10] Finally, a study of physician medical groups contracting with a large network healthcare maintenance organization found that the implementation of pay for performance did not result in major before and after improvements in clinical quality compared to a control group of medical groups.[26]
High‐Value Care Is Not Always Low‐Cost Care
Not surprisingly, the clinical process measures included in CMS' hospital VBP program evaluate a select and relatively small group of high‐value and low‐cost interventions (eg, appropriate administration of antibiotics and tight control of serum glucose in surgical patients). However, an important body of work has demonstrated that high‐cost care (eg, intensive inpatient hospital care for common acute medical conditions) may also be highly valuable in terms of improving survival.[20, 27, 28, 29, 30] As the hospital VBP program evolves, its overseers will need to consider whether to include additional incentives for high‐value high‐cost healthcare services. Such considerations will likely become increasingly salient as healthcare delivery organizations move toward capitated delivery models. In particular, the VBP program's Medicare Spending Per Beneficiary measure, which quantifies inpatient and subsequent outpatient spending per beneficiary after a given hospitalization episode, will need to distinguish between higher‐spending hospitals that provide highly effective care (eg, care that reduces mortality and readmissions) and facilities that provide less‐effective care.
FUTURE OF VBP
Although the future of VBP is unknown, CMS is likely to modify the program in a number of ways over the next 3 to 5 years. First, CMS will likely expand the breadth and focus of incentivized measures in the VBP program. In FY 2014, for example, CMS is adding a set of 3, 30‐day mortality outcome measures to VBP: 30‐day risk‐adjusted mortality for AMI, CHF, and pneumonia.[1] A hospital's performance with respect to these outcomes will represent 25% of its total performance score in 2014, whereas the clinical process of care and patient experience of care domains will account for 45% and 30% of this score, respectively. In 2015, patient experience and outcome measures will account for 30% each in a hospital's performance score, whereas process and efficiency measures will each account for 20% of this score, respectively. The composition of this performance score evidences a shift away from rewarding process‐based measures and toward incentivizing measures of clinical outcomes and patient satisfaction, the latter of which may be highly subjective and more representative of a hospital's catchment population than of a hospital's care itself.[31] Additional measures in the domains of patient safety, care coordination, population and community health, emergency room wait times, and cost control may also be added to the VBP program in FY 2015 to FY 2017. Furthermore, CMS will continue to reevaluate the appropriateness of measures that are already included in VBP and will stop incentivizing measures that have become topped out, or are no longer supported by the National Quality Forum.[1, 13]
Second, CMS has established an annual gradual increase of 0.25% in the percentage of each hospital's inpatient DRG‐based payment that is at stake under VBP. In FY 2014, for example, participating hospitals will be required to contribute 1.25% of inpatient DRG payments to the VBP program. This percentage is likely to increase to 2% or more by 2017.[1, 32]
Third, expansions of the VBP program complement a number of other quality improvement efforts overseen by CMS, including the Hospital Readmissions Reduction Program. Effective for discharges beginning on October 1, 2012, hospitals with excess readmissions for AMI, CHF, and pneumonia are at risk for reimbursement reductions for all Medicare admissions in proportion to the rate of excess rehospitalizations. Some of the same concerns about the hospital VBP program outlined above have also been raised for this program, namely, whether readmission penalties will be large enough to impact hospital behavior, whether readmissions are even preventable,[33, 34] and whether adjustments in hospital‐level policies will reduce admissions that are known to be heavily influenced by patient economic and social factors that are outside of a hospital's control.[35, 36] Despite the limitations of VBP and the challenges that lie ahead, there is optimism that rewarding hospitals that provide high‐value rather than high‐volume care will not only improve outcomes of hospitalized patients in the United States, but will potentially be able to do so at a lower cost. Encouraging hospitals to improve their quality of care may also have important spillover effects on other healthcare domains. For example, hospitals that adopt systems to ensure prompt delivery of antibiotics to patients with pneumonia may also observe positive spillover effects with the prompt antibiotic management of other acute infectious illnesses that are not covered by VBP. VBP may have spillover effects on medical malpractice liability and defensive medicine as well. Indeed, financial incentives to practice higher‐quality evidenced‐based care may reduce medical malpractice liability and defensive medicine.
The government's ultimate goal in implementing VBP is to identify a broad and clinically relevant set of outcome measures that can be used to incentivize hospitals to deliver high‐quality as opposed to high‐volume healthcare. The first wave of outcome measures has already been instituted. It remains to be seen whether the incentive rewards of Medicare's hospital VBP program will be large enough that hospitals feel compelled to improve and compete for them.
The Centers for Medicaid and Medicare Services' (CMS) Hospital Inpatient Value‐Based Purchasing (VBP) Program, which was signed into law as part of the Patient Protection and Affordable Care Act of 2010, aims to incentivize inpatient providers to deliver high‐value, as opposed to high‐volume, healthcare.[1] Beginning on October 1, 2012, the start of the 2013 fiscal year (FY), hospitals participating in the VBP program became eligible for a variety of performance‐based incentive payments from CMS. These payments are based on an acute care hospital's ability to meet performance measurements in 6 care domains: (1) patient safety, (2) care coordination, (3) clinical processes and outcomes, (4) population or community health, (5) efficiency and cost reduction, and (6) patient‐ and caregiver‐centered experience.[2] The VBP program's ultimate purpose is to enable CMS to improve the health of Medicare beneficiaries by purchasing better care for them at a lower cost. These 3 characteristics of careimproved health, improved care, and lower costsare the foundation of CMS' conception of value.[1, 2] They are closely related to an economic conception of value, which is the difference between an intervention's benefit and its cost.
Although in principle not a new idea, the formal mandate of hospitals to provide high‐value healthcare through financial incentives marks an important change in Medicare and Medicaid policy. In this opportune review of VBP, we first discuss the relevant historical changes in the reimbursement environment of US hospitals that have set the stage for VBP. We then describe the structure of CMS' VBP program, with a focus on which facilities are eligible to participate in the program, the specific outcomes measured and incentivized, how rewards and penalties are allocated, and how the program will be funded. In an effort to anticipate some of the issues that lie ahead, we then highlight a number of potential challenges to the success of VBP, and discuss how VBP will impact the delivery and reimbursement of inpatient care services. We conclude by examining how the VBP program is likely to evolve over time.
HISTORICAL CONTEXT FOR VBP
Over the last decade, CMS has embarked on a number of initiatives to incentivize the provision of higher‐quality and more cost‐effective care. For example, in 2003, CMS implemented a national pay‐for‐performance (P4P) pilot project called the Premier Hospital Quality Incentive Demonstration (HQID).[3, 4] HQID, which ran for 6 years, tracked and rewarded the performance of 216 hospitals in 6 healthcare service domains: (1) acute myocardial infarction (AMI), (2) congestive heart failure (CHF), (3) pneumonia, (4) coronary artery bypass graft surgery, (5) hip and knee replacement surgery, and (6) perioperative management of surgical patients (including prevention of surgical site infections).[4] CMS then introduced its Hospital Compare Web site in 2005 to facilitate public reporting of hospital‐level quality outcomes.[3, 5] This Web site provides the public with access to data on hospital performance across a wide array of measures of process quality, clinical outcomes, spending, and resource utilization.[5] Next, in October 2008, CMS stopped reimbursing hospitals for a number of costly and common hospital‐acquired complications, including hospital‐acquired bloodstream infections and urinary tract infections, patient falls, and pressure ulcers.[3, 6] VBP is the latest and most comprehensive step that CMS has taken in its decade‐long effort to shift from volume to value‐based compensation for inpatient care.
Although CMS appears fully invested in using performance incentives to increase healthcare value, existing evidence of the effects of P4P on patient outcomes remains quite mixed.[7] On one hand, an analysis of an inpatient P4P program sponsored by the United Kingdom's National Health Service's (NHS) suggests that P4P may improve quality and save lives; indeed, hospitals that participated in the NHS P4P program significantly reduced inpatient mortality from pneumonia, saving an estimated 890 lives.[8] Additional empirical work suggests that the HQID was also associated with early improvements in healthcare quality.[9] However, a subsequent long‐term analysis found that participation in HQID had no discernible effect on 30‐day mortality rates.[10] Moreover, a meta‐analysis of P4P incentives for individual practitioners found few methodologically robust studies of P4P for clinicians and concluded that P4P's effects on individual practice patterns and outcomes remain largely uncertain.[11]
VBP: STRUCTURE AND DESIGN
This section reviews the structure of the VBP program. We describe current VBP eligibility criteria and sources of funding for the program, how hospitals participating in VBP are evaluated, and how VBP incentives for FY 2013 have been calculated.
Hospital Eligibility for VBP
All acute care hospitals in the United States (excluding Maryland) that are not psychiatric hospitals, rehabilitation hospitals, long‐term care facilities, children's hospitals, or cancer hospitals are eligible to participate in VBP in FY 2013 (full eligibility criteria is outlined in Table 1). For FY 2013, CMS chose to incentivize measures in just 2 care domains: (1) clinical processes of care and (2) patient experience of care. To be eligible for VBP in FY 2013, a hospital must report at least 10 cases each in at least 4 of 12 measures included in the clinical processes of care domain (Table 2), and/or must have at least 100 completed Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). Designed and validated by CMS, the HCAHPS survey provides hospitals with a standardized instrument for gathering information about patient satisfaction with, and perspectives on, their hospital care.[12] HCAHPS will be used to assess 8 patient experience of care measures (Table 3).
|
| Inclusion criteria |
| Acute care hospital |
| Located in all 50 US states or District of Columbia (excluding Maryland) |
| Has at least 10 cases in at least 4 of 12 clinical process of care measures and/or at least 100 completed HCAHPS surveys |
| Exclusion criteria |
| Psychiatric, rehabilitation, long‐term care, children's or cancer hospital |
| Does not participate in Hospital Inpatient Quality Reporting Program during the VBP performance period |
| Cited by the Secretary of HHS for significant patient safety violations during performance period |
| Hospital does not meet minimum reporting requirements for number of cases, process measures, and surveys needed to participate in VBP |
| Disease Process | Process of Care Measure |
|---|---|
| |
| Acute myocardial infarction | Fibrinolytic therapy received within 30 minutes of hospital arrival |
| Primary percutaneous coronary intervention received within 90 minutes of hospital arrival | |
| Heart failure | Discharge instructions provided |
| Pneumonia | Blood cultures performed in the emergency department prior to initial antibiotic received in hospital |
| Initial antibiotic selection for community‐acquired pneumonia in immunocompetent patient | |
| Healthcare‐associated infections | Prophylactic antibiotic received within 1 hour prior to surgical incision |
| Prophylactic antibiotic selection for surgical patients | |
| Prophylactic antibiotics discontinued within 24 hours after surgery ends | |
| Cardiac surgery patients with controlled 6:00 am postoperative serum glucose | |
| Surgeries | Surgery patients on ‐blocker prior to arrival that received ‐blocker during perioperative period |
| Surgery patients with recommended venous thromboembolism prophylaxis ordered | |
| Surgery patients who received appropriate venous thromboembolism prophylaxis within 24 hours prior to surgery to 24 hours after surgery | |
| Communication with nurses |
| Communication with doctors |
| Responsiveness of hospital staff |
| Pain management |
| Communication about medicines |
| Cleanliness and quietness of hospital environment |
| Discharge information |
| Overall rating of hospital |
Participation in the program is mandatory for eligible hospitals, and CMS estimates that more than 3000 facilities across the United States will participate in FY 2013. Roughly $850 million dollars in VBP incentives will be paid out to these participating hospitals in FY 2013. The program is being financed through a 1% across‐the‐board reduction in FY 2013 diagnosis‐related group (DRG)‐based inpatient payments to participating hospitals. On December 20, 2012, CMS publically announced FY 2013 VBP incentives for all participating hospitals. Each hospital's incentive is retroactive and based on its performance between July 1, 2011 and March 31, 2012.
