User login
Managing maladaptive behaviors in fragile X patients
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
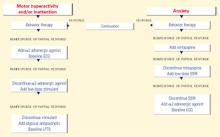
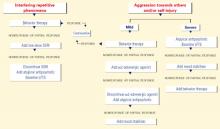
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
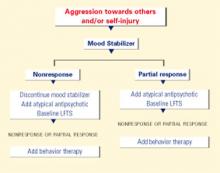
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
Are psychostimulants useful in pervasive developmental disorders?
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs
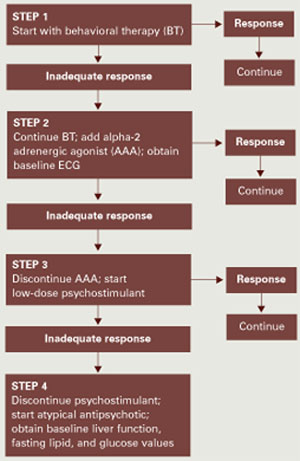
To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs

To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs

To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
Drug therapy algorithms target autism’s problem behaviors
Aggression, irritability, repetitive phenomena, or hyperactivity can interfere with the education and treatment of patients with autism.1 To assist clinicians in selecting drug therapies to control these behaviors, we developed a medication strategy based on controlled studies and our experience in an autism specialty clinic. In this article, we:
- offer an overview with two algorithms that show how to target maladaptive behaviors in patients of all ages with autism
- and discuss the controlled clinical evidence behind this approach.
Targeting behaviors
A multimodal approach is used to manage autistic disorder. Although behavioral techniques are useful and may decrease the need for drug therapy, one or more medications are often required to target severe maladaptive behaviors. Although we recommend that you consider all interfering behaviors when selecting medications, it is important to:
- initially focus on symptoms—such as self-injurious behaviors and aggression—that acutely affect the patient’s and caregiver’s safety
- consider the patient as being pre- or postpubertal, as developmental level may affect medication response.
Figure 1 BEHAVIOR-BASED DRUG THERAPY FOR CHILDREN WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc. Aggression or self-injury. When drug therapy is needed to control severe aggression or self-injurious behavior, we recommend a trial of an atypical antipsychotic for prepubertal patients (Figure 1). Alternately, you may consider a mood stabilizer such as lithium or divalproex for an adult patient with prominent symptoms (Figure 2). However, mood stabilizer use requires monitoring by venipuncture for potential blood cell or liver function abnormalities.
Atypical antipsychotics are associated with adverse effects including sedation, weight gain, extrapyramidal symptoms, tardive dyskinesia, and hyperprolactinemia. Therefore, you may need to monitor weight, lipid profile, glucose, liver function, and cardiac function when using this class of agent.
Repetitive phenomena can be addressed with selective serotonin reuptake inhibitors (SSRIs), although SSRIs may be activating in children and tend to be better tolerated in older adolescents and adults.
Anxiety. In children with anxiety, we suggest a trial of mirtazapine—because of its calming properties—before you try an SSRI. If both the SSRI and mirtazapine are ineffective, try an α2-adrenergic agonist. A baseline ECG is recommended before starting an α2-adrenergic agonist—particularly clonidine, which has been associated with rare cardiovascular events. Overall, these agents are well tolerated.
Hyperactivity, inattention. Anecdotal evidence indicates that stimulants may worsen symptoms of aggression, hyperactivity, and irritability in individuals with autism. Therefore, we suggest you start with an α2-adrenergic agonist when target symptoms include hyperactivity and inattention. Consider a stimulant trial if the α2-adrenergic agonist does not control the symptoms.
An atypical antipsychotic is generally recommended for autistic individuals with treatment-resistant hyperactivity and inattention, anxiety, and interfering repetitive behaviors.
Drug therapy. As summarized below, controlled evidence for behavior-based drug therapy of autism (Table.) suggests a role for antipsychotics, antidepressants, mood stabilizers, and psychostimulants in managing interfering behaviors.
Antipsychotics
Risperidone. Two controlled studies have reported the effect of risperidone on autism’s related symptoms.
McDougle et al conducted a 12-week, double-blind, placebo-controlled trial of risperidone, mean 2.9 mg/d, in adults with pervasive developmental disorders.2 Repetitive behavior, aggression, anxiety, irritability, depression, and general behavioral symptoms decreased in 8 of 14 patients, as measured by Clinical Global Impressions (CGI) scale ratings of “much improved” or “very much improved.” Sixteen subjects who received placebo showed no response. Transient sedation was the most common adverse effect.
More recently, the National Institutes of Mental Health-sponsored Research Units on Pediatric Psychopharmacology (RUPP) Autism Network completed a double-blind, placebo-controlled study of risperidone, 0.5 to 3.5 mg/d (mean 1.8 mg/d), in 101 children and adolescents with autism.3 After 8 weeks, 69% of the risperidone group responded, compared with 12% of the placebo group. Response was defined as:
- at least a 25% decrease in the Irritability subscale score of the Aberrant Behavior Checklist (ABC)
- and CGI ratings of “much improved” or “very much improved.”
Risperidone was effective for treating aggression, agitation, hyperactivity, and repetitive behavior. Adverse effects included weight gain (mean 2.7 kg vs. 0.8 kg with placebo), increased appetite, sedation, dizziness, and hypersalivation.
Olanzapine. A 12-week, open-label study of olanzapine, mean 7.8 mg/d, was conducted in eight patients ages 5 to 42 diagnosed with a pervasive developmental disorder.4 Hyperactivity, social relatedness, self-injurious behavior, aggression, anxiety, and depression improved significantly in six of seven patients who completed the trial, as measured by a CGI classification of “much improved” or “very much improved.” Adverse effects included weight gain (mean 18.4 lbs) in six patients and sedation in three.
Malone et al conducted a 6-week, open-label comparison of olanzapine, mean 7.9 mg/d, versus haloperidol, mean 1.4 mg/d, in 12 children with autistic disorder.5 Five of six subjects who received olanzapine responded, compared with three of six who received haloperidol, as determined by a CGI classification of “much improved” or “very much improved.” Anger/uncooperativeness and hyperactivity were reduced significantly only in the olanzapine group. Weight gain and sedation were the most common adverse effects with both medications. Weight gain was greater with olanzapine (mean 9 lbs) than with haloperidol (mean 3.2 lbs).
Quetiapine. Only one report addresses quetiapine in children and adolescents with autism.6 Two of six patients completed this 16-week, open-label trial of quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/d). The group showed no statistically significant improvement in behavioral symptoms. Two patients achieved CGI ratings of “much improved” or “very much improved.” One subject dropped out after a possible seizure during treatment, and three others withdrew because of sedation or lack of response.
