User login
Caring for patients with autism spectrum disorder
Autism spectrum disorder (ASD) is an umbrella term used to describe lifelong neurodevelopmental disorders characterized by impairment in social interactions and communication coupled with restricted, repetitive patterns of behaviors or interests that appear to share a common developmental course.1 In this article, we examine psychiatric care of patients with ASD and the most common symptom clusters treated with pharmacotherapy: irritability, anxiety, and hyperactivity/inattention.
First step: Keep the diagnosis in mind
Prior to 2013, ASD was comprised of 3 separate disorders distinguished by language delay and overall severity: autistic disorder, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified.2 With the release of DSM-5 in 2013, these disorders were essentially collapsed into a single ASD.3 ASD prevalence is estimated to be 1 in 59 children,4 which represents a 20- to 30-fold increase since the 1960s.
In order to provide adequate psychiatric care for individuals with ASD, the first step is to remember the diagnosis; keep it in mind. This may be particularly important for clinicians who primarily care for adults, because such clinicians often receive limited training in disorders first manifesting in childhood and may not consider ASD in patients who have not been previously diagnosed. However, ASD diagnostic criteria have become broader, and public knowledge of the diagnosis has grown. DSM-5 acknowledges that although symptoms begin in early childhood, they may become more recognizable later in life with increasing social demand. The result is that many adults are likely undiagnosed. The estimated prevalence of ASD in adult psychiatric settings range from 1.5% to 4%.5-7 These patients have different treatment needs and unfortunately are often misdiagnosed with other psychiatric conditions.
A recent study in a state psychiatric facility found that 10% of patients in this setting met criteria for ASD.8 Almost all of those patients had been misdiagnosed with some form of schizophrenia, including one patient who had been previously diagnosed with autism by the father of autism himself, Leo Kanner, MD. Through the years, this patient’s autism diagnosis had fallen away, and at the time of the study, the patient carried a diagnosis of undifferentiated schizophrenia and was prescribed 8 psychotropic medications. The patient had repeatedly denied auditory or visual hallucinations; however, his stereotypies and odd behaviors were taken as evidence that he was responding to internal stimuli. This case highlights the importance of keeping the ASD diagnosis in mind when evaluating and treating patients.
Addressing 3 key symptom clusters
Even for patients with an established ASD diagnosis, comprehensive treatment is complex. It typically involves a multimodal approach that includes speech therapy, occupational therapy, applied behavioral analysis (ABA), and vocational training and support as well as management of associated medical conditions. Because medical comorbidities may play an important role in exacerbation of severe behaviors in ASD, often leading to acute behavioral regression and psychiatric admission, it is essential that they not be overlooked during evaluations.9,10
There are no effective pharmacologic treatments for the core social deficits seen in ASD. Novel pharmacotherapies to improve social impairment are in the early stages of research,11,12 but currently social impairment is best addressed through behavioral therapy and social skills training. Our role as psychiatrists is most often to treat co-occurring psychiatric symptoms so that individuals with ASD can fully participate in behavioral and school-based treatments that lead to improved social skills, activities of daily living, and quality of life. Three of the most common of these symptoms are irritability, anxiety, and hyperactivity/inattention.
Irritability
Irritability, marked by aggression, self-injury, and severe tantrums, causes serious distress for both patients and families, and this behavior cluster is the most frequently reported comorbid symptom in ASD.13-15 Nonpharmacologic treatment of irritability often involves ABA-based therapy and communication training.
Continued to: ABA includes an initial functional behavior assessment...
ABA includes an initial functional behavior assessment (FBA) of maladaptive behavior followed by the application of specific schedules of reinforcement for positive behavior. The FBA allows the therapist to determine what desirable consequences maintain a behavior. Without this knowledge, there is the risk of inadvertently rewarding a maladaptive behavior. For instance, if you are recommending a time-out for escape-motivated aggression, the result will likely be an increase rather than decrease in aggression.
Communication training teaches the patient to use communicative means to request a desired outcome to reduce inappropriate behaviors and improve independent functioning. Communication training can include speech therapy, teaching sign language, using picture exchange programs, or navigating communication devices. Consideration of nonpharmacologic management is vital in treatment planning. Continual inadvertent reward of behaviors will limit the effects of medications. Evidence suggests that pharmacotherapy is more effective when it occurs in the context of appropriate behavioral management techniques.16
Irritability has been the focus of significant pharmacotherapy research in ASD. Second-generation antipsychotics (SGAs) are first-line pharmacotherapy for severe irritability. Risperidone and aripiprazole are both FDA-approved for addressing irritability in youth with ASD. Their efficacy has been established in several large, placebo-controlled trials.17-23
Given issues with tolerability and cases refractory to the use of first-line agents,24 other SGAs are frequently used off-label for this indication with limited safety or efficacy data. Olanzapine demonstrated high response rates in early open-label studies,25,26 followed by efficacy over an 8-week double-blind placebo-controlled trial, although with significant weight gain.27 No other SGAs have been examined in double-blind placebo-controlled trials. Paliperidone demonstrated a particularly high response rate (84%) in a prospective open-label study of 25 adolescents and young adults with ASD.28 In a retrospective study of ziprasidone in 42 youth with ASD and irritability, we reported a response rate of 40%, which is lower than that seen for some other SGAs; however, ziprasidone can be an appealing option for patients for whom SGA-associated weight gain has been significant, because it is much more likely to be weight-neutral.29,30 Open-label studies with quetiapine in ASD have generally revealed only minimal efficacy for aggression,31,32 although sleep improvement may be more substantial.32 The safety and tolerability of lurasidone in treating irritability in youth with ASD has yet to be established.33 It is the only SGA with a published negative placebo-controlled trial in ASD.34 Use of SGAs may be limited by adverse effects, including weight gain, increased appetite, sedation, enuresis, and elevated prolactin. Monitoring of body mass index and metabolic profiles is indicated with all SGAs.
Haloperidol is the only first-generation antipsychotic with significant evidence (from multiple studies dating back to 1978) to support its use for ASD-associated irritability.35 However, due to the high incidence of dyskinesias and potential dystonias, use of haloperidol is reserved for severe treatment-refractory symptoms that have often not improved after multiple SGA trials.
Continued to: When severe self-inury and aggression fail to improve...
When severe self-injury and aggression fail to improve with multiple medication trials, the next steps include combination treatment with multiple antipsychotics,36 followed by clozapine, often as a last option.37 Research suggests that clozapine is effective and well-tolerated in ASD38-42; however, it has many potential severe adverse effects, including cardiomyopathy, lowered seizure threshold, severe constipation, weight gain, and agranulocytosis; due to risk of the latter, patients require regular blood draws for monitoring.