All data used for calculating VBP incentives is reported to CMS through its Hospital Inpatient Quality Reporting (Hospital IQR) Program, a national program instituted in 2003 that rewards hospitals for reporting designated quality measures. As of 2007, approximately 95% of eligible US hospitals were using the Hospital IQR program.[1] Measures evaluated via chart abstracts and surveys reflect a hospital's performance for its entire patient population, whereas measures assessed with claims data reflect hospital performance only for Medicare patients.
Evaluation of Hospitals
In FY 2013, hospital VBP incentive payments will be based entirely on performance in 2 domains: (1) clinical processes of care (weighted 70%) and (2) patient experience of care (weighted 30%). For each domain, CMS will evaluate each hospital's improvement over time as well as achievement compared to other hospitals in the VBP program. By assessing and rewarding both achievement and improvement, CMS will ensure that lower‐performing hospitals will still be rewarded for making substantial improvements in quality. To evaluate the first metricimprovement over timeCMS will compare a hospital's performance during a given reporting period with its baseline performance 2 years prior to this block of time. A hospital receives improvement points for improving its performance over time. To assess the second metricachievement compared to other hospitals in the VBP programCMS will compare each hospital's performance during a reporting period with the baseline performance (eg, performance 2 years prior to reporting period) of all other hospitals in the VBP program. A hospital is awarded achievement points if its performance exceeds the 50th percentile of all hospitals during the baseline performance period. Improvement scores range from 0 to 9, whereas achievement scores range from 0 to 10. The greater of a hospital's improvement and achievement scores on each VBP measure are used to calculate each hospital's total earned clinical care domain score and total earned HCAHPS base score. Hospitals that lack baseline performance data, which is required to assess improvement, will be evaluated solely on the basis of achievement points.[1] The total earned clinical care domain score is multiplied by 70% to reach the clinical care domain's contribution to a hospital's total performance score.
Each hospital's total patient experience domain, or HCAHPS performance, score consists of 2 components: a total earned HCAHPS base score as described above and a consistency score. The consistency score evaluates the reliability of a hospital's performance across all 8 patient experience of care measures (Table 3). If a hospital is above the 50th percentile of all hospital scores during the baseline period on all 8 measures, then it receives 100% of its consistency points. If a hospital is at the 0 percentile for a given measure, then it receives 0 consistency points for all measures. This provision promotes consistency by harshly penalizing hospitals with extremely poor performance on any 1 specific measure. If 1 or more measures are between the 0 and 50th percentiles, then it will receive a consistency score that takes into account how many measures were below the 50th percentile and their distance from this threshold. Each hospital's total HCAHPS performance score (the sum of total earned HCAHPS base points and consistency points) is then multiplied by 30% to arrive at the patient experience of care domain's contribution to a hospital's total performance score.
Importantly, CMS excluded from its VBP initiative 10 clinical process measures reported in the Hospital IQR Program because they are topped out; that is, almost all hospitals already perform them at very high rates (Table 4). Examples of these topped out process measures include administration of aspirin to all patients with AMI on arrival at the hospital; counseling of patients with AMI, CHF, and pneumonia about smoking cessation; and prescribing angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers to patients with CHF and left ventricular dysfunction.[1]
| Disease Process | Measure |
|---|---|
| |
| Acute myocardial infarction | Aspirin administered on arrival to the emergency department |
| ACEI or ARB prescribed on discharge | |
| Patient counseled about smoking cessation | |
| ‐Blocker prescribed on discharge | |
| Aspirin prescribed at discharge | |
| Heart failure | Patient counseled about smoking cessation |
| Evaluation of left ventricular systolic function | |
| ACEI or ARB prescribed for left ventricular systolic dysfunction | |
| Pneumonia | Patient counseled about smoking cessation |
| Surgical Care Improvement Project | Surgery patients with appropriate hair removal |
Calculation of VBP Incentives and Public Reporting
A hospital's total performance score for FY 2013 is equal to the sum of 70% of its clinical care domain score and 30% of its total HCAHPS performance score. This total performance score is entered into a linear mathematical formula to calculate each hospital's incentive payment. CMS projects that VBP will lead to a net increase in Medicare payments for one‐half of hospitals and a net decrease in payments for the other half of participating facilities.[1]
In December 2012, CMS publicly disclosed information about the initial performance of each hospital in the VBP program. Reported information included: (1) hospital performance for each applicable performance measure, (2) hospital performance by disease condition or procedure, and (3) hospital's total performance score. Initial analyses of this performance data revealed that 1557 hospitals will receive bonus payments under VBP in FY 2013, whereas 1427 hospitals will lose money under this program. Treasure Valley Hospital, a 10‐bed physician‐owned hospital in Boise, Idaho, will receive a 0.83% increase in Medicare payments, the largest payment increase under VBP in 2013. Conversely, Auburn Community Hospital in upstate New York, will suffer the most severe payment reduction: 0.9% per Medicare admission. The penalty will cost Auburn Hospital about $100,000, which is slightly more than 0.1% of its yearly $85 million operating budget.[13] For almost two‐thirds of participating hospitals, FY 2013 Medicare payments will change by <0.25%.[13] Additional information about VBP payments for FY 2013, including the number of hospitals who received VBP incentives and the size and range of these payments, is now accessible to the public through CMS' Hospital Compare Web site (
CHALLENGES OF VBP
As the Medicare VBP program evolves, and hospitals confront ever‐larger financial incentives to deliver high‐value as opposed to high‐volume care, it will be important to recognize limitations of the VBP program as they arise. Here we briefly discuss several conceptual and implementation challenges that physicians and policymakers should consider when assessing the merits of VBP in promoting high‐quality healthcare.
Rigorous and Continuous Evaluation of VBP Programs
The main premise of using VBP to incentivize hospitals to deliver high‐quality cost‐effective care is that the process measures used to determine hospital quality do impact patient outcomes. However, it is already well established that improvements in measures of process quality are not always associated with improvements in patient outcomes.[14, 15, 16] Moreover, incentivizing specific process measures encourages hospitals to shift resources away from other aspects of care delivery, which may have ambiguous, or even deleterious, effects on patient outcomes. Although incentives ideally push hospitals to shift resources away from low‐quality care toward high‐quality care, in practice this is not always the case. Hospital resources may instead be drawn away from areas that are not yet incented by VBP, but for which improvements in quality of care are desperately needed. The same empirical focus behind using VBP to incentivize hospitals to improve patient outcomes efficiently should be used to evaluate whether VBP is continually meeting its stated goals: reducing overall patient morbidity and mortality and improving patient satisfaction at ideally lower cost. The experience of the US education system with public policies designed to improve student testing performance may serve as a cautionary example here. Such policies, which provide financial rewards to schools whose students perform well on standardized tests, can indeed raise testing performance. However, these policies also lead educators to teach to the test, and to neglect important topics that are not tested on standardized exams.[17]
Prioritization of Process Measures
As payment incentives for VBP currently stand, process measures are weighted equally regardless of the clinical benefits they generate and the resources required to achieve improvements in process quality. For instance, 2 process measures, continuing home ‐blocker medications for patients with coronary artery disease undergoing surgery and early percutaneous coronary intervention for patients with AMI, may be weighted equally as process measures although both their clinical benefits and the costs of implementation are very different. Some hospitals responding to VBP incentives may choose to invest in areas where their ability to earn VBP incentive payments is high and the costs of improvement are low, although those areas may not be where interventions are most needed because clinical outcomes could be most improved. Recognizing that process measures have heterogeneous benefits and costs of implementation is important when prioritizing their reimbursement in VBP.
Measuring Improvements in Hospital Quality
Tying hospital financial compensation to hospital quality implies that measures of hospital quality should be robust. To incentivize hospitals to improve quality not only relative to other hospitals but to themselves in the past, the VBP program has established a baseline performance for each hospital. Each hospital is compared to its baseline performance in subsequent evaluation periods. Thus, properly measuring a hospital's baseline performance is important. During a given baseline period, some hospitals may have better or worse outcomes than their steady state due to random variation alone. Some hospitals deemed to have a low baseline will experience improvements in quality that are not related to active efforts to improve quality but through chance alone. Similarly, some hospitals deemed to have a high baseline will experience reductions in quality through chance. Of course, neither of these changes should be subject to differences in reimbursement because they do not reflect actual organizational changes made by the hospitals. The VBP program has made significant efforts to address this issue by requiring participating hospitals to have a large enough sample of cases such that estimated rates of process quality adherence meet a reliability threshold (ie, are likely to be consistent over time rather than vary substantially through chance alone). However, not all process measures exhibit high reliability, particularly those for which adverse events are rare (eg, foreign objects retained after surgery, air embolisms, and blood incompatibility). Ultimately, CMS's decision to balance the need for statistically reliable data with the goal of including as many hospitals as possible in the VBP program will require ongoing reevaluation of this issue.
Choosing Hospital Comparators Appropriately
In the current VBP program, hospitals will be evaluated in part by how they compare to hospitals nationally. However, studies of regional variation in healthcare have demonstrated large variations in practice patterns across the United States,[18, 19, 20] raising the question of whether hospitals should, at least initially, be compared to hospitals in the same geographic area. Although the ultimate goal of VBP should be to hold hospitals to a national standard, local practice patterns are not easily modified within 1‐ to 2‐year timeframes. Initially comparing hospitals to a national rather than local standard may unfairly penalize hospitals that are relative underperformers nationally but overperformers regionally. Although CMS's policy to reward improvement within hospitals over time mitigates issues arising from a cross‐sectional comparison of hospitals, the issue still remains if many hospitals within a region not only underperform relative to other hospitals nationally but also fail to demonstrate improvement. More broadly, this issue extends to differences across hospitals in factors that impact their ability to meet VBP goals. These factors may include, for example, hospital size, profitability, patient case and insurance mix, and presence of an electronic medical record. Comparing hospitals with vastly different abilities to achieve VBP goals and improve quickly may amount to inequitable policy.
Continual Evaluation of Topped‐Out Measures
Process measures that are met at high rates at nearly all hospitals are not used in evaluations by CMS for VBP. An assumption underlying CMS' decision to not reward hospitals for achieving these topped‐out measures is that once physicians and hospitals make cognitive and system‐level improvements that improve process quality, these gains will persist after the incentive is removed. Thus, CMS hopes and anticipates that although performance incentives will make it easier for well‐meaning physicians to learn to do the right thing, doctors will continue to do the right things for patients after these incentives are removed.[21, 22] Although this assumption may generally be accurate, it is important to continue to evaluate whether measures that are currently topped out continue to remain adequately performed, because rewarding new quality measures will necessarily lead hospitals to reallocate resources away from other clinical activities. Although we hope that the continued public reporting of topped‐out measures will prevent declines in performance on these measures, policy makers and clinicians should be aware that the lack of financial incentives for topped‐out measures may result in declines in quality. To this point, an analysis of 35 Kaiser Permanente facilities from 1997 to 2007 demonstrated that the removal of financial incentives for diabetic retinopathy and cervical cancer screening was associated with subsequent declines in performance of 3% and 1.6% per year, respectively.[23]
Will VBP Incentives Be Large Enough to Change Practice Patterns?
The VBP Program's ability to influence change depends, at least in part, on how the incentives offered under this program compare to the magnitude of the investments that hospitals must make to achieve a given reward. In general, larger incentives are necessary to motivate more significant changes in behavior or to influence organizations to invest the resources needed to achieve change. The incentives offered under VBP in FY 2013 are quite modest. Almost two‐thirds of participating hospitals will see their FY 2013 Medicare revenues change by <0.25%, roughly $125,000 at most.[13, 24] Although these incentives may motivate hospitals that can improve performance and achievement with very modest investments, they may have little impact on organizations that need to make significant upfront investments in care processes to achieve sustainable improvements in care quality. As CMS increases the size of VBP incentives over the next 2 to 4 years, it will also hold hospitals accountable for a broader and increasingly complex set of outcomes. Improving these outcomes may require investments in areas such as information technology and process improvement that far surpass the VBP incentive reward.