Ziprasidone. McDougle et al conducted a naturalistic, open-label study of ziprasidone, 20 to 120 mg/d (mean 59.2 mg/d), in 12 subjects ages 8 to 20. Nine were diagnosed with autism and three with a pervasive developmental disorder not otherwise specified (NOS). Six subjects responded across 14 weeks of treatment, as determined by ratings of “much improved” or “very much improved” on the CGI.7
Transient sedation was the most common adverse effect. Mean weight change was −5.83 lbs (range −35 to +6 lbs). Despite ziprasidone’s reported risk of causing abnormal cardiac rhythms, no cardiovascular adverse effects were reported.
Clozapine. Three case reports but no controlled studies have addressed clozapine in autistic disorder:
- Zuddas et al used clozapine, 100 to 200 mg/d for 3 months, to treat three children ages 8 to 12.8 Previous trials of haloperidol were ineffective, but the patients’ aggression, hyperactivity, negativism, and language and communication skills improved with clozapine, as measured by the Children’s Psychiatric Rating Scale (CPRS) and the Self-Injurious Behavior Questionnaire.
- Chen et al used clozapine, 275 mg/d, to treat a 17-year-old male inpatient.9 Aggression was significantly reduced across 15 days, as determined by a 21-question modified version of the CPRS. Side effects included mild constipation and excessive salivation.
- Clozapine, 300 mg/d for 2 months, reduced aggression in an adult patient and was associated with progressive and marked improvement of aggression and social interaction, as measured by the CGI and the Visual Analog Scale (VAS). No agranulocytosis or extrapyramidal symptoms were observed across 5 years of therapy.10
Clozapine’s adverse effect profile probably explains why the literature contains so little data regarding its use in patients with autism. This drug’s propensity to lower the seizure threshold discourages its use in a population predisposed to seizures. Autistic patients also have a low tolerance for frequent venipuncture and limited ability to communicate agranulocytosis symptoms.
Antidepressants
Clomipramine—a tricyclic antidepressant with anti-obsessional properties—has shown some positive effects in treating autistic behaviors. Even so, this compound is prescribed infrequently to patients with autism because of low tolerability. In one recent double-blind, placebo-controlled, crossover study, 36 autistic patients ages 10 to 36 received:
- clomipramine, 100 to 150 mg/d (mean 128 mg/d)
- haloperidol, 1 to 1.5 mg/d (mean 1.3 mg/d)
or placebo for 7 weeks. Among patients who completed the trial, clomipramine and haloperidol were similarly effective in reducing overall autistic symptoms, irritability, and stereotypy. However, only 37.5% of those receiving clomipramine completed the study because of adverse effects, behavioral problems, and general lack of efficacy.11
Table
CONTROLLED EVIDENCE* FOR BEHAVIOR-BASED DRUG THERAPY OF AUTISM
| Medication | Behaviors improved | Significant adverse effects | Comments |
|---|---|---|---|
| Risperidone 2 | Aggression, irritability | Mild transient sedation | Conducted in adults |
| Risperidone 3 | Aggression, irritability | Weight gain, increased appetite, sedation, tremor, hypersalivation | Largest controlled study to date in children with autism |
| Clomipramine 11 | Irritability, stereotypy | Tachycardia, tremors, diaphoresis, insomnia, nausea | Conducted in children and adults |
| Fluvoxamine 12 | Aggression, repetitive phenomena | Nausea, sedation | No published controlled pediatric data; unpublished pediatric data unfavorable |
| Clonidine 24 | Hyperactivity, irritability, stereotypies, oppositional behavior, aggression, self-injury | Hypotension, sedation, decreased activity | Small number of subjects |
| Clonidine 25 | Impulsivity, self-stimulation, hyperarousal | Sedation and fatigue | Small number of subjects |
| Methylphenidate 28 | Hyperactivity, irritability | Social withdrawal and irritability | Adverse effects more common at 0.6 mg/kg/dose |
| • Double-blind, placebo-controlled studies | |||
Fluvoxamine. Only one double-blind, placebo-controlled study has examined the use of an SSRI in autistic disorder.12 In this 12-week trial, 30 adults with autism received fluvoxamine, mean 276.7 mg/d, or placebo. Eight of 15 subjects in the fluvoxamine group were categorized as “much improved” or “very much improved” on the CGI.
Fluvoxamine was much more effective than placebo in reducing repetitive thoughts and behavior, aggression, and maladaptive behavior. Adverse effects included minimal sedation and nausea.
Unpublished data of McDougle et al suggest that autistic children and adolescents respond less favorably than adults to fluvoxamine. In a 12-week, double-blind, placebocontrolled trial in patients ages 5 to 18, only 1 of 18 subjects responded to fluvoxamine at a mean dosage of 106.9 mg/d. Agitation, increased aggression, hyperactivity, and increased repetitive behavior were reported.
Fluoxetine. Seven autistic patients ages 9 to 20 were treated with fluoxetine, 20 to 80 mg/d (mean 37.1 mg/d) in an 18-month longitudinal open trial. Irritability, lethargy, and stereotypy improved, as measured by the ABC.13 Adverse effects included increased hyperactivity and transient appetite suppression.
In another open-label trial of 37 autistic children ages 2 to 7, response was rated by investigators as “excellent” in 11 and “good” in another 11 who received fluoxetine, 0.2 to 1.4 mg/kg/d. However, aggression was a frequent cause for drug discontinuation during the 21-month study.14
Sertraline. In an open-label trial, Steingard et al treated nine autistic children ages 6 to 12 with sertraline, 25 to 50 mg/d for 2 to 8 weeks. Clinical improvement was observed in irritability, transition-associated anxiety, and need for sameness in eight of the children.15
Figure 2 BEHAVIOR-BASED DRUG THERAPY FOR POSTPUBERTAL PATIENTS WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc.In an open-label trial, 42 adults with pervasive developmental disorders were treated for 12 weeks with sertraline, 50 to 200 mg/d (mean 122 mg/d). Twenty-four (57%) achieved CGI ratings of “much improved” or “very much improved” in aggressive and repetitive behavior.16
Paroxetine. Fifteen adults with profound mental retardation (including seven with pervasive developmental disorders) received paroxetine, 10 to 50 mg/d (mean 35 mg/d) in a 4-month open-label trial. Frequency and severity of aggression were rated as improved after 1 month, but not at 4 months.17
Mirtazapine. In an open-label trial, 26 patients ages 3 to 23 with pervasive developmental disorders were treated with mirtazapine, 7.5 to 45 mg/d (mean 30.3 mg/d). Aggression, self-injury, irritability, hyperactivity, anxiety, depression, and insomnia decreased in 9 patients (34%), as determined by ratings of “much improved” or “very much improved” on the CGI. Adverse effects included increased appetite and transient sedation.18
Mood stabilizers
Lithium. No recent studies of lithium in pervasive developmental disorders are known to exist, but several case reports have been published:
- two reports found lithium decreased manic symptoms in individuals with autism and a family history of bipolar disorder19,20
- one report of lithium augmentation of fluvoxamine in an adult with autistic disorder noted markedly improved aggression and impulsivity after 2 weeks of treatment, as measured by the CGI, Brown Aggression Scale, and Vineland Adaptive Behavior Scale.21
Divalproex. In an open-label trial, 14 subjects ages 5 to 40 with pervasive developmental disorders were given divalproex sodium, 125 to 2,500 mg/d (mean 768 mg/d; mean blood level 75.8 mcg/mL. Affective instability, repetitive behavior, impulsivity, and aggression improved in 10 patients (71%), as measured by CGI ratings of “much improved” or “very much improved.”22 Adverse effects included sedation, weight gain, hair loss, behavioral activation, and elevated liver enzymes.