There is very little evidence to support the use of antiepileptic medications (AEDs) and mood stabilizers for irritability in ASD.43 Placebo-controlled trials have had mixed results. Some evidence suggests that AEDS may have more utility in individuals with ASD and abnormal EEGs without epilepsy44 or as an adjunct to SGA treatment.45 One study found that lithium may be beneficial for patients with ASD whose clinical presentation includes 2 or more mood symptoms.46
Anxiety
Anxiety is a significant issue for many individuals with ASD.47 Anxiety symptoms and disorders, including specific phobias, obsessive-compulsive disorder (OCD), social anxiety, and generalized anxiety disorder, are commonly seen in persons with ASD.48 Anxiety is often combined with restricted, repetitive behaviors (RBs) in ASD literature. Some evidence suggests that in individuals with ASD, sameness behaviors may limit sensory input and modulate anxiety.49 However, the core RBs symptom domain may not be related solely to anxiety, but rather represents deficits in executive processes that include cognitive flexibility and inhibitory control seen across multiple disorders with prominent RBs.50-54 Research indicates that anxiety is an independent and separable construct in ASD.55
Studies of treatments for both RBs and anxiety have focused primarily on selective serotonin reuptake inhibitors (SSRIs), hoping that the promising results for anxiety and OCD behaviors seen in neurotypical patients would translate to patients with ASD.56 Unfortunately, there is little evidence for effective pharmacologic management of ASD-associated anxiety.57 Large, randomized controlled trials (RCTs) are lacking. A Cochrane Database review of SSRIs for ASD58 examined 9 RCTs with a total of 320 patients. The authors concluded that there is no evidence to support the use of SSRIs for children with ASD, and limited evidence of utility in adults. Youth with ASD are particularly vulnerable to adverse effects from SSRIs, specifically impulsivity and agitation.57,59 However, SSRIs are among the most commonly prescribed medications for youth with ASD. Because there is limited evidence supporting SSRIs’ efficacy for this indication and issues with tolerability, there is significant concern for the overprescribing of SSRIs to patients with ASD. In comparison, there is some compelling evidence of efficacy for modified cognitive-behavioral therapy (CBT) for patients with high-functioning ASD. Seven RCTs have shown that CBT is superior to treatment as usual and waiting list control groups, with most effect sizes >0.8 and with no treatment-associated adverse effects.57
Risperidone has been shown to reduce RBs17,60 and anxiety17 in patients with ASD. In young children with co-occurring irritability, risperidone monotherapy is likely best to address both symptoms. When anxiety occurs in isolation and is severe, clinical experience suggests that SSRIs can be effective in a limited percentage of cases, though we recommend starting at low doses with frequent monitoring for activation and irritability. Treatment of anxiety is further complicated by the significant challenges presented by the diagnosis of true anxiety in the context of ASD.
Continued to: Hyperactivity and impulsivity
Hyperactivity and impulsivity
Hyperactivity and impulsivity are common among patients with ASD, with rates estimated from 41% to 78%.61 Hyperactivity and inattention are treated with a variety of medications. Research examining methylphenidate in ASD has demonstrated modest effects compared with placebo, though with frequent adverse effects, such as increased irritability and insomnia62,63 Other smaller studies have confirmed these results.64-66 One additional study found improvements not only in hyperactivity but also in joint attention and self-regulation of affective state following stimulant treatment.67 There is limited data on the efficacy and tolerability of amphetamine for treating hyperactivity and impulsivity in ASD. Stimulant medications often are avoided as the first-line treatment for hyperactivity because of concerns about increased irritability. Alpha-2 adrenergic receptor agonists often are used before stimulants because of their relatively benign adverse effect profile. Clonidine, guanfacine, and guanfacine ER all have demonstrated effectiveness in double-blind, placebo-controls trials in patients with ASD.68-70 In these trails, sedation was the most common adverse effect, although some studies have reported increased irritability with guanfacine.70,71
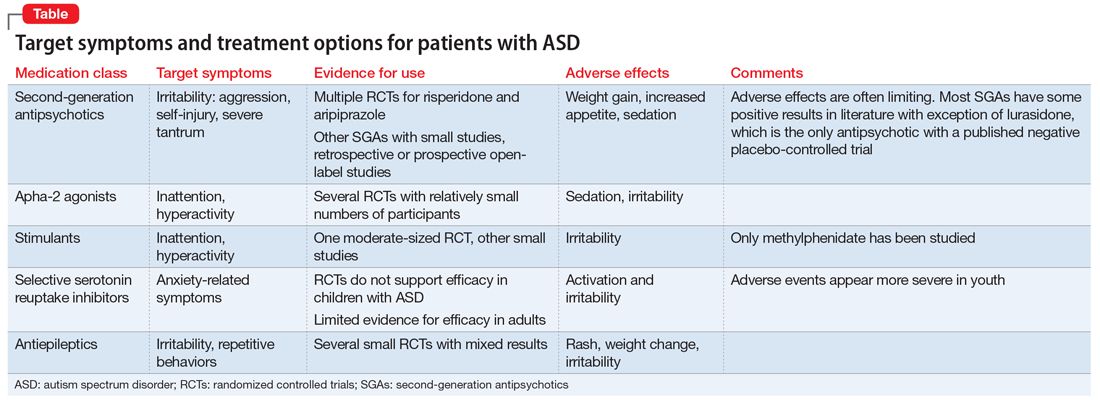
The Table provides a summary of the target symptoms and their treatment options for patients with ASD.
Improved diagnosis, but few evidence-based treatments
The rise in ASD cases observed over the past 20 years can be explained in part by a broader diagnostic algorithm and increased awareness. We are better at identifying ASD; however, there are still considerable gaps in identifying ASD in high-functioning patients and adults. One percent of the population has ASD,72,73 and this group is overrepresented in psychiatric clinic and hospital settings.74 Therefore, we must be aware of and understand the diagnosis.
Medication treatments are often less effective and less tolerable in patients with ASD than in patients without neurodevelopmental disability. There are differences in pharmacotherapy response and tolerability across development in ASD and limited evidence to guide prescribing in adults with ASD. SGAs appear to be effective across multiple symptom domains, but carry the risk of significant adverse effects. For anxiety and irritability, there is compelling evidence supporting the use of nonpharmacologic treatments.
Bottom Line
A subset of patients seen in psychiatry will have undiagnosed autism spectrum disorder (ASD). When evaluating worsening behaviors, first rule out organic causes. Second-generation antipsychotics have the most evidence for efficacy in ASD across multiple symptom domains. To sustain improvement in symptoms, it is vital to incorporate nonpharmacologic treatments.