Moreover, prior research suggests that financial incentives like those available under VBP may contribute only slightly to performance improvements when public reporting already exists. For example, in a 2‐year study of 613 US hospitals implementing pay‐for‐performance plus public reporting or public reporting only, pay for performance plus public reporting was associated with only a 2.6% to 4.1% increase in a composite measure of quality when compared to hospitals with public reporting only.[9] Similarly, a study of 54 hospitals participating in the CMS pay for performance pilot initiative found no significant improvement in quality of care or outcomes for AMI when compared to 446 control hospitals.[25] A long‐term analysis of pay for performance in the Medicare Premier Hospital Quality Incentive Demonstration found that participation in the program had no discernible effect on 30‐day mortality rates.[10] Finally, a study of physician medical groups contracting with a large network healthcare maintenance organization found that the implementation of pay for performance did not result in major before and after improvements in clinical quality compared to a control group of medical groups.[26]
High‐Value Care Is Not Always Low‐Cost Care
Not surprisingly, the clinical process measures included in CMS' hospital VBP program evaluate a select and relatively small group of high‐value and low‐cost interventions (eg, appropriate administration of antibiotics and tight control of serum glucose in surgical patients). However, an important body of work has demonstrated that high‐cost care (eg, intensive inpatient hospital care for common acute medical conditions) may also be highly valuable in terms of improving survival.[20, 27, 28, 29, 30] As the hospital VBP program evolves, its overseers will need to consider whether to include additional incentives for high‐value high‐cost healthcare services. Such considerations will likely become increasingly salient as healthcare delivery organizations move toward capitated delivery models. In particular, the VBP program's Medicare Spending Per Beneficiary measure, which quantifies inpatient and subsequent outpatient spending per beneficiary after a given hospitalization episode, will need to distinguish between higher‐spending hospitals that provide highly effective care (eg, care that reduces mortality and readmissions) and facilities that provide less‐effective care.
FUTURE OF VBP
Although the future of VBP is unknown, CMS is likely to modify the program in a number of ways over the next 3 to 5 years. First, CMS will likely expand the breadth and focus of incentivized measures in the VBP program. In FY 2014, for example, CMS is adding a set of 3, 30‐day mortality outcome measures to VBP: 30‐day risk‐adjusted mortality for AMI, CHF, and pneumonia.[1] A hospital's performance with respect to these outcomes will represent 25% of its total performance score in 2014, whereas the clinical process of care and patient experience of care domains will account for 45% and 30% of this score, respectively. In 2015, patient experience and outcome measures will account for 30% each in a hospital's performance score, whereas process and efficiency measures will each account for 20% of this score, respectively. The composition of this performance score evidences a shift away from rewarding process‐based measures and toward incentivizing measures of clinical outcomes and patient satisfaction, the latter of which may be highly subjective and more representative of a hospital's catchment population than of a hospital's care itself.[31] Additional measures in the domains of patient safety, care coordination, population and community health, emergency room wait times, and cost control may also be added to the VBP program in FY 2015 to FY 2017. Furthermore, CMS will continue to reevaluate the appropriateness of measures that are already included in VBP and will stop incentivizing measures that have become topped out, or are no longer supported by the National Quality Forum.[1, 13]
Second, CMS has established an annual gradual increase of 0.25% in the percentage of each hospital's inpatient DRG‐based payment that is at stake under VBP. In FY 2014, for example, participating hospitals will be required to contribute 1.25% of inpatient DRG payments to the VBP program. This percentage is likely to increase to 2% or more by 2017.[1, 32]
Third, expansions of the VBP program complement a number of other quality improvement efforts overseen by CMS, including the Hospital Readmissions Reduction Program. Effective for discharges beginning on October 1, 2012, hospitals with excess readmissions for AMI, CHF, and pneumonia are at risk for reimbursement reductions for all Medicare admissions in proportion to the rate of excess rehospitalizations. Some of the same concerns about the hospital VBP program outlined above have also been raised for this program, namely, whether readmission penalties will be large enough to impact hospital behavior, whether readmissions are even preventable,[33, 34] and whether adjustments in hospital‐level policies will reduce admissions that are known to be heavily influenced by patient economic and social factors that are outside of a hospital's control.[35, 36] Despite the limitations of VBP and the challenges that lie ahead, there is optimism that rewarding hospitals that provide high‐value rather than high‐volume care will not only improve outcomes of hospitalized patients in the United States, but will potentially be able to do so at a lower cost. Encouraging hospitals to improve their quality of care may also have important spillover effects on other healthcare domains. For example, hospitals that adopt systems to ensure prompt delivery of antibiotics to patients with pneumonia may also observe positive spillover effects with the prompt antibiotic management of other acute infectious illnesses that are not covered by VBP. VBP may have spillover effects on medical malpractice liability and defensive medicine as well. Indeed, financial incentives to practice higher‐quality evidenced‐based care may reduce medical malpractice liability and defensive medicine.
The government's ultimate goal in implementing VBP is to identify a broad and clinically relevant set of outcome measures that can be used to incentivize hospitals to deliver high‐quality as opposed to high‐volume healthcare. The first wave of outcome measures has already been instituted. It remains to be seen whether the incentive rewards of Medicare's hospital VBP program will be large enough that hospitals feel compelled to improve and compete for them.
- Centers for Medicare and Medicaid Services. Hospital Value‐Based Purchasing Web site. 2013. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/hospital‐value‐based‐purchasing/index.html. Accessed March 4, 2013.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367:292–295.
- , . Hospital value‐based purchasing: will Medicare's new policy exacerbate disparities? Circ Cardiovasc Qual Outcomes. 2012;5:148–149.
- Centers for Medicare and Medicaid Services. CMS/premier hospital quality incentive demonstration (QHID). 2013. Available at: https://www.premierinc.com/quality‐safety/tools‐services/p4p/hqi/faqs.jsp. Accessed March 5, 2013.
- Centers for Medicare and Medicaid Services. Hospital Compare Web site. 2013. Available at: http://www.medicare.gov/hospitalcompare. Accessed March 4, 2013.
- , , . “Never events”: not every hospital‐acquired infection is preventable. Clin Infect Dis. 2009;49:743–746.
- . Will pay for performance improve quality of care? The answer is in the details. N Engl J Med. 2012;367:1852–1853.
- , , , , , . Reduced mortality with hospital pay for performance in England. N Engl J Med. 2012;367:1821–1828.
- , , , et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–496.
- , , , . The long‐term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157:889–899.
- Centers for Medicare and Medicaid Services. Hospital Consumer Assessment Of Healthcare Providers and Systems Web site. 2013. Available at: http://www.hcahpsonline.org. Accessed March 5, 2013.
- . Medicare discloses hospitals' bonuses, penalties based on quality. Kaiser Health News. December 20, 2012. Available at: http://www.kaiserhealthnews.org/stories/2012/december/21/medicare‐hospitals‐value‐based‐purchasing.aspx?referrer=search. Accessed March 26, 2013.
- , , , . Hospital quality and intensity of spending: is there an association? Health Aff (Millwood). 2009;28:w566–w572.
- , , , et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70.
- , , . The advantages and disadvantages of process‐based measures of health care quality. Int J Qual Health Care. 2001;13:469–474.
- . Accountability, incentives and behavior: the impact of high‐stakes testing in the Chicago public schools. J Public Econ. 2005;89:761–796.
- , , , , , . The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287.
- . Medical care—is more always better? N Engl J Med. 2003;349:1665–1667.
- , , . Hospital spending and inpatient mortality: evidence from California: an observational study. Ann Intern Med. 2011;154:160–167.
- . Making it easy to do it right. N Engl J Med. 2001;345:991–993.
- , , , . A high rate of compliance with neonatal intensive care unit transfusion guidelines persists even after a program to improve transfusion guideline compliance ended. Transfusion. 2011;51:2519–2520.
- , , , et al. The impact of removing financial incentives from clinical quality indicators: longitudinal analysis of four Kaiser Permanente indicators. BMJ. 2010;340:c1898.
- , . Medicare's new hospital value‐based purchasing program is likely to have only a small impact on hospital payments. Health Aff (Millwood). 2012;31:1932–1940.
- , , , et al. Pay for performance, quality of care, and outcomes in acute myocardial infarction. JAMA. 2007;297:2373–2380.
- , , . Can you get what you pay for? Pay‐for‐performance and the quality of healthcare providers. Rand J Econ. 2010;41:64–91.
- , , , . Spending and mortality in US acute care hospitals. Am J Manag Care. 2013;19:e46–e54.
- , , , , , . Development and validation of hospital “end‐of‐life” treatment intensity measures. Med Care. 2009;47:1098–1105.
- , , , et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2:548–557.
- , , , et al. Association of hospital spending intensity with mortality and readmission rates in Ontario hospitals. JAMA. 2012;307:1037–1045.
- , , . Patient satisfaction with hospital care: effects of demographic and institutional characteristics. Med Care. 2000;38:325–334.
- , , . Linking performance with payment: implementing the Physician Value‐Based Payment Modifier. JAMA. 2012;308:2089–2090.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391–E402.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183:E1067–E1072.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366:1366–1369.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681.
- Centers for Medicare and Medicaid Services. Hospital Value‐Based Purchasing Web site. 2013. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/hospital‐value‐based‐purchasing/index.html. Accessed March 4, 2013.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367:292–295.
- , . Hospital value‐based purchasing: will Medicare's new policy exacerbate disparities? Circ Cardiovasc Qual Outcomes. 2012;5:148–149.
- Centers for Medicare and Medicaid Services. CMS/premier hospital quality incentive demonstration (QHID). 2013. Available at: https://www.premierinc.com/quality‐safety/tools‐services/p4p/hqi/faqs.jsp. Accessed March 5, 2013.
- Centers for Medicare and Medicaid Services. Hospital Compare Web site. 2013. Available at: http://www.medicare.gov/hospitalcompare. Accessed March 4, 2013.
- , , . “Never events”: not every hospital‐acquired infection is preventable. Clin Infect Dis. 2009;49:743–746.
- . Will pay for performance improve quality of care? The answer is in the details. N Engl J Med. 2012;367:1852–1853.
- , , , , , . Reduced mortality with hospital pay for performance in England. N Engl J Med. 2012;367:1821–1828.
- , , , et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–496.
- , , , . The long‐term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157:889–899.
- Centers for Medicare and Medicaid Services. Hospital Consumer Assessment Of Healthcare Providers and Systems Web site. 2013. Available at: http://www.hcahpsonline.org. Accessed March 5, 2013.
- . Medicare discloses hospitals' bonuses, penalties based on quality. Kaiser Health News. December 20, 2012. Available at: http://www.kaiserhealthnews.org/stories/2012/december/21/medicare‐hospitals‐value‐based‐purchasing.aspx?referrer=search. Accessed March 26, 2013.
- , , , . Hospital quality and intensity of spending: is there an association? Health Aff (Millwood). 2009;28:w566–w572.
- , , , et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70.
- , , . The advantages and disadvantages of process‐based measures of health care quality. Int J Qual Health Care. 2001;13:469–474.
- . Accountability, incentives and behavior: the impact of high‐stakes testing in the Chicago public schools. J Public Econ. 2005;89:761–796.
- , , , , , . The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287.
- . Medical care—is more always better? N Engl J Med. 2003;349:1665–1667.
- , , . Hospital spending and inpatient mortality: evidence from California: an observational study. Ann Intern Med. 2011;154:160–167.
- . Making it easy to do it right. N Engl J Med. 2001;345:991–993.
- , , , . A high rate of compliance with neonatal intensive care unit transfusion guidelines persists even after a program to improve transfusion guideline compliance ended. Transfusion. 2011;51:2519–2520.
- , , , et al. The impact of removing financial incentives from clinical quality indicators: longitudinal analysis of four Kaiser Permanente indicators. BMJ. 2010;340:c1898.
- , . Medicare's new hospital value‐based purchasing program is likely to have only a small impact on hospital payments. Health Aff (Millwood). 2012;31:1932–1940.
- , , , et al. Pay for performance, quality of care, and outcomes in acute myocardial infarction. JAMA. 2007;297:2373–2380.
- , , . Can you get what you pay for? Pay‐for‐performance and the quality of healthcare providers. Rand J Econ. 2010;41:64–91.
- , , , . Spending and mortality in US acute care hospitals. Am J Manag Care. 2013;19:e46–e54.