Lamotrigine. In a 4-week, double-blind, placebo-controlled trial, lamotrigine, 5.0 mg/kg/d, was given to 14 children ages 3 to 11 with autistic disorder.23 Lamotrigine and placebo showed no significant differences in effect, as measured by the ABC, Autism Behavior Checklist, Vineland scales, Childhood Autism Rating Scale, and PreLinguistic Autism Diagnostic Observation Scale. Insomnia and hyperactivity were the most common adverse effects.
α2-adrenergic agonists
Clonidine. Jaselskis et al administered clonidine, 4 to 10 mcg/kg/d, to eight children ages 5 to 13 with autism in a 6-week, double-blind, placebo-controlled, crossover study.24 Symptoms of hyperactivity, irritability, and oppositional behavior improved, as determined by teacher ratings on the ABC, Conners Abbreviated Parent-Teacher Questionnaire, and Attention Deficit Disorder with Hyperactivity Comprehensive Teacher’s Rating Scale. Adverse effects included hypotension, sedation, and decreased activity.
Nine autistic patients ages 5 to 33 received transdermal clonidine, 0.16 to 0.48 mcg/kg/d (mean 3.6 mcg/kg/d), in a 4-week, double-blind, placebo-controlled, crossover study. Hyperarousal, impulsivity, and self-stimulation improved, as measured by the Ritvo-Freeman Real Life Rating Scale and the CGI. Sedation and fatigue were reported, especially during the first 2 weeks of treatment.25
Guanfacine. The effect of guanfacine, 0.25 to 9.0 mg/d (mean 2.6 mg/d), was examined retrospectively in 80 children and adolescents ages 3 to 18 with pervasive developmental disorders.26 Hyperactivity, inattention, and tics improved in the 19 subjects (23.8%) who were rated “much improved” or “very much improved” on the CGI. Sedation was the most frequently reported adverse effect.
Psychostimulants
Early reports showed little benefit from stimulants in pervasive developmental disorders, but more recent studies suggest a modest effect. The RUPP Autism Network is conducting a large controlled investigation of methylphenidate to better understand the role of stimulants in children and adolescents in this diagnostic cluster.
Quintana et al conducted a double-blind, crossover study of methylphenidate, 10 or 20 mg bid for 2 weeks, in 10 children ages 7 to 11 with autism.27 Although irritability and hyperactivity showed statistically significant improvement as determined by the ABC and Conners Teacher Questionnaire, the authors reported only modest clinical effects. More recently, use of methylphenidate, 0.3 and 0.6 mg/kg/dose, was associated with a 50% decrease on the Connors Hyperactivity Index in 8 of 13 autistic children ages 5 to 11.28 Adverse effects—most common with the 0.6 mg/kg/dose—included social withdrawal and irritability in this double-blind, placebo-controlled, crossover study.
Related resources
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice. New York: Oxford University Press, 2002.
- Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- National Institute of Mental Health. Autism booklet. www.nimh.nih.gov/publicat/autism.cfm
- Autism Society of America. www.autism-society.org
Drug brand names
- Clomipramine • Anafranil
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith
- Methylphenidate • Ritalin
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives grant support from and is a consultant to Eli Lilly & Co.
Dr. McDougle receives grant support from and is a consultant to Pfizer Inc., Eli Lilly & Co., and Janssen Pharmaceutica, and is a speaker for Pfizer Inc. and Janssen Pharmaceutica.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression Young Investigator Award (Dr. Posey), a Research Unit on Pediatric Psychopharmacology contract (N01MH70001) from the National Institute of Mental Health to Indiana University (Drs. McDougle and Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development grant (B-01-SP-IN-0200).
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed, text revision). Washington, DC: American Psychiatric Association, 2000.
2. McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55:633-41.
3. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
4. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: An open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
5. Malone RP, Cater J, Sheikh RM, Choudhury MS, Delaney MA. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
6. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
7. McDougle CJ, Kem DL, Posey DJ. Case series: use of ziprasidone for maladaptive symptoms in youths with autism. J Am Acad Child Adolesc Psychiatry 2002;41(8):921-7.
8. Zuddas A, Ledda MG, Fratta A, Muglia P, Cianchetti C. Clinical effects of clozapine on autistic disorder. Am J Psychiatry 1996;153(5):738.-
9. Chen NC, Bedair HS, McKay B, Bowers MB, Mazure C. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry 2001;62(6):479-80.
10. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psych Neurol 2001;26(4):340-1.
11. Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: A double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol 2001;21(4):440-4.
12. McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
13. Fatemi SH, Realmuto GM, Khan L, Thuras P. Fluoxetine in treatment of adolescent patients with autism: a longitudinal open trial. J Autism Dev Disord 1998;28(4):303-7.
14. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
15. Steingard RJ, Zimnitzky B, DeMaso DR, Bauman ML, Bucci JP. Sertraline treatment of transition-associated anxiety and agitation in children with autistic disorder. J Child Adolesc Psychopharmacol 1997;7(1):9-15.
16. McDougle CJ, Brodkin ES, Naylor ST, Carlson DC, Cohen DJ, Price LH. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
17. Davanzo PA, Belin TR, Widawski MH, King BH. Paroxetine treatment of aggression and self-injury in persons with mental retardation. Am J Ment Retard 1998;102(5):427-37.
18. Posey DJ, Guenin KD, Kohn AE, Swiezy NB, McDougle CJ. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol 2001;11(3):267-77.
19. Kerbeshian J, Burd L, Fisher W. Lithium carbonate in the treatment of two patients with infantile autism and atypical bipolar symptomatology. J Clin Psychopharmacol 1987;7(6):401-5.