Related Resources
- National Institute of Mental Health. Autism spectrum disorder. https://www.nimh.nih.gov/health/publications/autismspectrum-disorder/index.shtml.
- Centers for Disease Control and Prevention. Autism spectrum disorder (ASD). https://www.cdc.gov/ncbddd/ autism/index.html.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Guanfacine • Tenex
Guanfacine Extended Release • Intuniv
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Volkmar FR, Lord C, Bailey A, et al. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135-170.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1-23.
5. Scragg P, Shah A. Prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165(5):679-682.
6. Hare DJ, Gould J, Mills R, et al. A preliminary study of individuals with autistic spectrum disorders in three special hospitals in England. London, UK: National Autistic Society; 1999.
7. Shah A, Holmes N, Wing L. Prevalence of autism and related conditions in adults in a mental handicap hospital. Appl Res Ment Retard. 1982;3(3):303-317.
8. Mandell DS, Lawer LJ, Branch K, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism. 2012;16(6):557-567.
9. Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Devel Disabil. 2015;38:242-255.
10. Perisse D, Amiet C, Consoli A, et al. Risk factors of acute behavioral regression in psychiatrically hospitalized adolescents with autism. J Can Acad Child Adolesc Psychiatry. 2010;19(2):100-108.
11. Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61.
12. Wink LK, Plawecki MH, Erickson CA, et al. Emerging drugs for the treatment of symptoms associated with autism spectrum disorders. Expert Opin Emerg Drugs. 2010;15(3):481-494.
13. Fitzpatrick SE, Srivorakiat L, Wink LK, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525-1538.
14. Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Disabil Res. 2006;50(pt 3):172-183.
15. Mills R, Wing L. Researching interventions in ASD and priorities for research: surveying the membership of the NAS. London, UK: National Autistic Society; 2005.
16. Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143-1154.
17. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633-641.
18. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361-1369.
19. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634-e641.
20. Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600-620.
21. Benton TD. Aripiprazole to treat irritability associated with autism: a placebo-controlled, fixed-dose trial. Curr Psychiatry Rep. 2011;13(2):77-79.
22. Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110-1119.
23. Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533-1540.
24. Adler BA, Wink LK, Early M, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102-106.
25. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887-894.
26. Potenza MN, Holmes JP, Kanes SJ, et al. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol. 1999;19(1):37-44.
27. Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541-548.
28. Stigler KA, Erickson CA, Mullett JE, et al. Paliperidone for irritability in autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):75-78.
29. Dominick K, Wink LK, McDougle CJ, et al. A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2015;25(5):397-401.
30. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
31. Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(2):287-294.
32. Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216-219.
33. McClellan L, Dominick KC, Pedapati EV, et al. Lurasidone for the treatment of irritability and anger in autism spectrum disorders. Expert Opin Investig Drugs. 2017;26(8):985-989.
34. Loebel A, Brams M, Goldman RS, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016;46(4):1153-1163.
35. Campbell M, Anderson LT, Meier M, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640-655.
36. Wink LK, Pedapati EV, Horn PS, et al. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91-94.
37. Wink LK, Badran I, Pedapati EV, et al. Clozapine for drug-refractory irritability in individuals with developmental disability. J Child Adolesc Psychopharmacol. 2016;26(9):843-846.
38. Chen NC, Bedair HS, McKay B, et al. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry. 2001;62(6):479-480.
39. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psychiatry Neurosci. 2001;26(4):340-341.
40. Lambrey S, Falissard B, Martin-Barrero M, et al. Effectiveness of clozapine for the treatment of aggression in an adolescent with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):79-80.
41. Yalcin O, Kaymak G, Erdogan A, et al. a retrospective investigation of clozapine treatment in autistic and nonautistic children and adolescents in an inpatient clinic in Turkey. J Child Adolesc Psychopharmacol. 2016;26(9):815-821.
42. Beherec L, Lambrey S, Quilici G, et al. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341-344.
43. Hirota T, Veenstra-Vanderweele J, Hollander E, et al, Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
44. Hollander E, Chaplin W, Soorya L, et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology. 2010;35(4):990-998.
45. Rezaei V, Mohammadi MR, Ghanizadeh A, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269-1272.
46. Siegel M, Beresford CA, Bunker M, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24(7):399-402.
47. Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631-648,vii.
48. van Steensel FJ, Deutschman AA, Bogels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism. 2013;17(6):681-692.
49. Lidstone J, Uljarevic M, Sullivan J, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8(2):82-92.
50. Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839-849.
51. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65(3):185-236.
52. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1-3):15-23.
53. Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20(1):58-65.
54. Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46(12):1327-1336.
55. Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(9):2135-2146.
56. Wink LK, Erickson CA, Stigler KA, et al. Riluzole in autistic disorder. J Child Adolesc Psychopharmacol. 2011;21(4):375-379.
57. Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215-3229.
58. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677.
59. Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12(6):529-538.
60. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148.
61. Murray MJ, Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382-388.
62. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266-1274.
63. Posey DJ, Aman MG, McCracken JT, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry. 2007;61(4):538-544.
64. Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30(5):451-459.
65. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30(3):245-255.
66. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25(3):283-294.
67. Jahromi LB, Kasari CL, McCracken JT, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39(3):395-404.
68. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77-82.
69. Scahill L, McCracken JT, King BH, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206.
70. Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29(4):303-308.
71. Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589-598.
72. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21.
73. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459-465.
74. Mandell DS, Psychiatric hospitalization among children with autism spectrum disorders. J Autism Dev Disord. 2008;38(6):1059-1065.
Autism spectrum disorder (ASD) is an umbrella term used to describe lifelong neurodevelopmental disorders characterized by impairment in social interactions and communication coupled with restricted, repetitive patterns of behaviors or interests that appear to share a common developmental course.1 In this article, we examine psychiatric care of patients with ASD and the most common symptom clusters treated with pharmacotherapy: irritability, anxiety, and hyperactivity/inattention.
First step: Keep the diagnosis in mind
Prior to 2013, ASD was comprised of 3 separate disorders distinguished by language delay and overall severity: autistic disorder, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified.2 With the release of DSM-5 in 2013, these disorders were essentially collapsed into a single ASD.3 ASD prevalence is estimated to be 1 in 59 children,4 which represents a 20- to 30-fold increase since the 1960s.
In order to provide adequate psychiatric care for individuals with ASD, the first step is to remember the diagnosis; keep it in mind. This may be particularly important for clinicians who primarily care for adults, because such clinicians often receive limited training in disorders first manifesting in childhood and may not consider ASD in patients who have not been previously diagnosed. However, ASD diagnostic criteria have become broader, and public knowledge of the diagnosis has grown. DSM-5 acknowledges that although symptoms begin in early childhood, they may become more recognizable later in life with increasing social demand. The result is that many adults are likely undiagnosed. The estimated prevalence of ASD in adult psychiatric settings range from 1.5% to 4%.5-7 These patients have different treatment needs and unfortunately are often misdiagnosed with other psychiatric conditions.