- , , , , , . Development and validation of hospital “end‐of‐life” treatment intensity measures. Med Care. 2009;47:1098–1105.
- , , , et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2:548–557.
- , , , et al. Association of hospital spending intensity with mortality and readmission rates in Ontario hospitals. JAMA. 2012;307:1037–1045.
- , , . Patient satisfaction with hospital care: effects of demographic and institutional characteristics. Med Care. 2000;38:325–334.
- , , . Linking performance with payment: implementing the Physician Value‐Based Payment Modifier. JAMA. 2012;308:2089–2090.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391–E402.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183:E1067–E1072.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366:1366–1369.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681.
Mortality After Therapeutic Hypothermia
There are over 350,000 cases of out‐of‐hospital cardiac arrest (OHCA) each year in the United States1, 2 and, with supportive therapy alone, only a fraction of victims survive to hospital discharge. Rapid intervention including cardiopulmonary resuscitation in the moments following arrest is critical to minimizing neurologic injury, morbidity, and mortality. In 2002, two small randomized controlled trials showed a survival benefit of therapeutic hypothermia (TH) when provided within 12 hours after return of circulation following an OHCA and, to date, TH remains one of the few interventions with proven mortality benefit after initial cardiopulmonary resuscitation.3, 4 Since 2003, TH has been incorporated into the American Heart Association practice guidelines57 and use of TH has steadily increased, but widespread clinical uptake remains low.8, 9
The initial studies that evaluated TH were small, with only 189 patients included in the TH arms of the 2 trials combined. To date, only a few studies have replicated this initial observation in real‐world settings, with little analysis of outcomes in US centers in particular.1013 Accordingly, we aimed to examine the real‐world experience with TH in the United States using a large administrative claims database of all California hospital admissions to describe utilization trends, hospital mortality, and volumeoutcome relationships associated with the intervention.
MATERIALS AND METHODS
Data
We identified all admissions to California hospitals during 19992008 based on discharge records from the California Office of Statewide Health Planning and Development. Our study period included cases of TH performed prior to the 2002 major clinical trials, since TH was in occasional use prior to the publication of these trials. The data was de‐identified and publicly available, and therefore exempt from review by the Institutional Review Board. In addition to hospital name, each discharge record included patient age, gender, admission year, International Classification of Disease, Ninth Revision (ICD‐9) code for presenting primary and secondary diagnoses, procedure codes, and disposition (discharge to home or rehabilitation, in‐hospital death). All California hospitals were included in the registry (n = 419). We defined teaching status for each hospital based on membership in the Council of Teaching Hospitals, as reported in the American Hospital Association's Annual Survey (n = 19 teaching hospitals).14
Setting and Participants
We used discharge diagnoses to identify patients who could be considered eligible for therapeutic hypothermia after cardiac arrest. We classified patients as eligible for therapeutic hypothermia after cardiac arrest based on ICD‐9 diagnosis codes that indicated the presence of both cardiac arrest and anoxic brain injury in the administrative diagnoses. Because of known imprecision in using billing codes to identify patients with cardiac arrest,15, 16 we broadly defined cardiac arrest to include those patients with ICD‐9 codes for cardiac arrest, ventricular fibrillation (VF), or ventricular tachycardia (VT) (see Supporting Table 1 in the online version of this article). We could not distinguish between out‐of‐hospital and in‐hospital cardiac arrest based on administrative diagnoses. To ensure that we included only patients with cardiac arrest complicated by neurologic insult, we required an ICD‐9 diagnosis of either anoxic brain injury, coma, or persistent vegetative state. Claims did not allow us to distinguish among initial cardiac arrest rhythms (VF vs pulseless VT vs asystole). Patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia were excluded.3, 17 We did not exclude patients based on coagulopathy (which is considered a contraindication to TH), since ICD‐9 coding did not allow us to determine the severity of the coagulopathy or whether it was a result of therapeutic hypothermia itself.
We used the ICD‐9 procedure code (99.81) for TH to first identify patients who underwent TH from 1999 to 2008. Since this code also applies to TH used during cardiac and neurosurgery, we examined each of these cases and excluded individuals who underwent cardiac surgery or neurosurgery during the hospitalization. As in our eligible for TH definition, we excluded patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia. Patients who underwent therapeutic hypothermia but for whom a specific procedure code was not recorded in the discharge abstractperhaps because the medical institution did not directly bill for the procedurecould not be identified.
Statistical Analysis
We used a multivariable logistic model to estimate differences in hospital mortality after cardiac arrest associated with use of therapeutic hypothermia. We conducted 2 specifications. In our baseline specification, we accounted for case‐mix differences between those who underwent TH and those who did not by adjusting for age, gender, year of admission, the number of Charlson‐Deyo comorbidities,1820 and hospital teaching status. Because mortality after cardiac arrest in centers that perform therapeutic hypothermia may be different from centers that do not, even for patients who do not undergo the procedure, we included indicators for volume tercile of therapeutic hypothermia cases performed. Volume of therapeutic hypothermia was defined at the hospital level as the total number of cases performed by that hospital from 1999 to 2008.
In order to explore how hospital teaching status, volume of therapeutic hypothermia procedures (broken into terciles), and year of admission affected the association between hospital mortality after cardiac arrest and therapeutic hypothermia, our baseline logistic model was expanded to include interactions between therapeutic hypothermia and each of these variables. The interaction between therapeutic hypothermia and year explored whether the effectiveness of the procedure changed over time, as case‐selection, method of therapeutic hypothermia (cold saline vs commercially available devices), and experience changed in California hospitals. For both specifications, we reported the odds ratio of hospital mortality among patients undergoing therapeutic hypothermia, as well as risk‐adjusted mortality for both TH and non‐TH groups.
STATA version 11 (STATA Corp, College Station, TX) was used for statistical analyses, and a 2‐sided P 0.05 was used.
RESULTS
Descriptive Data
Table 1 reports summary statistics for patients with cardiac arrest complicated by neurologic insult (anoxic brain injury, coma, or persistent vegetative state) between 1999 and 2008. Across all years, 204 patients were identified as undergoing TH. In comparison, 105 patients were identified as undergoing TH in 2008 alone. Patients who underwent TH were less likely to be male (30.7% vs 44.6% male, P < 0.01), were younger (63.9 15.0 years vs 67.3 15.7 years, P = 0.03), and had equivalent numbers of Charlson‐Deyo comorbidities (2.5 2.0 diagnoses vs 2.5 2.0 diagnoses, P = 0.89). Therapeutic hypothermia was more commonly employed at teaching hospitals (51/3709 [1.4%] vs 153/42,942 [0.4%], P < 0.01). There was a trend toward decreased unadjusted mortality among patients who underwent therapeutic hypothermia compared with those who did not (56.9% vs 62.8%, P = 0.08).
| Therapeutic Hypothermia | No Therapeutic Hypothermia | P Value | |
|---|---|---|---|
| |||
| No. observations | 204 | 46,629 | |
| No. cases in teaching hospitals | 51 | 3,658 | |
| No. cases in non‐teaching hospitals | 153 | 42,789 | |
| Age, y | 63.9 15.0 | 67.3 15.7 | 0.06 |
| Male | 30.7 | 44.6 | <0.01 |
| Hospital mortality, % | 56.9 | 62.8 | 0.08 |
| Comorbidities | |||
| No. Charlson‐Deyo comorbidities | 2.5 2.0 | 2.5 2.0 | 0.89 |
| Coronary artery disease, % | 48.0 | 38.0 | <0.01 |
| Acute myocardial infarction, % | 42.6 | 28.9 | <0.01 |
| Congestive heart failure, % | 27.9 | 35.3 | 0.03 |
| Hypertension, % | 36.3 | 33.2 | 0.83 |
| Acute renal failure, % | 33.3 | 26.6 | 0.03 |
| Diabetes mellitus, % | 30.9 | 23.0 | <0.01 |
| Chronic obstructive pulmonary disease, % | 10.3 | 19.3 | <0.01 |
Figures 1 and 2 provide additional aggregate statistics on therapeutic hypothermia in California hospitals. Figure 1 plots the number of therapeutic hypothermia cases recorded in the administrative discharge registry between 1999 and 2008. Of the 204 total cases identified during this period, 178 (87.3%) were performed between 2006 and 2008. Figure 2 shows the distribution of TH cases across centers that performed therapeutic hypothermia (n = 47 hospitals, 11.3 % of all hospitals). Ten centers accounted for 124/204 (60.7%) of the total patients treated with TH after cardiac arrest; the top 3 centers accounted for 64 (31.4%) of the treated patients. Twenty‐seven hospitals were identified as performing therapeutic hypothermia on only 1 or 2 patients between 1999 and 2008.
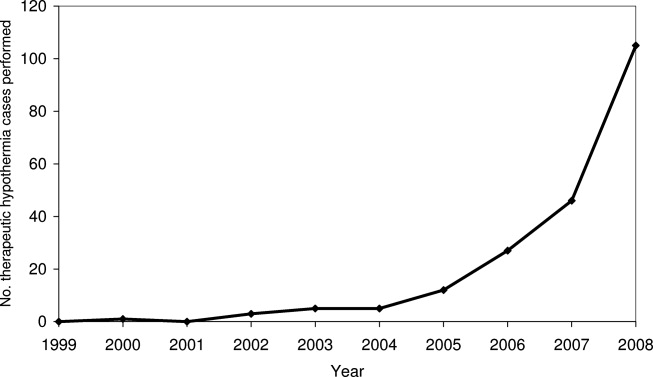
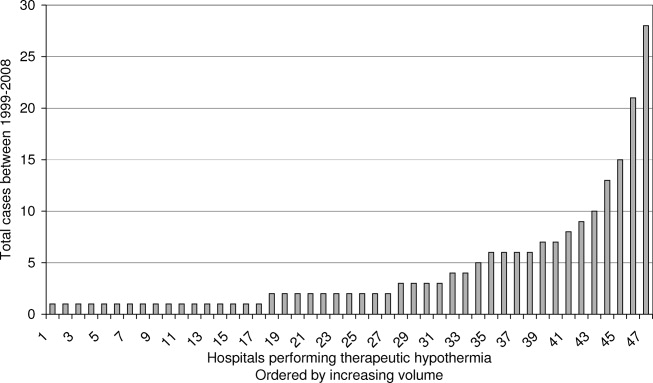
Risk‐Adjusted Mortality
Table 2 presents the odds ratio of factors predicting in‐hospital mortality after cardiac arrest complicated by neurologic insult. Factors include use of TH after cardiac arrest, age, gender, year of admission, number of Charlson‐Deyo comorbidities, hospital teaching status, and volume tercile of hospitals that performed therapeutic hypothermia. Overall, patients who were older, male, and had greater comorbidities were statistically more likely to die after cardiac arrest complicated by neurologic insult. Regardless of whether they underwent TH, patients admitted to hospitals in the highest volume tercile of TH use were more likely to die after cardiac arrest. Adjusting for volume tercile, teaching hospital status was not independently associated with mortality after cardiac arrest. The adjusted odds ratio of mortality among patients undergoing therapeutic hypothermia was 0.80 (95% confidence interval [CI] 0.601.06, P = 0.11). The adjusted probability of inpatient mortality among patients undergoing therapeutic hypothermia was 57.5% (95% CI 50.764.3%) compared to those who did not 62.8% (95% CI 61.763.9%, P = 0.11).