20. Steingard R, Biederman J. Lithium-responsive manic-like symptoms in two individuals with autism and mental retardation. J Am Acad Child Adolesc Psychiatry 1987;26:932-5.
21. Epperson CN, McDougle CJ, Anand A, et al. Lithium augmentation of fluvoxamine in autistic disorder: a case report. J Child Adolesc Psychopharmacol 1994;4:201-7.
22. Hollander E, Dolgoff-Kaspar R, Cartwright C, Rawitt R, Novotny S. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62(7):530-4.
23. Belsito KM, Law PA, Kirk KS, Landa RJ, Zimmerman AW. Lamotrigine therapy for autistic disorder: a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord 2001;31(2):175-81.
24. Jaselskis CA, Cook EH, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
25. Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
26. Posey DJ, Decker J, Sasher TM, Kohburn A, Swiezy NB, McDougle CJ. A retrospective analysis of guanfacine in the treatment of autism. New Orleans: American Psychiatric Association annual meeting, 2001; new research abstracts no. 816.
27. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-95.
28. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
Aggression, irritability, repetitive phenomena, or hyperactivity can interfere with the education and treatment of patients with autism.1 To assist clinicians in selecting drug therapies to control these behaviors, we developed a medication strategy based on controlled studies and our experience in an autism specialty clinic. In this article, we:
- offer an overview with two algorithms that show how to target maladaptive behaviors in patients of all ages with autism
- and discuss the controlled clinical evidence behind this approach.
Targeting behaviors
A multimodal approach is used to manage autistic disorder. Although behavioral techniques are useful and may decrease the need for drug therapy, one or more medications are often required to target severe maladaptive behaviors. Although we recommend that you consider all interfering behaviors when selecting medications, it is important to:
- initially focus on symptoms—such as self-injurious behaviors and aggression—that acutely affect the patient’s and caregiver’s safety
- consider the patient as being pre- or postpubertal, as developmental level may affect medication response.
Figure 1 BEHAVIOR-BASED DRUG THERAPY FOR CHILDREN WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc. Aggression or self-injury. When drug therapy is needed to control severe aggression or self-injurious behavior, we recommend a trial of an atypical antipsychotic for prepubertal patients (Figure 1). Alternately, you may consider a mood stabilizer such as lithium or divalproex for an adult patient with prominent symptoms (Figure 2). However, mood stabilizer use requires monitoring by venipuncture for potential blood cell or liver function abnormalities.
Atypical antipsychotics are associated with adverse effects including sedation, weight gain, extrapyramidal symptoms, tardive dyskinesia, and hyperprolactinemia. Therefore, you may need to monitor weight, lipid profile, glucose, liver function, and cardiac function when using this class of agent.
Repetitive phenomena can be addressed with selective serotonin reuptake inhibitors (SSRIs), although SSRIs may be activating in children and tend to be better tolerated in older adolescents and adults.
Anxiety. In children with anxiety, we suggest a trial of mirtazapine—because of its calming properties—before you try an SSRI. If both the SSRI and mirtazapine are ineffective, try an α2-adrenergic agonist. A baseline ECG is recommended before starting an α2-adrenergic agonist—particularly clonidine, which has been associated with rare cardiovascular events. Overall, these agents are well tolerated.
Hyperactivity, inattention. Anecdotal evidence indicates that stimulants may worsen symptoms of aggression, hyperactivity, and irritability in individuals with autism. Therefore, we suggest you start with an α2-adrenergic agonist when target symptoms include hyperactivity and inattention. Consider a stimulant trial if the α2-adrenergic agonist does not control the symptoms.
An atypical antipsychotic is generally recommended for autistic individuals with treatment-resistant hyperactivity and inattention, anxiety, and interfering repetitive behaviors.
Drug therapy. As summarized below, controlled evidence for behavior-based drug therapy of autism (Table.) suggests a role for antipsychotics, antidepressants, mood stabilizers, and psychostimulants in managing interfering behaviors.
Antipsychotics
Risperidone. Two controlled studies have reported the effect of risperidone on autism’s related symptoms.
McDougle et al conducted a 12-week, double-blind, placebo-controlled trial of risperidone, mean 2.9 mg/d, in adults with pervasive developmental disorders.2 Repetitive behavior, aggression, anxiety, irritability, depression, and general behavioral symptoms decreased in 8 of 14 patients, as measured by Clinical Global Impressions (CGI) scale ratings of “much improved” or “very much improved.” Sixteen subjects who received placebo showed no response. Transient sedation was the most common adverse effect.
More recently, the National Institutes of Mental Health-sponsored Research Units on Pediatric Psychopharmacology (RUPP) Autism Network completed a double-blind, placebo-controlled study of risperidone, 0.5 to 3.5 mg/d (mean 1.8 mg/d), in 101 children and adolescents with autism.3 After 8 weeks, 69% of the risperidone group responded, compared with 12% of the placebo group. Response was defined as:
- at least a 25% decrease in the Irritability subscale score of the Aberrant Behavior Checklist (ABC)
- and CGI ratings of “much improved” or “very much improved.”
Risperidone was effective for treating aggression, agitation, hyperactivity, and repetitive behavior. Adverse effects included weight gain (mean 2.7 kg vs. 0.8 kg with placebo), increased appetite, sedation, dizziness, and hypersalivation.
Olanzapine. A 12-week, open-label study of olanzapine, mean 7.8 mg/d, was conducted in eight patients ages 5 to 42 diagnosed with a pervasive developmental disorder.4 Hyperactivity, social relatedness, self-injurious behavior, aggression, anxiety, and depression improved significantly in six of seven patients who completed the trial, as measured by a CGI classification of “much improved” or “very much improved.” Adverse effects included weight gain (mean 18.4 lbs) in six patients and sedation in three.
Malone et al conducted a 6-week, open-label comparison of olanzapine, mean 7.9 mg/d, versus haloperidol, mean 1.4 mg/d, in 12 children with autistic disorder.5 Five of six subjects who received olanzapine responded, compared with three of six who received haloperidol, as determined by a CGI classification of “much improved” or “very much improved.” Anger/uncooperativeness and hyperactivity were reduced significantly only in the olanzapine group. Weight gain and sedation were the most common adverse effects with both medications. Weight gain was greater with olanzapine (mean 9 lbs) than with haloperidol (mean 3.2 lbs).
Quetiapine. Only one report addresses quetiapine in children and adolescents with autism.6 Two of six patients completed this 16-week, open-label trial of quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/d). The group showed no statistically significant improvement in behavioral symptoms. Two patients achieved CGI ratings of “much improved” or “very much improved.” One subject dropped out after a possible seizure during treatment, and three others withdrew because of sedation or lack of response.