A recent study in a state psychiatric facility found that 10% of patients in this setting met criteria for ASD.8 Almost all of those patients had been misdiagnosed with some form of schizophrenia, including one patient who had been previously diagnosed with autism by the father of autism himself, Leo Kanner, MD. Through the years, this patient’s autism diagnosis had fallen away, and at the time of the study, the patient carried a diagnosis of undifferentiated schizophrenia and was prescribed 8 psychotropic medications. The patient had repeatedly denied auditory or visual hallucinations; however, his stereotypies and odd behaviors were taken as evidence that he was responding to internal stimuli. This case highlights the importance of keeping the ASD diagnosis in mind when evaluating and treating patients.
Addressing 3 key symptom clusters
Even for patients with an established ASD diagnosis, comprehensive treatment is complex. It typically involves a multimodal approach that includes speech therapy, occupational therapy, applied behavioral analysis (ABA), and vocational training and support as well as management of associated medical conditions. Because medical comorbidities may play an important role in exacerbation of severe behaviors in ASD, often leading to acute behavioral regression and psychiatric admission, it is essential that they not be overlooked during evaluations.9,10
There are no effective pharmacologic treatments for the core social deficits seen in ASD. Novel pharmacotherapies to improve social impairment are in the early stages of research,11,12 but currently social impairment is best addressed through behavioral therapy and social skills training. Our role as psychiatrists is most often to treat co-occurring psychiatric symptoms so that individuals with ASD can fully participate in behavioral and school-based treatments that lead to improved social skills, activities of daily living, and quality of life. Three of the most common of these symptoms are irritability, anxiety, and hyperactivity/inattention.
Irritability
Irritability, marked by aggression, self-injury, and severe tantrums, causes serious distress for both patients and families, and this behavior cluster is the most frequently reported comorbid symptom in ASD.13-15 Nonpharmacologic treatment of irritability often involves ABA-based therapy and communication training.
Continued to: ABA includes an initial functional behavior assessment...
ABA includes an initial functional behavior assessment (FBA) of maladaptive behavior followed by the application of specific schedules of reinforcement for positive behavior. The FBA allows the therapist to determine what desirable consequences maintain a behavior. Without this knowledge, there is the risk of inadvertently rewarding a maladaptive behavior. For instance, if you are recommending a time-out for escape-motivated aggression, the result will likely be an increase rather than decrease in aggression.
Communication training teaches the patient to use communicative means to request a desired outcome to reduce inappropriate behaviors and improve independent functioning. Communication training can include speech therapy, teaching sign language, using picture exchange programs, or navigating communication devices. Consideration of nonpharmacologic management is vital in treatment planning. Continual inadvertent reward of behaviors will limit the effects of medications. Evidence suggests that pharmacotherapy is more effective when it occurs in the context of appropriate behavioral management techniques.16
Irritability has been the focus of significant pharmacotherapy research in ASD. Second-generation antipsychotics (SGAs) are first-line pharmacotherapy for severe irritability. Risperidone and aripiprazole are both FDA-approved for addressing irritability in youth with ASD. Their efficacy has been established in several large, placebo-controlled trials.17-23
Given issues with tolerability and cases refractory to the use of first-line agents,24 other SGAs are frequently used off-label for this indication with limited safety or efficacy data. Olanzapine demonstrated high response rates in early open-label studies,25,26 followed by efficacy over an 8-week double-blind placebo-controlled trial, although with significant weight gain.27 No other SGAs have been examined in double-blind placebo-controlled trials. Paliperidone demonstrated a particularly high response rate (84%) in a prospective open-label study of 25 adolescents and young adults with ASD.28 In a retrospective study of ziprasidone in 42 youth with ASD and irritability, we reported a response rate of 40%, which is lower than that seen for some other SGAs; however, ziprasidone can be an appealing option for patients for whom SGA-associated weight gain has been significant, because it is much more likely to be weight-neutral.29,30 Open-label studies with quetiapine in ASD have generally revealed only minimal efficacy for aggression,31,32 although sleep improvement may be more substantial.32 The safety and tolerability of lurasidone in treating irritability in youth with ASD has yet to be established.33 It is the only SGA with a published negative placebo-controlled trial in ASD.34 Use of SGAs may be limited by adverse effects, including weight gain, increased appetite, sedation, enuresis, and elevated prolactin. Monitoring of body mass index and metabolic profiles is indicated with all SGAs.
Haloperidol is the only first-generation antipsychotic with significant evidence (from multiple studies dating back to 1978) to support its use for ASD-associated irritability.35 However, due to the high incidence of dyskinesias and potential dystonias, use of haloperidol is reserved for severe treatment-refractory symptoms that have often not improved after multiple SGA trials.
Continued to: When severe self-inury and aggression fail to improve...
When severe self-injury and aggression fail to improve with multiple medication trials, the next steps include combination treatment with multiple antipsychotics,36 followed by clozapine, often as a last option.37 Research suggests that clozapine is effective and well-tolerated in ASD38-42; however, it has many potential severe adverse effects, including cardiomyopathy, lowered seizure threshold, severe constipation, weight gain, and agranulocytosis; due to risk of the latter, patients require regular blood draws for monitoring.