| Variable | Odds Ratio of Hospital Mortality (95% CI) | P Value |
|---|---|---|
| ||
| No. observations | 46,651 | |
| Age* | ||
| 6569 | 1.19 (1.121.28) | <0.001 |
| 7074 | 1.29 (1.201.39) | <0.001 |
| 7579 | 1.55 (1.441.67) | <0.001 |
| 8084 | 1.79 (1.651.93) | <0.001 |
| 85 and over | 2.06 (1.892.25) | <0.001 |
| Male | 1.15 (1.101.21) | <0.001 |
| Teaching hospital | 1.13 (0.951.34) | 0.17 |
| No. Charlson‐Deyo comorbidities | 1.09 (1.081.10) | <0.001 |
| Year trend | 0.98 (0.970.99) | <0.001 |
| Volume tercile among hospitals performing TH | ||
| First tercile | 0.94 (0.791.12) | 0.48 |
| Second tercile | 1.03 (0.801.33) | 0.82 |
| Third tercile | 1.20 (1.051.36) | 0.006 |
| Therapeutic hypothermia | 0.80 (0.601.06) | 0.11 |
Figure 3 presents adjusted mortality after cardiac arrest in hospitals that did not perform TH, as well as adjusted mortality associated with TH for each volume tercile of hospitals that performed the procedure. Hospital mortality rates among patients not receiving TH after cardiac arrest were slightly higher in hospitals in the high volume tercile of TH (66.3%, 95% CI 63.868.8%) compared to hospitals in low and moderate volume terciles and to hospitals not performing TH (P < 0.001). Hospital mortality rates among low and moderate TH volume centers and in centers not performing TH were similar (62.3%, 61.3%, and 63.4%, respectively). Among both the low volume and moderate volume terciles, however, patients who underwent TH after cardiac arrest were significantly less likely to die in‐hospital compared to those who did not. For patients admitted to hospitals in the low volume tercile, those undergoing therapeutic hypothermia had an adjusted hospital mortality rate of 25.5% (95% CI 3.047.9%) compared to those who did not undergo TH (adjusted mortality 61.3%, 95% CI 57.465.1%), P < 0.001. In the moderate volume tercile, patients receiving therapeutic hypothermia had an adjusted hospital mortality rate of 31.0% (95% CI 9.2%52.8%) compared to 63.4% (95% CI 57.769.1%), P < 0.001, among those not undergoing the procedure. There was no statistically significant difference in adjusted mortality between those who underwent TH and those who did not, in hospitals in the highest volume tercile (P = 0.211). In addition to examining how volume of therapeutic hypothermia performed by hospitals affected the association between TH and hospital mortality, we also examined whether year of admission and teaching hospital independently modified the association. Neither year of admission nor teaching hospital statistically significantly affected the association between therapeutic hypothermia and hospital mortality after cardiac arrest at the P < 0.10 level.
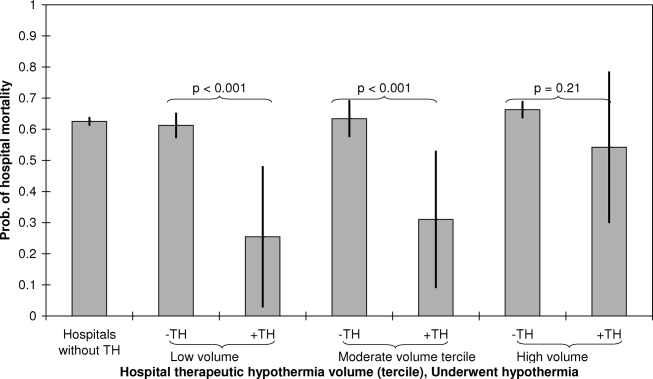
DISCUSSION
In an administrative database of all admissions to California hospitals, we demonstrated that use of TH increased steadily since the publication of the initial clinical trials in 2002. The absolute level of TH utilization in our study undoubtedly represents a significant underestimation of actual TH utilization, however, our study does provide an assessment of the utilization trends over time. The bulk of TH use appears to be performed in a small group of high volume centers, and 89% of California hospitals did not perform TH during the study period (as assessed by procedure billing codes). Additionally, within the limitations of a retrospective, administrative claims‐based study design, TH appears to be associated with a similar in‐hospital mortality rate to that seen in clinical trials.3, 4 In exploratory analyses, there appears to be a particular benefit of TH in low and moderate volume centers, though these findings should be considered hypothesis‐generating.
Despite the body of evidence in favor of TH, utilization in our study and others appears quite low. In a 2005 survey of physicians, 87% of respondents had never used therapeutic hypothermia, citing inadequate data, technical limitations, and lack of incorporation in the Advanced Cardiac Life Support (ACLS) protocol as principal justifications.8 Other surveys have shown similar results and noted that critical care physicians and those working in large medical centers were more likely to adopt the therapy.9 Advocates of the therapy have suggested that an explicit hospital‐based plan developed by key stakeholders can help facilitate implementation.21 Accordingly, there is growing interest in developing centers of expertise in highly intensive therapies such as TH. For instance, the New York City Emergency Medical Service has begun to explore a protocol to divert TH candidates to specialized centers.22, 23 Some favorable results have been reported in individual hospitals and local hospital systems.2428
Our data suggest that TH is associated with an in‐hospital mortality rate that is comparable to that reported in the clinical trials. For example, in a 2009 meta‐analysis of 4 clinical trials and 1 abstract (481 patients in total), TH was associated with a 35% relative mortality benefit as compared to standard post‐resuscitation care.29 It has been estimated that broad TH implementation could save thousands of lives30 and many authors have advocated for its use and outlined explicit protocols for implementation.17 Furthermore, TH appears to be cost‐effective in line with other accepted therapies. Assuming the Hypothermia After Cardiac Arrest (HACA) trial inclusion criteria, even at extreme estimates for costs, the cost‐effectiveness of hypothermia remains less than $100,000 per quality‐adjusted life year.31
There are important limitations of this study. Our use of administrative claims data certainly underestimates the level of TH utilization, since we could only identify cases in which TH was included in the billing codes for the hospitalization. Hospitals may vary in utilization of this particular billing code for TH in ways that bias our estimated associations. The ICD‐9 code 99.81 for therapeutic hypothermia was also not developed for post‐cardiac arrest TH specifically, so use of the code may actually lag clinical utilization. Although the observed trend in TH utilization is likely mainly due to a true increase in utilization, it is possible that some of the observed increase is due to an increase in utilization of TH procedure billing codes. Our TH utilization estimates should be construed as a lower bound of the actual rates. Additionally, although the estimated real‐world mortality benefit of TH may be comparable to that of clinical trials, the equivalence of patients in our sample to those in published randomized trials is uncertain. Similarly, even after adjusting for age, gender, year of admission, comorbidities, hospital teaching status, and TH volume, there are likely many unmeasured variables that influence mortality in both the TH and comparison groups. There are also likely patients included in our comparison group who had both cardiac arrest or ventricular tachycardia and anoxic brain injury, but who were not candidates for TH as the episode of cardiac arrest followed rather than preceded the anoxic brain injury. Since we lack detailed clinical data about the TH cases (initial rhythm, time before return of circulation, preexisting disease states, etc.), we are unable to match controls directly to cases. Additionally, we lack data to assess neurologic recovery or quality of life after arrest.
The observation that a mortality benefit in our study could be detected only in low and moderate volume centers requires further exploration. Indeed, one might expect that high volume centers may have better outcomes with TH as a result of more robust infrastructure, technical experience, and available resources. Our finding that mortality benefits of TH appear concentrated in centers with low to moderate volume of TH utilization suggest at least 1 of 2 possibilities. First, low and moderate volume centers may perform TH in a subset of patients who benefit most from the intervention or, alternatively, in the most viable cardiac arrest cases (those who may fare well with or without the therapy). Consequently, we may observe relatively favorable outcomes in this group due to this selection bias. Second, high volume centersdespite having more expertisemay also attract patients at higher mortality risk due to referral bias. This would lead us to estimate lower mortality benefits associated with TH in these high volume centers. Indeed, greater observed mortality at high volume centers regardless of TH status suggests that overall acuity is higher at high volume centers. While our inferences are greatly affected by issues of case selection and referral bias, it also important to consider the possibility that the estimated mortality benefit of TH in higher volume centers is lower because of the selection of patients who do not meet current guidelines for treatment with TH. Distinguishing whether the selection of patients undergoing TH at high volume centers is appropriate or inappropriate based on current guidelines is an important issue that merits further research with datasets with more refined patient clinical information.
In summary, therapeutic hypothermia utilization is low, but the rate of implementation has increased since the publication of the initial clinical trials in 2002. The bulk of TH utilization appears limited to a subset of high volume centers, and most centers in California appear to have not used the therapy. Real‐world in‐hospital mortality associated with TH is comparable to that reported in randomized clinical trials.
Acknowledgements
Disclosures: Dr Romley received support from NIH grant R03AG031990‐A1. Dr Noseworthy received support from the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology granted by the Heart Rhythm Society. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources. The contents of this article have not been published in any other peer‐reviewed media, and the manuscript is not under review elsewhere. All authors listed have contributed sufficiently to this project to be included as authors. The authors have no conflict of interest, financial or otherwise.
- , , , et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163.
- , . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- , , , et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563.
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556.
- , , , et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 7.5: postresuscitation support. Circulation. 2005;112(suppl I):IV‐84–IV‐88.
- , , , et al. Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(suppl 1):e93–e174.
- , , , et al. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186.
- , , , et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940.
- , , , et al. Adverse events and their relation to mortality in out‐of‐hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med.39(1):57–64.
- , , , et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934.
- . Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–1047.
- , , , et al. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–214.
- American Hospital Association (AHA). American Hospital Association 2001 Annual Survey. Chicago, IL: Health Forum, LLC.
- , , . A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296.
- . Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604.
- . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–1264.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- , , , et al. Practical implementation of therapeutic hypothermia after cardiac arrest. Hosp Pract (Minneap). 2009;37(1):71–83.
- . Paramedics will employ new therapy in cardiac arrest cases. New York Times. August 3, 2010:A18.
- , , , et al. Resuscitation center designation: recommendations for emergency medical services practices. Prehosp Emerg Care. 2010;14(1):51–61.
- , , , et al. Implementation of a hospital‐wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol. 2010;9(4):216–220.
- , , , et al. Therapeutic hypothermia protocol in a community emergency department. West J Emerg Med. 2010;11(4):367–372.
- , , , et al. Implementation of a post‐cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out‐of‐hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366.
- , , , et al. Improved out‐of‐hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med. 2010;56(4):348–357.
- , , , et al. Community‐based application of mild therapeutic hypothermia for survivors of cardiac arrest. South Med J. 2010;103(4):295–300.
- , , , et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009(4):CD004128.
- , . Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22(4):711–728.
- , , , et al. Cost‐effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–428.
There are over 350,000 cases of out‐of‐hospital cardiac arrest (OHCA) each year in the United States1, 2 and, with supportive therapy alone, only a fraction of victims survive to hospital discharge. Rapid intervention including cardiopulmonary resuscitation in the moments following arrest is critical to minimizing neurologic injury, morbidity, and mortality. In 2002, two small randomized controlled trials showed a survival benefit of therapeutic hypothermia (TH) when provided within 12 hours after return of circulation following an OHCA and, to date, TH remains one of the few interventions with proven mortality benefit after initial cardiopulmonary resuscitation.3, 4 Since 2003, TH has been incorporated into the American Heart Association practice guidelines57 and use of TH has steadily increased, but widespread clinical uptake remains low.8, 9
The initial studies that evaluated TH were small, with only 189 patients included in the TH arms of the 2 trials combined. To date, only a few studies have replicated this initial observation in real‐world settings, with little analysis of outcomes in US centers in particular.1013 Accordingly, we aimed to examine the real‐world experience with TH in the United States using a large administrative claims database of all California hospital admissions to describe utilization trends, hospital mortality, and volumeoutcome relationships associated with the intervention.