Ziprasidone. McDougle et al conducted a naturalistic, open-label study of ziprasidone, 20 to 120 mg/d (mean 59.2 mg/d), in 12 subjects ages 8 to 20. Nine were diagnosed with autism and three with a pervasive developmental disorder not otherwise specified (NOS). Six subjects responded across 14 weeks of treatment, as determined by ratings of “much improved” or “very much improved” on the CGI.7
Transient sedation was the most common adverse effect. Mean weight change was −5.83 lbs (range −35 to +6 lbs). Despite ziprasidone’s reported risk of causing abnormal cardiac rhythms, no cardiovascular adverse effects were reported.
Clozapine. Three case reports but no controlled studies have addressed clozapine in autistic disorder:
- Zuddas et al used clozapine, 100 to 200 mg/d for 3 months, to treat three children ages 8 to 12.8 Previous trials of haloperidol were ineffective, but the patients’ aggression, hyperactivity, negativism, and language and communication skills improved with clozapine, as measured by the Children’s Psychiatric Rating Scale (CPRS) and the Self-Injurious Behavior Questionnaire.
- Chen et al used clozapine, 275 mg/d, to treat a 17-year-old male inpatient.9 Aggression was significantly reduced across 15 days, as determined by a 21-question modified version of the CPRS. Side effects included mild constipation and excessive salivation.
- Clozapine, 300 mg/d for 2 months, reduced aggression in an adult patient and was associated with progressive and marked improvement of aggression and social interaction, as measured by the CGI and the Visual Analog Scale (VAS). No agranulocytosis or extrapyramidal symptoms were observed across 5 years of therapy.10
Clozapine’s adverse effect profile probably explains why the literature contains so little data regarding its use in patients with autism. This drug’s propensity to lower the seizure threshold discourages its use in a population predisposed to seizures. Autistic patients also have a low tolerance for frequent venipuncture and limited ability to communicate agranulocytosis symptoms.
Antidepressants
Clomipramine—a tricyclic antidepressant with anti-obsessional properties—has shown some positive effects in treating autistic behaviors. Even so, this compound is prescribed infrequently to patients with autism because of low tolerability. In one recent double-blind, placebo-controlled, crossover study, 36 autistic patients ages 10 to 36 received:
- clomipramine, 100 to 150 mg/d (mean 128 mg/d)
- haloperidol, 1 to 1.5 mg/d (mean 1.3 mg/d)
or placebo for 7 weeks. Among patients who completed the trial, clomipramine and haloperidol were similarly effective in reducing overall autistic symptoms, irritability, and stereotypy. However, only 37.5% of those receiving clomipramine completed the study because of adverse effects, behavioral problems, and general lack of efficacy.11
Table
CONTROLLED EVIDENCE* FOR BEHAVIOR-BASED DRUG THERAPY OF AUTISM
| Medication | Behaviors improved | Significant adverse effects | Comments |
|---|---|---|---|
| Risperidone 2 | Aggression, irritability | Mild transient sedation | Conducted in adults |
| Risperidone 3 | Aggression, irritability | Weight gain, increased appetite, sedation, tremor, hypersalivation | Largest controlled study to date in children with autism |
| Clomipramine 11 | Irritability, stereotypy | Tachycardia, tremors, diaphoresis, insomnia, nausea | Conducted in children and adults |
| Fluvoxamine 12 | Aggression, repetitive phenomena | Nausea, sedation | No published controlled pediatric data; unpublished pediatric data unfavorable |
| Clonidine 24 | Hyperactivity, irritability, stereotypies, oppositional behavior, aggression, self-injury | Hypotension, sedation, decreased activity | Small number of subjects |
| Clonidine 25 | Impulsivity, self-stimulation, hyperarousal | Sedation and fatigue | Small number of subjects |
| Methylphenidate 28 | Hyperactivity, irritability | Social withdrawal and irritability | Adverse effects more common at 0.6 mg/kg/dose |
| • Double-blind, placebo-controlled studies | |||
Fluvoxamine. Only one double-blind, placebo-controlled study has examined the use of an SSRI in autistic disorder.12 In this 12-week trial, 30 adults with autism received fluvoxamine, mean 276.7 mg/d, or placebo. Eight of 15 subjects in the fluvoxamine group were categorized as “much improved” or “very much improved” on the CGI.
Fluvoxamine was much more effective than placebo in reducing repetitive thoughts and behavior, aggression, and maladaptive behavior. Adverse effects included minimal sedation and nausea.
Unpublished data of McDougle et al suggest that autistic children and adolescents respond less favorably than adults to fluvoxamine. In a 12-week, double-blind, placebocontrolled trial in patients ages 5 to 18, only 1 of 18 subjects responded to fluvoxamine at a mean dosage of 106.9 mg/d. Agitation, increased aggression, hyperactivity, and increased repetitive behavior were reported.
Fluoxetine. Seven autistic patients ages 9 to 20 were treated with fluoxetine, 20 to 80 mg/d (mean 37.1 mg/d) in an 18-month longitudinal open trial. Irritability, lethargy, and stereotypy improved, as measured by the ABC.13 Adverse effects included increased hyperactivity and transient appetite suppression.
In another open-label trial of 37 autistic children ages 2 to 7, response was rated by investigators as “excellent” in 11 and “good” in another 11 who received fluoxetine, 0.2 to 1.4 mg/kg/d. However, aggression was a frequent cause for drug discontinuation during the 21-month study.14
Sertraline. In an open-label trial, Steingard et al treated nine autistic children ages 6 to 12 with sertraline, 25 to 50 mg/d for 2 to 8 weeks. Clinical improvement was observed in irritability, transition-associated anxiety, and need for sameness in eight of the children.15
Figure 2 BEHAVIOR-BASED DRUG THERAPY FOR POSTPUBERTAL PATIENTS WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc.In an open-label trial, 42 adults with pervasive developmental disorders were treated for 12 weeks with sertraline, 50 to 200 mg/d (mean 122 mg/d). Twenty-four (57%) achieved CGI ratings of “much improved” or “very much improved” in aggressive and repetitive behavior.16
Paroxetine. Fifteen adults with profound mental retardation (including seven with pervasive developmental disorders) received paroxetine, 10 to 50 mg/d (mean 35 mg/d) in a 4-month open-label trial. Frequency and severity of aggression were rated as improved after 1 month, but not at 4 months.17
Mirtazapine. In an open-label trial, 26 patients ages 3 to 23 with pervasive developmental disorders were treated with mirtazapine, 7.5 to 45 mg/d (mean 30.3 mg/d). Aggression, self-injury, irritability, hyperactivity, anxiety, depression, and insomnia decreased in 9 patients (34%), as determined by ratings of “much improved” or “very much improved” on the CGI. Adverse effects included increased appetite and transient sedation.18
Mood stabilizers
Lithium. No recent studies of lithium in pervasive developmental disorders are known to exist, but several case reports have been published:
- two reports found lithium decreased manic symptoms in individuals with autism and a family history of bipolar disorder19,20
- one report of lithium augmentation of fluvoxamine in an adult with autistic disorder noted markedly improved aggression and impulsivity after 2 weeks of treatment, as measured by the CGI, Brown Aggression Scale, and Vineland Adaptive Behavior Scale.21
Divalproex. In an open-label trial, 14 subjects ages 5 to 40 with pervasive developmental disorders were given divalproex sodium, 125 to 2,500 mg/d (mean 768 mg/d; mean blood level 75.8 mcg/mL. Affective instability, repetitive behavior, impulsivity, and aggression improved in 10 patients (71%), as measured by CGI ratings of “much improved” or “very much improved.”22 Adverse effects included sedation, weight gain, hair loss, behavioral activation, and elevated liver enzymes.