There is very little evidence to support the use of antiepileptic medications (AEDs) and mood stabilizers for irritability in ASD.43 Placebo-controlled trials have had mixed results. Some evidence suggests that AEDS may have more utility in individuals with ASD and abnormal EEGs without epilepsy44 or as an adjunct to SGA treatment.45 One study found that lithium may be beneficial for patients with ASD whose clinical presentation includes 2 or more mood symptoms.46
Anxiety
Anxiety is a significant issue for many individuals with ASD.47 Anxiety symptoms and disorders, including specific phobias, obsessive-compulsive disorder (OCD), social anxiety, and generalized anxiety disorder, are commonly seen in persons with ASD.48 Anxiety is often combined with restricted, repetitive behaviors (RBs) in ASD literature. Some evidence suggests that in individuals with ASD, sameness behaviors may limit sensory input and modulate anxiety.49 However, the core RBs symptom domain may not be related solely to anxiety, but rather represents deficits in executive processes that include cognitive flexibility and inhibitory control seen across multiple disorders with prominent RBs.50-54 Research indicates that anxiety is an independent and separable construct in ASD.55
Studies of treatments for both RBs and anxiety have focused primarily on selective serotonin reuptake inhibitors (SSRIs), hoping that the promising results for anxiety and OCD behaviors seen in neurotypical patients would translate to patients with ASD.56 Unfortunately, there is little evidence for effective pharmacologic management of ASD-associated anxiety.57 Large, randomized controlled trials (RCTs) are lacking. A Cochrane Database review of SSRIs for ASD58 examined 9 RCTs with a total of 320 patients. The authors concluded that there is no evidence to support the use of SSRIs for children with ASD, and limited evidence of utility in adults. Youth with ASD are particularly vulnerable to adverse effects from SSRIs, specifically impulsivity and agitation.57,59 However, SSRIs are among the most commonly prescribed medications for youth with ASD. Because there is limited evidence supporting SSRIs’ efficacy for this indication and issues with tolerability, there is significant concern for the overprescribing of SSRIs to patients with ASD. In comparison, there is some compelling evidence of efficacy for modified cognitive-behavioral therapy (CBT) for patients with high-functioning ASD. Seven RCTs have shown that CBT is superior to treatment as usual and waiting list control groups, with most effect sizes >0.8 and with no treatment-associated adverse effects.57
Risperidone has been shown to reduce RBs17,60 and anxiety17 in patients with ASD. In young children with co-occurring irritability, risperidone monotherapy is likely best to address both symptoms. When anxiety occurs in isolation and is severe, clinical experience suggests that SSRIs can be effective in a limited percentage of cases, though we recommend starting at low doses with frequent monitoring for activation and irritability. Treatment of anxiety is further complicated by the significant challenges presented by the diagnosis of true anxiety in the context of ASD.
Continued to: Hyperactivity and impulsivity
Hyperactivity and impulsivity
Hyperactivity and impulsivity are common among patients with ASD, with rates estimated from 41% to 78%.61 Hyperactivity and inattention are treated with a variety of medications. Research examining methylphenidate in ASD has demonstrated modest effects compared with placebo, though with frequent adverse effects, such as increased irritability and insomnia62,63 Other smaller studies have confirmed these results.64-66 One additional study found improvements not only in hyperactivity but also in joint attention and self-regulation of affective state following stimulant treatment.67 There is limited data on the efficacy and tolerability of amphetamine for treating hyperactivity and impulsivity in ASD. Stimulant medications often are avoided as the first-line treatment for hyperactivity because of concerns about increased irritability. Alpha-2 adrenergic receptor agonists often are used before stimulants because of their relatively benign adverse effect profile. Clonidine, guanfacine, and guanfacine ER all have demonstrated effectiveness in double-blind, placebo-controls trials in patients with ASD.68-70 In these trails, sedation was the most common adverse effect, although some studies have reported increased irritability with guanfacine.70,71
The Table provides a summary of the target symptoms and their treatment options for patients with ASD.
Improved diagnosis, but few evidence-based treatments
The rise in ASD cases observed over the past 20 years can be explained in part by a broader diagnostic algorithm and increased awareness. We are better at identifying ASD; however, there are still considerable gaps in identifying ASD in high-functioning patients and adults. One percent of the population has ASD,72,73 and this group is overrepresented in psychiatric clinic and hospital settings.74 Therefore, we must be aware of and understand the diagnosis.
Medication treatments are often less effective and less tolerable in patients with ASD than in patients without neurodevelopmental disability. There are differences in pharmacotherapy response and tolerability across development in ASD and limited evidence to guide prescribing in adults with ASD. SGAs appear to be effective across multiple symptom domains, but carry the risk of significant adverse effects. For anxiety and irritability, there is compelling evidence supporting the use of nonpharmacologic treatments.
Bottom Line
A subset of patients seen in psychiatry will have undiagnosed autism spectrum disorder (ASD). When evaluating worsening behaviors, first rule out organic causes. Second-generation antipsychotics have the most evidence for efficacy in ASD across multiple symptom domains. To sustain improvement in symptoms, it is vital to incorporate nonpharmacologic treatments.
Related Resources
- National Institute of Mental Health. Autism spectrum disorder. https://www.nimh.nih.gov/health/publications/autismspectrum-disorder/index.shtml.
- Centers for Disease Control and Prevention. Autism spectrum disorder (ASD). https://www.cdc.gov/ncbddd/ autism/index.html.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Guanfacine • Tenex
Guanfacine Extended Release • Intuniv
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
Autism spectrum disorder (ASD) is an umbrella term used to describe lifelong neurodevelopmental disorders characterized by impairment in social interactions and communication coupled with restricted, repetitive patterns of behaviors or interests that appear to share a common developmental course.1 In this article, we examine psychiatric care of patients with ASD and the most common symptom clusters treated with pharmacotherapy: irritability, anxiety, and hyperactivity/inattention.
First step: Keep the diagnosis in mind
Prior to 2013, ASD was comprised of 3 separate disorders distinguished by language delay and overall severity: autistic disorder, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified.2 With the release of DSM-5 in 2013, these disorders were essentially collapsed into a single ASD.3 ASD prevalence is estimated to be 1 in 59 children,4 which represents a 20- to 30-fold increase since the 1960s.
In order to provide adequate psychiatric care for individuals with ASD, the first step is to remember the diagnosis; keep it in mind. This may be particularly important for clinicians who primarily care for adults, because such clinicians often receive limited training in disorders first manifesting in childhood and may not consider ASD in patients who have not been previously diagnosed. However, ASD diagnostic criteria have become broader, and public knowledge of the diagnosis has grown. DSM-5 acknowledges that although symptoms begin in early childhood, they may become more recognizable later in life with increasing social demand. The result is that many adults are likely undiagnosed. The estimated prevalence of ASD in adult psychiatric settings range from 1.5% to 4%.5-7 These patients have different treatment needs and unfortunately are often misdiagnosed with other psychiatric conditions.
A recent study in a state psychiatric facility found that 10% of patients in this setting met criteria for ASD.8 Almost all of those patients had been misdiagnosed with some form of schizophrenia, including one patient who had been previously diagnosed with autism by the father of autism himself, Leo Kanner, MD. Through the years, this patient’s autism diagnosis had fallen away, and at the time of the study, the patient carried a diagnosis of undifferentiated schizophrenia and was prescribed 8 psychotropic medications. The patient had repeatedly denied auditory or visual hallucinations; however, his stereotypies and odd behaviors were taken as evidence that he was responding to internal stimuli. This case highlights the importance of keeping the ASD diagnosis in mind when evaluating and treating patients.