MATERIALS AND METHODS
Data
We identified all admissions to California hospitals during 19992008 based on discharge records from the California Office of Statewide Health Planning and Development. Our study period included cases of TH performed prior to the 2002 major clinical trials, since TH was in occasional use prior to the publication of these trials. The data was de‐identified and publicly available, and therefore exempt from review by the Institutional Review Board. In addition to hospital name, each discharge record included patient age, gender, admission year, International Classification of Disease, Ninth Revision (ICD‐9) code for presenting primary and secondary diagnoses, procedure codes, and disposition (discharge to home or rehabilitation, in‐hospital death). All California hospitals were included in the registry (n = 419). We defined teaching status for each hospital based on membership in the Council of Teaching Hospitals, as reported in the American Hospital Association's Annual Survey (n = 19 teaching hospitals).14
Setting and Participants
We used discharge diagnoses to identify patients who could be considered eligible for therapeutic hypothermia after cardiac arrest. We classified patients as eligible for therapeutic hypothermia after cardiac arrest based on ICD‐9 diagnosis codes that indicated the presence of both cardiac arrest and anoxic brain injury in the administrative diagnoses. Because of known imprecision in using billing codes to identify patients with cardiac arrest,15, 16 we broadly defined cardiac arrest to include those patients with ICD‐9 codes for cardiac arrest, ventricular fibrillation (VF), or ventricular tachycardia (VT) (see Supporting Table 1 in the online version of this article). We could not distinguish between out‐of‐hospital and in‐hospital cardiac arrest based on administrative diagnoses. To ensure that we included only patients with cardiac arrest complicated by neurologic insult, we required an ICD‐9 diagnosis of either anoxic brain injury, coma, or persistent vegetative state. Claims did not allow us to distinguish among initial cardiac arrest rhythms (VF vs pulseless VT vs asystole). Patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia were excluded.3, 17 We did not exclude patients based on coagulopathy (which is considered a contraindication to TH), since ICD‐9 coding did not allow us to determine the severity of the coagulopathy or whether it was a result of therapeutic hypothermia itself.
We used the ICD‐9 procedure code (99.81) for TH to first identify patients who underwent TH from 1999 to 2008. Since this code also applies to TH used during cardiac and neurosurgery, we examined each of these cases and excluded individuals who underwent cardiac surgery or neurosurgery during the hospitalization. As in our eligible for TH definition, we excluded patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia. Patients who underwent therapeutic hypothermia but for whom a specific procedure code was not recorded in the discharge abstractperhaps because the medical institution did not directly bill for the procedurecould not be identified.
Statistical Analysis
We used a multivariable logistic model to estimate differences in hospital mortality after cardiac arrest associated with use of therapeutic hypothermia. We conducted 2 specifications. In our baseline specification, we accounted for case‐mix differences between those who underwent TH and those who did not by adjusting for age, gender, year of admission, the number of Charlson‐Deyo comorbidities,1820 and hospital teaching status. Because mortality after cardiac arrest in centers that perform therapeutic hypothermia may be different from centers that do not, even for patients who do not undergo the procedure, we included indicators for volume tercile of therapeutic hypothermia cases performed. Volume of therapeutic hypothermia was defined at the hospital level as the total number of cases performed by that hospital from 1999 to 2008.
In order to explore how hospital teaching status, volume of therapeutic hypothermia procedures (broken into terciles), and year of admission affected the association between hospital mortality after cardiac arrest and therapeutic hypothermia, our baseline logistic model was expanded to include interactions between therapeutic hypothermia and each of these variables. The interaction between therapeutic hypothermia and year explored whether the effectiveness of the procedure changed over time, as case‐selection, method of therapeutic hypothermia (cold saline vs commercially available devices), and experience changed in California hospitals. For both specifications, we reported the odds ratio of hospital mortality among patients undergoing therapeutic hypothermia, as well as risk‐adjusted mortality for both TH and non‐TH groups.
STATA version 11 (STATA Corp, College Station, TX) was used for statistical analyses, and a 2‐sided P 0.05 was used.
RESULTS
Descriptive Data
Table 1 reports summary statistics for patients with cardiac arrest complicated by neurologic insult (anoxic brain injury, coma, or persistent vegetative state) between 1999 and 2008. Across all years, 204 patients were identified as undergoing TH. In comparison, 105 patients were identified as undergoing TH in 2008 alone. Patients who underwent TH were less likely to be male (30.7% vs 44.6% male, P < 0.01), were younger (63.9 15.0 years vs 67.3 15.7 years, P = 0.03), and had equivalent numbers of Charlson‐Deyo comorbidities (2.5 2.0 diagnoses vs 2.5 2.0 diagnoses, P = 0.89). Therapeutic hypothermia was more commonly employed at teaching hospitals (51/3709 [1.4%] vs 153/42,942 [0.4%], P < 0.01). There was a trend toward decreased unadjusted mortality among patients who underwent therapeutic hypothermia compared with those who did not (56.9% vs 62.8%, P = 0.08).
| Therapeutic Hypothermia | No Therapeutic Hypothermia | P Value | |
|---|---|---|---|
| |||
| No. observations | 204 | 46,629 | |
| No. cases in teaching hospitals | 51 | 3,658 | |
| No. cases in non‐teaching hospitals | 153 | 42,789 | |
| Age, y | 63.9 15.0 | 67.3 15.7 | 0.06 |
| Male | 30.7 | 44.6 | <0.01 |
| Hospital mortality, % | 56.9 | 62.8 | 0.08 |
| Comorbidities | |||
| No. Charlson‐Deyo comorbidities | 2.5 2.0 | 2.5 2.0 | 0.89 |
| Coronary artery disease, % | 48.0 | 38.0 | <0.01 |
| Acute myocardial infarction, % | 42.6 | 28.9 | <0.01 |
| Congestive heart failure, % | 27.9 | 35.3 | 0.03 |
| Hypertension, % | 36.3 | 33.2 | 0.83 |
| Acute renal failure, % | 33.3 | 26.6 | 0.03 |
| Diabetes mellitus, % | 30.9 | 23.0 | <0.01 |
| Chronic obstructive pulmonary disease, % | 10.3 | 19.3 | <0.01 |
Figures 1 and 2 provide additional aggregate statistics on therapeutic hypothermia in California hospitals. Figure 1 plots the number of therapeutic hypothermia cases recorded in the administrative discharge registry between 1999 and 2008. Of the 204 total cases identified during this period, 178 (87.3%) were performed between 2006 and 2008. Figure 2 shows the distribution of TH cases across centers that performed therapeutic hypothermia (n = 47 hospitals, 11.3 % of all hospitals). Ten centers accounted for 124/204 (60.7%) of the total patients treated with TH after cardiac arrest; the top 3 centers accounted for 64 (31.4%) of the treated patients. Twenty‐seven hospitals were identified as performing therapeutic hypothermia on only 1 or 2 patients between 1999 and 2008.


Risk‐Adjusted Mortality
Table 2 presents the odds ratio of factors predicting in‐hospital mortality after cardiac arrest complicated by neurologic insult. Factors include use of TH after cardiac arrest, age, gender, year of admission, number of Charlson‐Deyo comorbidities, hospital teaching status, and volume tercile of hospitals that performed therapeutic hypothermia. Overall, patients who were older, male, and had greater comorbidities were statistically more likely to die after cardiac arrest complicated by neurologic insult. Regardless of whether they underwent TH, patients admitted to hospitals in the highest volume tercile of TH use were more likely to die after cardiac arrest. Adjusting for volume tercile, teaching hospital status was not independently associated with mortality after cardiac arrest. The adjusted odds ratio of mortality among patients undergoing therapeutic hypothermia was 0.80 (95% confidence interval [CI] 0.601.06, P = 0.11). The adjusted probability of inpatient mortality among patients undergoing therapeutic hypothermia was 57.5% (95% CI 50.764.3%) compared to those who did not 62.8% (95% CI 61.763.9%, P = 0.11).
| Variable | Odds Ratio of Hospital Mortality (95% CI) | P Value |
|---|---|---|
| ||
| No. observations | 46,651 | |
| Age* | ||
| 6569 | 1.19 (1.121.28) | <0.001 |
| 7074 | 1.29 (1.201.39) | <0.001 |
| 7579 | 1.55 (1.441.67) | <0.001 |
| 8084 | 1.79 (1.651.93) | <0.001 |
| 85 and over | 2.06 (1.892.25) | <0.001 |
| Male | 1.15 (1.101.21) | <0.001 |
| Teaching hospital | 1.13 (0.951.34) | 0.17 |
| No. Charlson‐Deyo comorbidities | 1.09 (1.081.10) | <0.001 |
| Year trend | 0.98 (0.970.99) | <0.001 |
| Volume tercile among hospitals performing TH | ||
| First tercile | 0.94 (0.791.12) | 0.48 |
| Second tercile | 1.03 (0.801.33) | 0.82 |
| Third tercile | 1.20 (1.051.36) | 0.006 |
| Therapeutic hypothermia | 0.80 (0.601.06) | 0.11 |
Figure 3 presents adjusted mortality after cardiac arrest in hospitals that did not perform TH, as well as adjusted mortality associated with TH for each volume tercile of hospitals that performed the procedure. Hospital mortality rates among patients not receiving TH after cardiac arrest were slightly higher in hospitals in the high volume tercile of TH (66.3%, 95% CI 63.868.8%) compared to hospitals in low and moderate volume terciles and to hospitals not performing TH (P < 0.001). Hospital mortality rates among low and moderate TH volume centers and in centers not performing TH were similar (62.3%, 61.3%, and 63.4%, respectively). Among both the low volume and moderate volume terciles, however, patients who underwent TH after cardiac arrest were significantly less likely to die in‐hospital compared to those who did not. For patients admitted to hospitals in the low volume tercile, those undergoing therapeutic hypothermia had an adjusted hospital mortality rate of 25.5% (95% CI 3.047.9%) compared to those who did not undergo TH (adjusted mortality 61.3%, 95% CI 57.465.1%), P < 0.001. In the moderate volume tercile, patients receiving therapeutic hypothermia had an adjusted hospital mortality rate of 31.0% (95% CI 9.2%52.8%) compared to 63.4% (95% CI 57.769.1%), P < 0.001, among those not undergoing the procedure. There was no statistically significant difference in adjusted mortality between those who underwent TH and those who did not, in hospitals in the highest volume tercile (P = 0.211). In addition to examining how volume of therapeutic hypothermia performed by hospitals affected the association between TH and hospital mortality, we also examined whether year of admission and teaching hospital independently modified the association. Neither year of admission nor teaching hospital statistically significantly affected the association between therapeutic hypothermia and hospital mortality after cardiac arrest at the P < 0.10 level.

DISCUSSION
In an administrative database of all admissions to California hospitals, we demonstrated that use of TH increased steadily since the publication of the initial clinical trials in 2002. The absolute level of TH utilization in our study undoubtedly represents a significant underestimation of actual TH utilization, however, our study does provide an assessment of the utilization trends over time. The bulk of TH use appears to be performed in a small group of high volume centers, and 89% of California hospitals did not perform TH during the study period (as assessed by procedure billing codes). Additionally, within the limitations of a retrospective, administrative claims‐based study design, TH appears to be associated with a similar in‐hospital mortality rate to that seen in clinical trials.3, 4 In exploratory analyses, there appears to be a particular benefit of TH in low and moderate volume centers, though these findings should be considered hypothesis‐generating.
Despite the body of evidence in favor of TH, utilization in our study and others appears quite low. In a 2005 survey of physicians, 87% of respondents had never used therapeutic hypothermia, citing inadequate data, technical limitations, and lack of incorporation in the Advanced Cardiac Life Support (ACLS) protocol as principal justifications.8 Other surveys have shown similar results and noted that critical care physicians and those working in large medical centers were more likely to adopt the therapy.9 Advocates of the therapy have suggested that an explicit hospital‐based plan developed by key stakeholders can help facilitate implementation.21 Accordingly, there is growing interest in developing centers of expertise in highly intensive therapies such as TH. For instance, the New York City Emergency Medical Service has begun to explore a protocol to divert TH candidates to specialized centers.22, 23 Some favorable results have been reported in individual hospitals and local hospital systems.2428
Our data suggest that TH is associated with an in‐hospital mortality rate that is comparable to that reported in the clinical trials. For example, in a 2009 meta‐analysis of 4 clinical trials and 1 abstract (481 patients in total), TH was associated with a 35% relative mortality benefit as compared to standard post‐resuscitation care.29 It has been estimated that broad TH implementation could save thousands of lives30 and many authors have advocated for its use and outlined explicit protocols for implementation.17 Furthermore, TH appears to be cost‐effective in line with other accepted therapies. Assuming the Hypothermia After Cardiac Arrest (HACA) trial inclusion criteria, even at extreme estimates for costs, the cost‐effectiveness of hypothermia remains less than $100,000 per quality‐adjusted life year.31
There are important limitations of this study. Our use of administrative claims data certainly underestimates the level of TH utilization, since we could only identify cases in which TH was included in the billing codes for the hospitalization. Hospitals may vary in utilization of this particular billing code for TH in ways that bias our estimated associations. The ICD‐9 code 99.81 for therapeutic hypothermia was also not developed for post‐cardiac arrest TH specifically, so use of the code may actually lag clinical utilization. Although the observed trend in TH utilization is likely mainly due to a true increase in utilization, it is possible that some of the observed increase is due to an increase in utilization of TH procedure billing codes. Our TH utilization estimates should be construed as a lower bound of the actual rates. Additionally, although the estimated real‐world mortality benefit of TH may be comparable to that of clinical trials, the equivalence of patients in our sample to those in published randomized trials is uncertain. Similarly, even after adjusting for age, gender, year of admission, comorbidities, hospital teaching status, and TH volume, there are likely many unmeasured variables that influence mortality in both the TH and comparison groups. There are also likely patients included in our comparison group who had both cardiac arrest or ventricular tachycardia and anoxic brain injury, but who were not candidates for TH as the episode of cardiac arrest followed rather than preceded the anoxic brain injury. Since we lack detailed clinical data about the TH cases (initial rhythm, time before return of circulation, preexisting disease states, etc.), we are unable to match controls directly to cases. Additionally, we lack data to assess neurologic recovery or quality of life after arrest.