Lamotrigine. In a 4-week, double-blind, placebo-controlled trial, lamotrigine, 5.0 mg/kg/d, was given to 14 children ages 3 to 11 with autistic disorder.23 Lamotrigine and placebo showed no significant differences in effect, as measured by the ABC, Autism Behavior Checklist, Vineland scales, Childhood Autism Rating Scale, and PreLinguistic Autism Diagnostic Observation Scale. Insomnia and hyperactivity were the most common adverse effects.
α2-adrenergic agonists
Clonidine. Jaselskis et al administered clonidine, 4 to 10 mcg/kg/d, to eight children ages 5 to 13 with autism in a 6-week, double-blind, placebo-controlled, crossover study.24 Symptoms of hyperactivity, irritability, and oppositional behavior improved, as determined by teacher ratings on the ABC, Conners Abbreviated Parent-Teacher Questionnaire, and Attention Deficit Disorder with Hyperactivity Comprehensive Teacher’s Rating Scale. Adverse effects included hypotension, sedation, and decreased activity.
Nine autistic patients ages 5 to 33 received transdermal clonidine, 0.16 to 0.48 mcg/kg/d (mean 3.6 mcg/kg/d), in a 4-week, double-blind, placebo-controlled, crossover study. Hyperarousal, impulsivity, and self-stimulation improved, as measured by the Ritvo-Freeman Real Life Rating Scale and the CGI. Sedation and fatigue were reported, especially during the first 2 weeks of treatment.25
Guanfacine. The effect of guanfacine, 0.25 to 9.0 mg/d (mean 2.6 mg/d), was examined retrospectively in 80 children and adolescents ages 3 to 18 with pervasive developmental disorders.26 Hyperactivity, inattention, and tics improved in the 19 subjects (23.8%) who were rated “much improved” or “very much improved” on the CGI. Sedation was the most frequently reported adverse effect.
Psychostimulants
Early reports showed little benefit from stimulants in pervasive developmental disorders, but more recent studies suggest a modest effect. The RUPP Autism Network is conducting a large controlled investigation of methylphenidate to better understand the role of stimulants in children and adolescents in this diagnostic cluster.
Quintana et al conducted a double-blind, crossover study of methylphenidate, 10 or 20 mg bid for 2 weeks, in 10 children ages 7 to 11 with autism.27 Although irritability and hyperactivity showed statistically significant improvement as determined by the ABC and Conners Teacher Questionnaire, the authors reported only modest clinical effects. More recently, use of methylphenidate, 0.3 and 0.6 mg/kg/dose, was associated with a 50% decrease on the Connors Hyperactivity Index in 8 of 13 autistic children ages 5 to 11.28 Adverse effects—most common with the 0.6 mg/kg/dose—included social withdrawal and irritability in this double-blind, placebo-controlled, crossover study.
Related resources
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice. New York: Oxford University Press, 2002.
- Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- National Institute of Mental Health. Autism booklet. www.nimh.nih.gov/publicat/autism.cfm
- Autism Society of America. www.autism-society.org
Drug brand names
- Clomipramine • Anafranil
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith
- Methylphenidate • Ritalin
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives grant support from and is a consultant to Eli Lilly & Co.
Dr. McDougle receives grant support from and is a consultant to Pfizer Inc., Eli Lilly & Co., and Janssen Pharmaceutica, and is a speaker for Pfizer Inc. and Janssen Pharmaceutica.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression Young Investigator Award (Dr. Posey), a Research Unit on Pediatric Psychopharmacology contract (N01MH70001) from the National Institute of Mental Health to Indiana University (Drs. McDougle and Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development grant (B-01-SP-IN-0200).
Aggression, irritability, repetitive phenomena, or hyperactivity can interfere with the education and treatment of patients with autism.1 To assist clinicians in selecting drug therapies to control these behaviors, we developed a medication strategy based on controlled studies and our experience in an autism specialty clinic. In this article, we:
- offer an overview with two algorithms that show how to target maladaptive behaviors in patients of all ages with autism
- and discuss the controlled clinical evidence behind this approach.
Targeting behaviors
A multimodal approach is used to manage autistic disorder. Although behavioral techniques are useful and may decrease the need for drug therapy, one or more medications are often required to target severe maladaptive behaviors. Although we recommend that you consider all interfering behaviors when selecting medications, it is important to:
- initially focus on symptoms—such as self-injurious behaviors and aggression—that acutely affect the patient’s and caregiver’s safety
- consider the patient as being pre- or postpubertal, as developmental level may affect medication response.
Figure 1 BEHAVIOR-BASED DRUG THERAPY FOR CHILDREN WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc. Aggression or self-injury. When drug therapy is needed to control severe aggression or self-injurious behavior, we recommend a trial of an atypical antipsychotic for prepubertal patients (Figure 1). Alternately, you may consider a mood stabilizer such as lithium or divalproex for an adult patient with prominent symptoms (Figure 2). However, mood stabilizer use requires monitoring by venipuncture for potential blood cell or liver function abnormalities.
Atypical antipsychotics are associated with adverse effects including sedation, weight gain, extrapyramidal symptoms, tardive dyskinesia, and hyperprolactinemia. Therefore, you may need to monitor weight, lipid profile, glucose, liver function, and cardiac function when using this class of agent.
Repetitive phenomena can be addressed with selective serotonin reuptake inhibitors (SSRIs), although SSRIs may be activating in children and tend to be better tolerated in older adolescents and adults.