Addressing 3 key symptom clusters
Even for patients with an established ASD diagnosis, comprehensive treatment is complex. It typically involves a multimodal approach that includes speech therapy, occupational therapy, applied behavioral analysis (ABA), and vocational training and support as well as management of associated medical conditions. Because medical comorbidities may play an important role in exacerbation of severe behaviors in ASD, often leading to acute behavioral regression and psychiatric admission, it is essential that they not be overlooked during evaluations.9,10
There are no effective pharmacologic treatments for the core social deficits seen in ASD. Novel pharmacotherapies to improve social impairment are in the early stages of research,11,12 but currently social impairment is best addressed through behavioral therapy and social skills training. Our role as psychiatrists is most often to treat co-occurring psychiatric symptoms so that individuals with ASD can fully participate in behavioral and school-based treatments that lead to improved social skills, activities of daily living, and quality of life. Three of the most common of these symptoms are irritability, anxiety, and hyperactivity/inattention.
Irritability
Irritability, marked by aggression, self-injury, and severe tantrums, causes serious distress for both patients and families, and this behavior cluster is the most frequently reported comorbid symptom in ASD.13-15 Nonpharmacologic treatment of irritability often involves ABA-based therapy and communication training.
Continued to: ABA includes an initial functional behavior assessment...
ABA includes an initial functional behavior assessment (FBA) of maladaptive behavior followed by the application of specific schedules of reinforcement for positive behavior. The FBA allows the therapist to determine what desirable consequences maintain a behavior. Without this knowledge, there is the risk of inadvertently rewarding a maladaptive behavior. For instance, if you are recommending a time-out for escape-motivated aggression, the result will likely be an increase rather than decrease in aggression.
Communication training teaches the patient to use communicative means to request a desired outcome to reduce inappropriate behaviors and improve independent functioning. Communication training can include speech therapy, teaching sign language, using picture exchange programs, or navigating communication devices. Consideration of nonpharmacologic management is vital in treatment planning. Continual inadvertent reward of behaviors will limit the effects of medications. Evidence suggests that pharmacotherapy is more effective when it occurs in the context of appropriate behavioral management techniques.16
Irritability has been the focus of significant pharmacotherapy research in ASD. Second-generation antipsychotics (SGAs) are first-line pharmacotherapy for severe irritability. Risperidone and aripiprazole are both FDA-approved for addressing irritability in youth with ASD. Their efficacy has been established in several large, placebo-controlled trials.17-23
Given issues with tolerability and cases refractory to the use of first-line agents,24 other SGAs are frequently used off-label for this indication with limited safety or efficacy data. Olanzapine demonstrated high response rates in early open-label studies,25,26 followed by efficacy over an 8-week double-blind placebo-controlled trial, although with significant weight gain.27 No other SGAs have been examined in double-blind placebo-controlled trials. Paliperidone demonstrated a particularly high response rate (84%) in a prospective open-label study of 25 adolescents and young adults with ASD.28 In a retrospective study of ziprasidone in 42 youth with ASD and irritability, we reported a response rate of 40%, which is lower than that seen for some other SGAs; however, ziprasidone can be an appealing option for patients for whom SGA-associated weight gain has been significant, because it is much more likely to be weight-neutral.29,30 Open-label studies with quetiapine in ASD have generally revealed only minimal efficacy for aggression,31,32 although sleep improvement may be more substantial.32 The safety and tolerability of lurasidone in treating irritability in youth with ASD has yet to be established.33 It is the only SGA with a published negative placebo-controlled trial in ASD.34 Use of SGAs may be limited by adverse effects, including weight gain, increased appetite, sedation, enuresis, and elevated prolactin. Monitoring of body mass index and metabolic profiles is indicated with all SGAs.
Haloperidol is the only first-generation antipsychotic with significant evidence (from multiple studies dating back to 1978) to support its use for ASD-associated irritability.35 However, due to the high incidence of dyskinesias and potential dystonias, use of haloperidol is reserved for severe treatment-refractory symptoms that have often not improved after multiple SGA trials.
Continued to: When severe self-inury and aggression fail to improve...
When severe self-injury and aggression fail to improve with multiple medication trials, the next steps include combination treatment with multiple antipsychotics,36 followed by clozapine, often as a last option.37 Research suggests that clozapine is effective and well-tolerated in ASD38-42; however, it has many potential severe adverse effects, including cardiomyopathy, lowered seizure threshold, severe constipation, weight gain, and agranulocytosis; due to risk of the latter, patients require regular blood draws for monitoring.
There is very little evidence to support the use of antiepileptic medications (AEDs) and mood stabilizers for irritability in ASD.43 Placebo-controlled trials have had mixed results. Some evidence suggests that AEDS may have more utility in individuals with ASD and abnormal EEGs without epilepsy44 or as an adjunct to SGA treatment.45 One study found that lithium may be beneficial for patients with ASD whose clinical presentation includes 2 or more mood symptoms.46
Anxiety
Anxiety is a significant issue for many individuals with ASD.47 Anxiety symptoms and disorders, including specific phobias, obsessive-compulsive disorder (OCD), social anxiety, and generalized anxiety disorder, are commonly seen in persons with ASD.48 Anxiety is often combined with restricted, repetitive behaviors (RBs) in ASD literature. Some evidence suggests that in individuals with ASD, sameness behaviors may limit sensory input and modulate anxiety.49 However, the core RBs symptom domain may not be related solely to anxiety, but rather represents deficits in executive processes that include cognitive flexibility and inhibitory control seen across multiple disorders with prominent RBs.50-54 Research indicates that anxiety is an independent and separable construct in ASD.55
Studies of treatments for both RBs and anxiety have focused primarily on selective serotonin reuptake inhibitors (SSRIs), hoping that the promising results for anxiety and OCD behaviors seen in neurotypical patients would translate to patients with ASD.56 Unfortunately, there is little evidence for effective pharmacologic management of ASD-associated anxiety.57 Large, randomized controlled trials (RCTs) are lacking. A Cochrane Database review of SSRIs for ASD58 examined 9 RCTs with a total of 320 patients. The authors concluded that there is no evidence to support the use of SSRIs for children with ASD, and limited evidence of utility in adults. Youth with ASD are particularly vulnerable to adverse effects from SSRIs, specifically impulsivity and agitation.57,59 However, SSRIs are among the most commonly prescribed medications for youth with ASD. Because there is limited evidence supporting SSRIs’ efficacy for this indication and issues with tolerability, there is significant concern for the overprescribing of SSRIs to patients with ASD. In comparison, there is some compelling evidence of efficacy for modified cognitive-behavioral therapy (CBT) for patients with high-functioning ASD. Seven RCTs have shown that CBT is superior to treatment as usual and waiting list control groups, with most effect sizes >0.8 and with no treatment-associated adverse effects.57
Risperidone has been shown to reduce RBs17,60 and anxiety17 in patients with ASD. In young children with co-occurring irritability, risperidone monotherapy is likely best to address both symptoms. When anxiety occurs in isolation and is severe, clinical experience suggests that SSRIs can be effective in a limited percentage of cases, though we recommend starting at low doses with frequent monitoring for activation and irritability. Treatment of anxiety is further complicated by the significant challenges presented by the diagnosis of true anxiety in the context of ASD.