The observation that a mortality benefit in our study could be detected only in low and moderate volume centers requires further exploration. Indeed, one might expect that high volume centers may have better outcomes with TH as a result of more robust infrastructure, technical experience, and available resources. Our finding that mortality benefits of TH appear concentrated in centers with low to moderate volume of TH utilization suggest at least 1 of 2 possibilities. First, low and moderate volume centers may perform TH in a subset of patients who benefit most from the intervention or, alternatively, in the most viable cardiac arrest cases (those who may fare well with or without the therapy). Consequently, we may observe relatively favorable outcomes in this group due to this selection bias. Second, high volume centersdespite having more expertisemay also attract patients at higher mortality risk due to referral bias. This would lead us to estimate lower mortality benefits associated with TH in these high volume centers. Indeed, greater observed mortality at high volume centers regardless of TH status suggests that overall acuity is higher at high volume centers. While our inferences are greatly affected by issues of case selection and referral bias, it also important to consider the possibility that the estimated mortality benefit of TH in higher volume centers is lower because of the selection of patients who do not meet current guidelines for treatment with TH. Distinguishing whether the selection of patients undergoing TH at high volume centers is appropriate or inappropriate based on current guidelines is an important issue that merits further research with datasets with more refined patient clinical information.
In summary, therapeutic hypothermia utilization is low, but the rate of implementation has increased since the publication of the initial clinical trials in 2002. The bulk of TH utilization appears limited to a subset of high volume centers, and most centers in California appear to have not used the therapy. Real‐world in‐hospital mortality associated with TH is comparable to that reported in randomized clinical trials.
Acknowledgements
Disclosures: Dr Romley received support from NIH grant R03AG031990‐A1. Dr Noseworthy received support from the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology granted by the Heart Rhythm Society. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources. The contents of this article have not been published in any other peer‐reviewed media, and the manuscript is not under review elsewhere. All authors listed have contributed sufficiently to this project to be included as authors. The authors have no conflict of interest, financial or otherwise.
There are over 350,000 cases of out‐of‐hospital cardiac arrest (OHCA) each year in the United States1, 2 and, with supportive therapy alone, only a fraction of victims survive to hospital discharge. Rapid intervention including cardiopulmonary resuscitation in the moments following arrest is critical to minimizing neurologic injury, morbidity, and mortality. In 2002, two small randomized controlled trials showed a survival benefit of therapeutic hypothermia (TH) when provided within 12 hours after return of circulation following an OHCA and, to date, TH remains one of the few interventions with proven mortality benefit after initial cardiopulmonary resuscitation.3, 4 Since 2003, TH has been incorporated into the American Heart Association practice guidelines57 and use of TH has steadily increased, but widespread clinical uptake remains low.8, 9
The initial studies that evaluated TH were small, with only 189 patients included in the TH arms of the 2 trials combined. To date, only a few studies have replicated this initial observation in real‐world settings, with little analysis of outcomes in US centers in particular.1013 Accordingly, we aimed to examine the real‐world experience with TH in the United States using a large administrative claims database of all California hospital admissions to describe utilization trends, hospital mortality, and volumeoutcome relationships associated with the intervention.
MATERIALS AND METHODS
Data
We identified all admissions to California hospitals during 19992008 based on discharge records from the California Office of Statewide Health Planning and Development. Our study period included cases of TH performed prior to the 2002 major clinical trials, since TH was in occasional use prior to the publication of these trials. The data was de‐identified and publicly available, and therefore exempt from review by the Institutional Review Board. In addition to hospital name, each discharge record included patient age, gender, admission year, International Classification of Disease, Ninth Revision (ICD‐9) code for presenting primary and secondary diagnoses, procedure codes, and disposition (discharge to home or rehabilitation, in‐hospital death). All California hospitals were included in the registry (n = 419). We defined teaching status for each hospital based on membership in the Council of Teaching Hospitals, as reported in the American Hospital Association's Annual Survey (n = 19 teaching hospitals).14
Setting and Participants
We used discharge diagnoses to identify patients who could be considered eligible for therapeutic hypothermia after cardiac arrest. We classified patients as eligible for therapeutic hypothermia after cardiac arrest based on ICD‐9 diagnosis codes that indicated the presence of both cardiac arrest and anoxic brain injury in the administrative diagnoses. Because of known imprecision in using billing codes to identify patients with cardiac arrest,15, 16 we broadly defined cardiac arrest to include those patients with ICD‐9 codes for cardiac arrest, ventricular fibrillation (VF), or ventricular tachycardia (VT) (see Supporting Table 1 in the online version of this article). We could not distinguish between out‐of‐hospital and in‐hospital cardiac arrest based on administrative diagnoses. To ensure that we included only patients with cardiac arrest complicated by neurologic insult, we required an ICD‐9 diagnosis of either anoxic brain injury, coma, or persistent vegetative state. Claims did not allow us to distinguish among initial cardiac arrest rhythms (VF vs pulseless VT vs asystole). Patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia were excluded.3, 17 We did not exclude patients based on coagulopathy (which is considered a contraindication to TH), since ICD‐9 coding did not allow us to determine the severity of the coagulopathy or whether it was a result of therapeutic hypothermia itself.
We used the ICD‐9 procedure code (99.81) for TH to first identify patients who underwent TH from 1999 to 2008. Since this code also applies to TH used during cardiac and neurosurgery, we examined each of these cases and excluded individuals who underwent cardiac surgery or neurosurgery during the hospitalization. As in our eligible for TH definition, we excluded patients younger than 18 years of age, and those who were pregnant, suffered traumatic brain injury, intracranial hemorrhage, metastatic cancer, or dementia. Patients who underwent therapeutic hypothermia but for whom a specific procedure code was not recorded in the discharge abstractperhaps because the medical institution did not directly bill for the procedurecould not be identified.
Statistical Analysis
We used a multivariable logistic model to estimate differences in hospital mortality after cardiac arrest associated with use of therapeutic hypothermia. We conducted 2 specifications. In our baseline specification, we accounted for case‐mix differences between those who underwent TH and those who did not by adjusting for age, gender, year of admission, the number of Charlson‐Deyo comorbidities,1820 and hospital teaching status. Because mortality after cardiac arrest in centers that perform therapeutic hypothermia may be different from centers that do not, even for patients who do not undergo the procedure, we included indicators for volume tercile of therapeutic hypothermia cases performed. Volume of therapeutic hypothermia was defined at the hospital level as the total number of cases performed by that hospital from 1999 to 2008.
In order to explore how hospital teaching status, volume of therapeutic hypothermia procedures (broken into terciles), and year of admission affected the association between hospital mortality after cardiac arrest and therapeutic hypothermia, our baseline logistic model was expanded to include interactions between therapeutic hypothermia and each of these variables. The interaction between therapeutic hypothermia and year explored whether the effectiveness of the procedure changed over time, as case‐selection, method of therapeutic hypothermia (cold saline vs commercially available devices), and experience changed in California hospitals. For both specifications, we reported the odds ratio of hospital mortality among patients undergoing therapeutic hypothermia, as well as risk‐adjusted mortality for both TH and non‐TH groups.
STATA version 11 (STATA Corp, College Station, TX) was used for statistical analyses, and a 2‐sided P 0.05 was used.
RESULTS
Descriptive Data
Table 1 reports summary statistics for patients with cardiac arrest complicated by neurologic insult (anoxic brain injury, coma, or persistent vegetative state) between 1999 and 2008. Across all years, 204 patients were identified as undergoing TH. In comparison, 105 patients were identified as undergoing TH in 2008 alone. Patients who underwent TH were less likely to be male (30.7% vs 44.6% male, P < 0.01), were younger (63.9 15.0 years vs 67.3 15.7 years, P = 0.03), and had equivalent numbers of Charlson‐Deyo comorbidities (2.5 2.0 diagnoses vs 2.5 2.0 diagnoses, P = 0.89). Therapeutic hypothermia was more commonly employed at teaching hospitals (51/3709 [1.4%] vs 153/42,942 [0.4%], P < 0.01). There was a trend toward decreased unadjusted mortality among patients who underwent therapeutic hypothermia compared with those who did not (56.9% vs 62.8%, P = 0.08).
| Therapeutic Hypothermia | No Therapeutic Hypothermia | P Value | |
|---|---|---|---|
| |||
| No. observations | 204 | 46,629 | |
| No. cases in teaching hospitals | 51 | 3,658 | |
| No. cases in non‐teaching hospitals | 153 | 42,789 | |
| Age, y | 63.9 15.0 | 67.3 15.7 | 0.06 |
| Male | 30.7 | 44.6 | <0.01 |
| Hospital mortality, % | 56.9 | 62.8 | 0.08 |
| Comorbidities | |||
| No. Charlson‐Deyo comorbidities | 2.5 2.0 | 2.5 2.0 | 0.89 |
| Coronary artery disease, % | 48.0 | 38.0 | <0.01 |
| Acute myocardial infarction, % | 42.6 | 28.9 | <0.01 |
| Congestive heart failure, % | 27.9 | 35.3 | 0.03 |
| Hypertension, % | 36.3 | 33.2 | 0.83 |
| Acute renal failure, % | 33.3 | 26.6 | 0.03 |
| Diabetes mellitus, % | 30.9 | 23.0 | <0.01 |
| Chronic obstructive pulmonary disease, % | 10.3 | 19.3 | <0.01 |
Figures 1 and 2 provide additional aggregate statistics on therapeutic hypothermia in California hospitals. Figure 1 plots the number of therapeutic hypothermia cases recorded in the administrative discharge registry between 1999 and 2008. Of the 204 total cases identified during this period, 178 (87.3%) were performed between 2006 and 2008. Figure 2 shows the distribution of TH cases across centers that performed therapeutic hypothermia (n = 47 hospitals, 11.3 % of all hospitals). Ten centers accounted for 124/204 (60.7%) of the total patients treated with TH after cardiac arrest; the top 3 centers accounted for 64 (31.4%) of the treated patients. Twenty‐seven hospitals were identified as performing therapeutic hypothermia on only 1 or 2 patients between 1999 and 2008.