Anxiety. In children with anxiety, we suggest a trial of mirtazapine—because of its calming properties—before you try an SSRI. If both the SSRI and mirtazapine are ineffective, try an α2-adrenergic agonist. A baseline ECG is recommended before starting an α2-adrenergic agonist—particularly clonidine, which has been associated with rare cardiovascular events. Overall, these agents are well tolerated.
Hyperactivity, inattention. Anecdotal evidence indicates that stimulants may worsen symptoms of aggression, hyperactivity, and irritability in individuals with autism. Therefore, we suggest you start with an α2-adrenergic agonist when target symptoms include hyperactivity and inattention. Consider a stimulant trial if the α2-adrenergic agonist does not control the symptoms.
An atypical antipsychotic is generally recommended for autistic individuals with treatment-resistant hyperactivity and inattention, anxiety, and interfering repetitive behaviors.
Drug therapy. As summarized below, controlled evidence for behavior-based drug therapy of autism (Table.) suggests a role for antipsychotics, antidepressants, mood stabilizers, and psychostimulants in managing interfering behaviors.
Antipsychotics
Risperidone. Two controlled studies have reported the effect of risperidone on autism’s related symptoms.
McDougle et al conducted a 12-week, double-blind, placebo-controlled trial of risperidone, mean 2.9 mg/d, in adults with pervasive developmental disorders.2 Repetitive behavior, aggression, anxiety, irritability, depression, and general behavioral symptoms decreased in 8 of 14 patients, as measured by Clinical Global Impressions (CGI) scale ratings of “much improved” or “very much improved.” Sixteen subjects who received placebo showed no response. Transient sedation was the most common adverse effect.
More recently, the National Institutes of Mental Health-sponsored Research Units on Pediatric Psychopharmacology (RUPP) Autism Network completed a double-blind, placebo-controlled study of risperidone, 0.5 to 3.5 mg/d (mean 1.8 mg/d), in 101 children and adolescents with autism.3 After 8 weeks, 69% of the risperidone group responded, compared with 12% of the placebo group. Response was defined as:
- at least a 25% decrease in the Irritability subscale score of the Aberrant Behavior Checklist (ABC)
- and CGI ratings of “much improved” or “very much improved.”
Risperidone was effective for treating aggression, agitation, hyperactivity, and repetitive behavior. Adverse effects included weight gain (mean 2.7 kg vs. 0.8 kg with placebo), increased appetite, sedation, dizziness, and hypersalivation.
Olanzapine. A 12-week, open-label study of olanzapine, mean 7.8 mg/d, was conducted in eight patients ages 5 to 42 diagnosed with a pervasive developmental disorder.4 Hyperactivity, social relatedness, self-injurious behavior, aggression, anxiety, and depression improved significantly in six of seven patients who completed the trial, as measured by a CGI classification of “much improved” or “very much improved.” Adverse effects included weight gain (mean 18.4 lbs) in six patients and sedation in three.
Malone et al conducted a 6-week, open-label comparison of olanzapine, mean 7.9 mg/d, versus haloperidol, mean 1.4 mg/d, in 12 children with autistic disorder.5 Five of six subjects who received olanzapine responded, compared with three of six who received haloperidol, as determined by a CGI classification of “much improved” or “very much improved.” Anger/uncooperativeness and hyperactivity were reduced significantly only in the olanzapine group. Weight gain and sedation were the most common adverse effects with both medications. Weight gain was greater with olanzapine (mean 9 lbs) than with haloperidol (mean 3.2 lbs).
Quetiapine. Only one report addresses quetiapine in children and adolescents with autism.6 Two of six patients completed this 16-week, open-label trial of quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/d). The group showed no statistically significant improvement in behavioral symptoms. Two patients achieved CGI ratings of “much improved” or “very much improved.” One subject dropped out after a possible seizure during treatment, and three others withdrew because of sedation or lack of response.
Ziprasidone. McDougle et al conducted a naturalistic, open-label study of ziprasidone, 20 to 120 mg/d (mean 59.2 mg/d), in 12 subjects ages 8 to 20. Nine were diagnosed with autism and three with a pervasive developmental disorder not otherwise specified (NOS). Six subjects responded across 14 weeks of treatment, as determined by ratings of “much improved” or “very much improved” on the CGI.7
Transient sedation was the most common adverse effect. Mean weight change was −5.83 lbs (range −35 to +6 lbs). Despite ziprasidone’s reported risk of causing abnormal cardiac rhythms, no cardiovascular adverse effects were reported.
Clozapine. Three case reports but no controlled studies have addressed clozapine in autistic disorder:
- Zuddas et al used clozapine, 100 to 200 mg/d for 3 months, to treat three children ages 8 to 12.8 Previous trials of haloperidol were ineffective, but the patients’ aggression, hyperactivity, negativism, and language and communication skills improved with clozapine, as measured by the Children’s Psychiatric Rating Scale (CPRS) and the Self-Injurious Behavior Questionnaire.
- Chen et al used clozapine, 275 mg/d, to treat a 17-year-old male inpatient.9 Aggression was significantly reduced across 15 days, as determined by a 21-question modified version of the CPRS. Side effects included mild constipation and excessive salivation.
- Clozapine, 300 mg/d for 2 months, reduced aggression in an adult patient and was associated with progressive and marked improvement of aggression and social interaction, as measured by the CGI and the Visual Analog Scale (VAS). No agranulocytosis or extrapyramidal symptoms were observed across 5 years of therapy.10
Clozapine’s adverse effect profile probably explains why the literature contains so little data regarding its use in patients with autism. This drug’s propensity to lower the seizure threshold discourages its use in a population predisposed to seizures. Autistic patients also have a low tolerance for frequent venipuncture and limited ability to communicate agranulocytosis symptoms.