Continued to: Hyperactivity and impulsivity
Hyperactivity and impulsivity
Hyperactivity and impulsivity are common among patients with ASD, with rates estimated from 41% to 78%.61 Hyperactivity and inattention are treated with a variety of medications. Research examining methylphenidate in ASD has demonstrated modest effects compared with placebo, though with frequent adverse effects, such as increased irritability and insomnia62,63 Other smaller studies have confirmed these results.64-66 One additional study found improvements not only in hyperactivity but also in joint attention and self-regulation of affective state following stimulant treatment.67 There is limited data on the efficacy and tolerability of amphetamine for treating hyperactivity and impulsivity in ASD. Stimulant medications often are avoided as the first-line treatment for hyperactivity because of concerns about increased irritability. Alpha-2 adrenergic receptor agonists often are used before stimulants because of their relatively benign adverse effect profile. Clonidine, guanfacine, and guanfacine ER all have demonstrated effectiveness in double-blind, placebo-controls trials in patients with ASD.68-70 In these trails, sedation was the most common adverse effect, although some studies have reported increased irritability with guanfacine.70,71
The Table provides a summary of the target symptoms and their treatment options for patients with ASD.
Improved diagnosis, but few evidence-based treatments
The rise in ASD cases observed over the past 20 years can be explained in part by a broader diagnostic algorithm and increased awareness. We are better at identifying ASD; however, there are still considerable gaps in identifying ASD in high-functioning patients and adults. One percent of the population has ASD,72,73 and this group is overrepresented in psychiatric clinic and hospital settings.74 Therefore, we must be aware of and understand the diagnosis.
Medication treatments are often less effective and less tolerable in patients with ASD than in patients without neurodevelopmental disability. There are differences in pharmacotherapy response and tolerability across development in ASD and limited evidence to guide prescribing in adults with ASD. SGAs appear to be effective across multiple symptom domains, but carry the risk of significant adverse effects. For anxiety and irritability, there is compelling evidence supporting the use of nonpharmacologic treatments.
Bottom Line
A subset of patients seen in psychiatry will have undiagnosed autism spectrum disorder (ASD). When evaluating worsening behaviors, first rule out organic causes. Second-generation antipsychotics have the most evidence for efficacy in ASD across multiple symptom domains. To sustain improvement in symptoms, it is vital to incorporate nonpharmacologic treatments.
Related Resources
- National Institute of Mental Health. Autism spectrum disorder. https://www.nimh.nih.gov/health/publications/autismspectrum-disorder/index.shtml.
- Centers for Disease Control and Prevention. Autism spectrum disorder (ASD). https://www.cdc.gov/ncbddd/ autism/index.html.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Guanfacine • Tenex
Guanfacine Extended Release • Intuniv
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Volkmar FR, Lord C, Bailey A, et al. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135-170.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1-23.
5. Scragg P, Shah A. Prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165(5):679-682.
6. Hare DJ, Gould J, Mills R, et al. A preliminary study of individuals with autistic spectrum disorders in three special hospitals in England. London, UK: National Autistic Society; 1999.
7. Shah A, Holmes N, Wing L. Prevalence of autism and related conditions in adults in a mental handicap hospital. Appl Res Ment Retard. 1982;3(3):303-317.
8. Mandell DS, Lawer LJ, Branch K, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism. 2012;16(6):557-567.
9. Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Devel Disabil. 2015;38:242-255.
10. Perisse D, Amiet C, Consoli A, et al. Risk factors of acute behavioral regression in psychiatrically hospitalized adolescents with autism. J Can Acad Child Adolesc Psychiatry. 2010;19(2):100-108.
11. Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61.
12. Wink LK, Plawecki MH, Erickson CA, et al. Emerging drugs for the treatment of symptoms associated with autism spectrum disorders. Expert Opin Emerg Drugs. 2010;15(3):481-494.
13. Fitzpatrick SE, Srivorakiat L, Wink LK, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525-1538.
14. Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Disabil Res. 2006;50(pt 3):172-183.
15. Mills R, Wing L. Researching interventions in ASD and priorities for research: surveying the membership of the NAS. London, UK: National Autistic Society; 2005.
16. Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143-1154.
17. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633-641.
18. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361-1369.
19. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634-e641.
20. Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600-620.
21. Benton TD. Aripiprazole to treat irritability associated with autism: a placebo-controlled, fixed-dose trial. Curr Psychiatry Rep. 2011;13(2):77-79.
22. Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110-1119.
23. Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533-1540.
24. Adler BA, Wink LK, Early M, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102-106.
25. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887-894.
26. Potenza MN, Holmes JP, Kanes SJ, et al. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol. 1999;19(1):37-44.
27. Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541-548.
28. Stigler KA, Erickson CA, Mullett JE, et al. Paliperidone for irritability in autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):75-78.
29. Dominick K, Wink LK, McDougle CJ, et al. A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2015;25(5):397-401.
30. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
31. Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(2):287-294.
32. Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216-219.
33. McClellan L, Dominick KC, Pedapati EV, et al. Lurasidone for the treatment of irritability and anger in autism spectrum disorders. Expert Opin Investig Drugs. 2017;26(8):985-989.
34. Loebel A, Brams M, Goldman RS, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016;46(4):1153-1163.
35. Campbell M, Anderson LT, Meier M, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640-655.
36. Wink LK, Pedapati EV, Horn PS, et al. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91-94.
37. Wink LK, Badran I, Pedapati EV, et al. Clozapine for drug-refractory irritability in individuals with developmental disability. J Child Adolesc Psychopharmacol. 2016;26(9):843-846.
38. Chen NC, Bedair HS, McKay B, et al. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry. 2001;62(6):479-480.
39. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psychiatry Neurosci. 2001;26(4):340-341.
40. Lambrey S, Falissard B, Martin-Barrero M, et al. Effectiveness of clozapine for the treatment of aggression in an adolescent with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):79-80.
41. Yalcin O, Kaymak G, Erdogan A, et al. a retrospective investigation of clozapine treatment in autistic and nonautistic children and adolescents in an inpatient clinic in Turkey. J Child Adolesc Psychopharmacol. 2016;26(9):815-821.