Risk‐Adjusted Mortality
Table 2 presents the odds ratio of factors predicting in‐hospital mortality after cardiac arrest complicated by neurologic insult. Factors include use of TH after cardiac arrest, age, gender, year of admission, number of Charlson‐Deyo comorbidities, hospital teaching status, and volume tercile of hospitals that performed therapeutic hypothermia. Overall, patients who were older, male, and had greater comorbidities were statistically more likely to die after cardiac arrest complicated by neurologic insult. Regardless of whether they underwent TH, patients admitted to hospitals in the highest volume tercile of TH use were more likely to die after cardiac arrest. Adjusting for volume tercile, teaching hospital status was not independently associated with mortality after cardiac arrest. The adjusted odds ratio of mortality among patients undergoing therapeutic hypothermia was 0.80 (95% confidence interval [CI] 0.601.06, P = 0.11). The adjusted probability of inpatient mortality among patients undergoing therapeutic hypothermia was 57.5% (95% CI 50.764.3%) compared to those who did not 62.8% (95% CI 61.763.9%, P = 0.11).
| Variable | Odds Ratio of Hospital Mortality (95% CI) | P Value |
|---|---|---|
| ||
| No. observations | 46,651 | |
| Age* | ||
| 6569 | 1.19 (1.121.28) | <0.001 |
| 7074 | 1.29 (1.201.39) | <0.001 |
| 7579 | 1.55 (1.441.67) | <0.001 |
| 8084 | 1.79 (1.651.93) | <0.001 |
| 85 and over | 2.06 (1.892.25) | <0.001 |
| Male | 1.15 (1.101.21) | <0.001 |
| Teaching hospital | 1.13 (0.951.34) | 0.17 |
| No. Charlson‐Deyo comorbidities | 1.09 (1.081.10) | <0.001 |
| Year trend | 0.98 (0.970.99) | <0.001 |
| Volume tercile among hospitals performing TH | ||
| First tercile | 0.94 (0.791.12) | 0.48 |
| Second tercile | 1.03 (0.801.33) | 0.82 |
| Third tercile | 1.20 (1.051.36) | 0.006 |
| Therapeutic hypothermia | 0.80 (0.601.06) | 0.11 |
Figure 3 presents adjusted mortality after cardiac arrest in hospitals that did not perform TH, as well as adjusted mortality associated with TH for each volume tercile of hospitals that performed the procedure. Hospital mortality rates among patients not receiving TH after cardiac arrest were slightly higher in hospitals in the high volume tercile of TH (66.3%, 95% CI 63.868.8%) compared to hospitals in low and moderate volume terciles and to hospitals not performing TH (P < 0.001). Hospital mortality rates among low and moderate TH volume centers and in centers not performing TH were similar (62.3%, 61.3%, and 63.4%, respectively). Among both the low volume and moderate volume terciles, however, patients who underwent TH after cardiac arrest were significantly less likely to die in‐hospital compared to those who did not. For patients admitted to hospitals in the low volume tercile, those undergoing therapeutic hypothermia had an adjusted hospital mortality rate of 25.5% (95% CI 3.047.9%) compared to those who did not undergo TH (adjusted mortality 61.3%, 95% CI 57.465.1%), P < 0.001. In the moderate volume tercile, patients receiving therapeutic hypothermia had an adjusted hospital mortality rate of 31.0% (95% CI 9.2%52.8%) compared to 63.4% (95% CI 57.769.1%), P < 0.001, among those not undergoing the procedure. There was no statistically significant difference in adjusted mortality between those who underwent TH and those who did not, in hospitals in the highest volume tercile (P = 0.211). In addition to examining how volume of therapeutic hypothermia performed by hospitals affected the association between TH and hospital mortality, we also examined whether year of admission and teaching hospital independently modified the association. Neither year of admission nor teaching hospital statistically significantly affected the association between therapeutic hypothermia and hospital mortality after cardiac arrest at the P < 0.10 level.

DISCUSSION
In an administrative database of all admissions to California hospitals, we demonstrated that use of TH increased steadily since the publication of the initial clinical trials in 2002. The absolute level of TH utilization in our study undoubtedly represents a significant underestimation of actual TH utilization, however, our study does provide an assessment of the utilization trends over time. The bulk of TH use appears to be performed in a small group of high volume centers, and 89% of California hospitals did not perform TH during the study period (as assessed by procedure billing codes). Additionally, within the limitations of a retrospective, administrative claims‐based study design, TH appears to be associated with a similar in‐hospital mortality rate to that seen in clinical trials.3, 4 In exploratory analyses, there appears to be a particular benefit of TH in low and moderate volume centers, though these findings should be considered hypothesis‐generating.
Despite the body of evidence in favor of TH, utilization in our study and others appears quite low. In a 2005 survey of physicians, 87% of respondents had never used therapeutic hypothermia, citing inadequate data, technical limitations, and lack of incorporation in the Advanced Cardiac Life Support (ACLS) protocol as principal justifications.8 Other surveys have shown similar results and noted that critical care physicians and those working in large medical centers were more likely to adopt the therapy.9 Advocates of the therapy have suggested that an explicit hospital‐based plan developed by key stakeholders can help facilitate implementation.21 Accordingly, there is growing interest in developing centers of expertise in highly intensive therapies such as TH. For instance, the New York City Emergency Medical Service has begun to explore a protocol to divert TH candidates to specialized centers.22, 23 Some favorable results have been reported in individual hospitals and local hospital systems.2428
Our data suggest that TH is associated with an in‐hospital mortality rate that is comparable to that reported in the clinical trials. For example, in a 2009 meta‐analysis of 4 clinical trials and 1 abstract (481 patients in total), TH was associated with a 35% relative mortality benefit as compared to standard post‐resuscitation care.29 It has been estimated that broad TH implementation could save thousands of lives30 and many authors have advocated for its use and outlined explicit protocols for implementation.17 Furthermore, TH appears to be cost‐effective in line with other accepted therapies. Assuming the Hypothermia After Cardiac Arrest (HACA) trial inclusion criteria, even at extreme estimates for costs, the cost‐effectiveness of hypothermia remains less than $100,000 per quality‐adjusted life year.31
There are important limitations of this study. Our use of administrative claims data certainly underestimates the level of TH utilization, since we could only identify cases in which TH was included in the billing codes for the hospitalization. Hospitals may vary in utilization of this particular billing code for TH in ways that bias our estimated associations. The ICD‐9 code 99.81 for therapeutic hypothermia was also not developed for post‐cardiac arrest TH specifically, so use of the code may actually lag clinical utilization. Although the observed trend in TH utilization is likely mainly due to a true increase in utilization, it is possible that some of the observed increase is due to an increase in utilization of TH procedure billing codes. Our TH utilization estimates should be construed as a lower bound of the actual rates. Additionally, although the estimated real‐world mortality benefit of TH may be comparable to that of clinical trials, the equivalence of patients in our sample to those in published randomized trials is uncertain. Similarly, even after adjusting for age, gender, year of admission, comorbidities, hospital teaching status, and TH volume, there are likely many unmeasured variables that influence mortality in both the TH and comparison groups. There are also likely patients included in our comparison group who had both cardiac arrest or ventricular tachycardia and anoxic brain injury, but who were not candidates for TH as the episode of cardiac arrest followed rather than preceded the anoxic brain injury. Since we lack detailed clinical data about the TH cases (initial rhythm, time before return of circulation, preexisting disease states, etc.), we are unable to match controls directly to cases. Additionally, we lack data to assess neurologic recovery or quality of life after arrest.
The observation that a mortality benefit in our study could be detected only in low and moderate volume centers requires further exploration. Indeed, one might expect that high volume centers may have better outcomes with TH as a result of more robust infrastructure, technical experience, and available resources. Our finding that mortality benefits of TH appear concentrated in centers with low to moderate volume of TH utilization suggest at least 1 of 2 possibilities. First, low and moderate volume centers may perform TH in a subset of patients who benefit most from the intervention or, alternatively, in the most viable cardiac arrest cases (those who may fare well with or without the therapy). Consequently, we may observe relatively favorable outcomes in this group due to this selection bias. Second, high volume centersdespite having more expertisemay also attract patients at higher mortality risk due to referral bias. This would lead us to estimate lower mortality benefits associated with TH in these high volume centers. Indeed, greater observed mortality at high volume centers regardless of TH status suggests that overall acuity is higher at high volume centers. While our inferences are greatly affected by issues of case selection and referral bias, it also important to consider the possibility that the estimated mortality benefit of TH in higher volume centers is lower because of the selection of patients who do not meet current guidelines for treatment with TH. Distinguishing whether the selection of patients undergoing TH at high volume centers is appropriate or inappropriate based on current guidelines is an important issue that merits further research with datasets with more refined patient clinical information.
In summary, therapeutic hypothermia utilization is low, but the rate of implementation has increased since the publication of the initial clinical trials in 2002. The bulk of TH utilization appears limited to a subset of high volume centers, and most centers in California appear to have not used the therapy. Real‐world in‐hospital mortality associated with TH is comparable to that reported in randomized clinical trials.
Acknowledgements
Disclosures: Dr Romley received support from NIH grant R03AG031990‐A1. Dr Noseworthy received support from the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology granted by the Heart Rhythm Society. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources. The contents of this article have not been published in any other peer‐reviewed media, and the manuscript is not under review elsewhere. All authors listed have contributed sufficiently to this project to be included as authors. The authors have no conflict of interest, financial or otherwise.
- , , , et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163.
- , . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- , , , et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563.
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556.
- , , , et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 7.5: postresuscitation support. Circulation. 2005;112(suppl I):IV‐84–IV‐88.
- , , , et al. Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(suppl 1):e93–e174.
- , , , et al. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186.
- , , , et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940.
- , , , et al. Adverse events and their relation to mortality in out‐of‐hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med.39(1):57–64.
- , , , et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934.
- . Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–1047.
- , , , et al. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–214.
- American Hospital Association (AHA). American Hospital Association 2001 Annual Survey. Chicago, IL: Health Forum, LLC.
- , , . A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296.
- . Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604.
- . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–1264.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- , , , et al. Practical implementation of therapeutic hypothermia after cardiac arrest. Hosp Pract (Minneap). 2009;37(1):71–83.
- . Paramedics will employ new therapy in cardiac arrest cases. New York Times. August 3, 2010:A18.
- , , , et al. Resuscitation center designation: recommendations for emergency medical services practices. Prehosp Emerg Care. 2010;14(1):51–61.
- , , , et al. Implementation of a hospital‐wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol. 2010;9(4):216–220.
- , , , et al. Therapeutic hypothermia protocol in a community emergency department. West J Emerg Med. 2010;11(4):367–372.
- , , , et al. Implementation of a post‐cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out‐of‐hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366.
- , , , et al. Improved out‐of‐hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med. 2010;56(4):348–357.
- , , , et al. Community‐based application of mild therapeutic hypothermia for survivors of cardiac arrest. South Med J. 2010;103(4):295–300.
- , , , et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009(4):CD004128.
- , . Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22(4):711–728.
- , , , et al. Cost‐effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–428.
- , , , et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163.
- , . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- , , , et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563.
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556.
- , , , et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 7.5: postresuscitation support. Circulation. 2005;112(suppl I):IV‐84–IV‐88.
- , , , et al. Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(suppl 1):e93–e174.
- , , , et al. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186.
- , , , et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935–1940.
- , , , et al. Adverse events and their relation to mortality in out‐of‐hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med.39(1):57–64.
- , , , et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934.
- . Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–1047.
- , , , et al. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–214.
- American Hospital Association (AHA). American Hospital Association 2001 Annual Survey. Chicago, IL: Health Forum, LLC.
- , , . A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296.
- . Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604.
- . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–1264.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- , , , et al. Practical implementation of therapeutic hypothermia after cardiac arrest. Hosp Pract (Minneap). 2009;37(1):71–83.
- . Paramedics will employ new therapy in cardiac arrest cases. New York Times. August 3, 2010:A18.
- , , , et al. Resuscitation center designation: recommendations for emergency medical services practices. Prehosp Emerg Care. 2010;14(1):51–61.
- , , , et al. Implementation of a hospital‐wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol. 2010;9(4):216–220.
- , , , et al. Therapeutic hypothermia protocol in a community emergency department. West J Emerg Med. 2010;11(4):367–372.
- , , , et al. Implementation of a post‐cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out‐of‐hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366.
- , , , et al. Improved out‐of‐hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med. 2010;56(4):348–357.
- , , , et al. Community‐based application of mild therapeutic hypothermia for survivors of cardiac arrest. South Med J. 2010;103(4):295–300.
- , , , et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009(4):CD004128.
- , . Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22(4):711–728.
- , , , et al. Cost‐effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–428.
Copyright © 2012 Society of Hospital Medicine