42. Beherec L, Lambrey S, Quilici G, et al. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341-344.
43. Hirota T, Veenstra-Vanderweele J, Hollander E, et al, Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
44. Hollander E, Chaplin W, Soorya L, et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology. 2010;35(4):990-998.
45. Rezaei V, Mohammadi MR, Ghanizadeh A, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269-1272.
46. Siegel M, Beresford CA, Bunker M, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24(7):399-402.
47. Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631-648,vii.
48. van Steensel FJ, Deutschman AA, Bogels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism. 2013;17(6):681-692.
49. Lidstone J, Uljarevic M, Sullivan J, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8(2):82-92.
50. Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839-849.
51. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65(3):185-236.
52. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1-3):15-23.
53. Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20(1):58-65.
54. Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46(12):1327-1336.
55. Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(9):2135-2146.
56. Wink LK, Erickson CA, Stigler KA, et al. Riluzole in autistic disorder. J Child Adolesc Psychopharmacol. 2011;21(4):375-379.
57. Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215-3229.
58. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677.
59. Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12(6):529-538.
60. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148.
61. Murray MJ, Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382-388.
62. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266-1274.
63. Posey DJ, Aman MG, McCracken JT, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry. 2007;61(4):538-544.
64. Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30(5):451-459.
65. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30(3):245-255.
66. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25(3):283-294.
67. Jahromi LB, Kasari CL, McCracken JT, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39(3):395-404.
68. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77-82.
69. Scahill L, McCracken JT, King BH, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206.
70. Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29(4):303-308.
71. Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589-598.
72. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21.
73. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459-465.
74. Mandell DS, Psychiatric hospitalization among children with autism spectrum disorders. J Autism Dev Disord. 2008;38(6):1059-1065.
1. Volkmar FR, Lord C, Bailey A, et al. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135-170.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1-23.
5. Scragg P, Shah A. Prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165(5):679-682.
6. Hare DJ, Gould J, Mills R, et al. A preliminary study of individuals with autistic spectrum disorders in three special hospitals in England. London, UK: National Autistic Society; 1999.
7. Shah A, Holmes N, Wing L. Prevalence of autism and related conditions in adults in a mental handicap hospital. Appl Res Ment Retard. 1982;3(3):303-317.
8. Mandell DS, Lawer LJ, Branch K, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism. 2012;16(6):557-567.
9. Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Devel Disabil. 2015;38:242-255.
10. Perisse D, Amiet C, Consoli A, et al. Risk factors of acute behavioral regression in psychiatrically hospitalized adolescents with autism. J Can Acad Child Adolesc Psychiatry. 2010;19(2):100-108.
11. Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61.
12. Wink LK, Plawecki MH, Erickson CA, et al. Emerging drugs for the treatment of symptoms associated with autism spectrum disorders. Expert Opin Emerg Drugs. 2010;15(3):481-494.
13. Fitzpatrick SE, Srivorakiat L, Wink LK, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525-1538.
14. Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Disabil Res. 2006;50(pt 3):172-183.
15. Mills R, Wing L. Researching interventions in ASD and priorities for research: surveying the membership of the NAS. London, UK: National Autistic Society; 2005.
16. Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143-1154.
17. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633-641.
18. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361-1369.
19. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634-e641.
20. Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600-620.
21. Benton TD. Aripiprazole to treat irritability associated with autism: a placebo-controlled, fixed-dose trial. Curr Psychiatry Rep. 2011;13(2):77-79.
22. Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110-1119.
23. Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533-1540.
24. Adler BA, Wink LK, Early M, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102-106.
25. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887-894.
26. Potenza MN, Holmes JP, Kanes SJ, et al. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol. 1999;19(1):37-44.
27. Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541-548.
28. Stigler KA, Erickson CA, Mullett JE, et al. Paliperidone for irritability in autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):75-78.
29. Dominick K, Wink LK, McDougle CJ, et al. A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2015;25(5):397-401.
30. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
31. Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(2):287-294.
32. Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216-219.
33. McClellan L, Dominick KC, Pedapati EV, et al. Lurasidone for the treatment of irritability and anger in autism spectrum disorders. Expert Opin Investig Drugs. 2017;26(8):985-989.
34. Loebel A, Brams M, Goldman RS, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016;46(4):1153-1163.
35. Campbell M, Anderson LT, Meier M, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640-655.
36. Wink LK, Pedapati EV, Horn PS, et al. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91-94.
37. Wink LK, Badran I, Pedapati EV, et al. Clozapine for drug-refractory irritability in individuals with developmental disability. J Child Adolesc Psychopharmacol. 2016;26(9):843-846.
38. Chen NC, Bedair HS, McKay B, et al. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry. 2001;62(6):479-480.
39. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psychiatry Neurosci. 2001;26(4):340-341.
40. Lambrey S, Falissard B, Martin-Barrero M, et al. Effectiveness of clozapine for the treatment of aggression in an adolescent with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):79-80.
41. Yalcin O, Kaymak G, Erdogan A, et al. a retrospective investigation of clozapine treatment in autistic and nonautistic children and adolescents in an inpatient clinic in Turkey. J Child Adolesc Psychopharmacol. 2016;26(9):815-821.
42. Beherec L, Lambrey S, Quilici G, et al. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341-344.
43. Hirota T, Veenstra-Vanderweele J, Hollander E, et al, Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
44. Hollander E, Chaplin W, Soorya L, et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology. 2010;35(4):990-998.
45. Rezaei V, Mohammadi MR, Ghanizadeh A, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269-1272.
46. Siegel M, Beresford CA, Bunker M, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24(7):399-402.
47. Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631-648,vii.
48. van Steensel FJ, Deutschman AA, Bogels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism. 2013;17(6):681-692.
49. Lidstone J, Uljarevic M, Sullivan J, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8(2):82-92.
50. Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839-849.
51. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65(3):185-236.
52. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1-3):15-23.
53. Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20(1):58-65.
54. Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46(12):1327-1336.
55. Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(9):2135-2146.
56. Wink LK, Erickson CA, Stigler KA, et al. Riluzole in autistic disorder. J Child Adolesc Psychopharmacol. 2011;21(4):375-379.
57. Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215-3229.
58. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677.
59. Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12(6):529-538.
60. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148.
61. Murray MJ, Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382-388.
62. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266-1274.
63. Posey DJ, Aman MG, McCracken JT, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry. 2007;61(4):538-544.
64. Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30(5):451-459.
65. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30(3):245-255.
66. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25(3):283-294.
67. Jahromi LB, Kasari CL, McCracken JT, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39(3):395-404.
68. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77-82.
69. Scahill L, McCracken JT, King BH, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206.
70. Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29(4):303-308.
71. Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589-598.
72. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21.
73. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459-465.
74. Mandell DS, Psychiatric hospitalization among children with autism spectrum disorders. J Autism Dev Disord. 2008;38(6):1059-1065.
Managing maladaptive behaviors in fragile X patients
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.