Antidepressants
Clomipramine—a tricyclic antidepressant with anti-obsessional properties—has shown some positive effects in treating autistic behaviors. Even so, this compound is prescribed infrequently to patients with autism because of low tolerability. In one recent double-blind, placebo-controlled, crossover study, 36 autistic patients ages 10 to 36 received:
- clomipramine, 100 to 150 mg/d (mean 128 mg/d)
- haloperidol, 1 to 1.5 mg/d (mean 1.3 mg/d)
or placebo for 7 weeks. Among patients who completed the trial, clomipramine and haloperidol were similarly effective in reducing overall autistic symptoms, irritability, and stereotypy. However, only 37.5% of those receiving clomipramine completed the study because of adverse effects, behavioral problems, and general lack of efficacy.11
Table
CONTROLLED EVIDENCE* FOR BEHAVIOR-BASED DRUG THERAPY OF AUTISM
| Medication | Behaviors improved | Significant adverse effects | Comments |
|---|---|---|---|
| Risperidone 2 | Aggression, irritability | Mild transient sedation | Conducted in adults |
| Risperidone 3 | Aggression, irritability | Weight gain, increased appetite, sedation, tremor, hypersalivation | Largest controlled study to date in children with autism |
| Clomipramine 11 | Irritability, stereotypy | Tachycardia, tremors, diaphoresis, insomnia, nausea | Conducted in children and adults |
| Fluvoxamine 12 | Aggression, repetitive phenomena | Nausea, sedation | No published controlled pediatric data; unpublished pediatric data unfavorable |
| Clonidine 24 | Hyperactivity, irritability, stereotypies, oppositional behavior, aggression, self-injury | Hypotension, sedation, decreased activity | Small number of subjects |
| Clonidine 25 | Impulsivity, self-stimulation, hyperarousal | Sedation and fatigue | Small number of subjects |
| Methylphenidate 28 | Hyperactivity, irritability | Social withdrawal and irritability | Adverse effects more common at 0.6 mg/kg/dose |
| • Double-blind, placebo-controlled studies | |||
Fluvoxamine. Only one double-blind, placebo-controlled study has examined the use of an SSRI in autistic disorder.12 In this 12-week trial, 30 adults with autism received fluvoxamine, mean 276.7 mg/d, or placebo. Eight of 15 subjects in the fluvoxamine group were categorized as “much improved” or “very much improved” on the CGI.
Fluvoxamine was much more effective than placebo in reducing repetitive thoughts and behavior, aggression, and maladaptive behavior. Adverse effects included minimal sedation and nausea.
Unpublished data of McDougle et al suggest that autistic children and adolescents respond less favorably than adults to fluvoxamine. In a 12-week, double-blind, placebocontrolled trial in patients ages 5 to 18, only 1 of 18 subjects responded to fluvoxamine at a mean dosage of 106.9 mg/d. Agitation, increased aggression, hyperactivity, and increased repetitive behavior were reported.
Fluoxetine. Seven autistic patients ages 9 to 20 were treated with fluoxetine, 20 to 80 mg/d (mean 37.1 mg/d) in an 18-month longitudinal open trial. Irritability, lethargy, and stereotypy improved, as measured by the ABC.13 Adverse effects included increased hyperactivity and transient appetite suppression.
In another open-label trial of 37 autistic children ages 2 to 7, response was rated by investigators as “excellent” in 11 and “good” in another 11 who received fluoxetine, 0.2 to 1.4 mg/kg/d. However, aggression was a frequent cause for drug discontinuation during the 21-month study.14
Sertraline. In an open-label trial, Steingard et al treated nine autistic children ages 6 to 12 with sertraline, 25 to 50 mg/d for 2 to 8 weeks. Clinical improvement was observed in irritability, transition-associated anxiety, and need for sameness in eight of the children.15
Figure 2 BEHAVIOR-BASED DRUG THERAPY FOR POSTPUBERTAL PATIENTS WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc.In an open-label trial, 42 adults with pervasive developmental disorders were treated for 12 weeks with sertraline, 50 to 200 mg/d (mean 122 mg/d). Twenty-four (57%) achieved CGI ratings of “much improved” or “very much improved” in aggressive and repetitive behavior.16
Paroxetine. Fifteen adults with profound mental retardation (including seven with pervasive developmental disorders) received paroxetine, 10 to 50 mg/d (mean 35 mg/d) in a 4-month open-label trial. Frequency and severity of aggression were rated as improved after 1 month, but not at 4 months.17
Mirtazapine. In an open-label trial, 26 patients ages 3 to 23 with pervasive developmental disorders were treated with mirtazapine, 7.5 to 45 mg/d (mean 30.3 mg/d). Aggression, self-injury, irritability, hyperactivity, anxiety, depression, and insomnia decreased in 9 patients (34%), as determined by ratings of “much improved” or “very much improved” on the CGI. Adverse effects included increased appetite and transient sedation.18
Mood stabilizers
Lithium. No recent studies of lithium in pervasive developmental disorders are known to exist, but several case reports have been published:
- two reports found lithium decreased manic symptoms in individuals with autism and a family history of bipolar disorder19,20
- one report of lithium augmentation of fluvoxamine in an adult with autistic disorder noted markedly improved aggression and impulsivity after 2 weeks of treatment, as measured by the CGI, Brown Aggression Scale, and Vineland Adaptive Behavior Scale.21
Divalproex. In an open-label trial, 14 subjects ages 5 to 40 with pervasive developmental disorders were given divalproex sodium, 125 to 2,500 mg/d (mean 768 mg/d; mean blood level 75.8 mcg/mL. Affective instability, repetitive behavior, impulsivity, and aggression improved in 10 patients (71%), as measured by CGI ratings of “much improved” or “very much improved.”22 Adverse effects included sedation, weight gain, hair loss, behavioral activation, and elevated liver enzymes.
Lamotrigine. In a 4-week, double-blind, placebo-controlled trial, lamotrigine, 5.0 mg/kg/d, was given to 14 children ages 3 to 11 with autistic disorder.23 Lamotrigine and placebo showed no significant differences in effect, as measured by the ABC, Autism Behavior Checklist, Vineland scales, Childhood Autism Rating Scale, and PreLinguistic Autism Diagnostic Observation Scale. Insomnia and hyperactivity were the most common adverse effects.
α2-adrenergic agonists
Clonidine. Jaselskis et al administered clonidine, 4 to 10 mcg/kg/d, to eight children ages 5 to 13 with autism in a 6-week, double-blind, placebo-controlled, crossover study.24 Symptoms of hyperactivity, irritability, and oppositional behavior improved, as determined by teacher ratings on the ABC, Conners Abbreviated Parent-Teacher Questionnaire, and Attention Deficit Disorder with Hyperactivity Comprehensive Teacher’s Rating Scale. Adverse effects included hypotension, sedation, and decreased activity.
Nine autistic patients ages 5 to 33 received transdermal clonidine, 0.16 to 0.48 mcg/kg/d (mean 3.6 mcg/kg/d), in a 4-week, double-blind, placebo-controlled, crossover study. Hyperarousal, impulsivity, and self-stimulation improved, as measured by the Ritvo-Freeman Real Life Rating Scale and the CGI. Sedation and fatigue were reported, especially during the first 2 weeks of treatment.25
Guanfacine. The effect of guanfacine, 0.25 to 9.0 mg/d (mean 2.6 mg/d), was examined retrospectively in 80 children and adolescents ages 3 to 18 with pervasive developmental disorders.26 Hyperactivity, inattention, and tics improved in the 19 subjects (23.8%) who were rated “much improved” or “very much improved” on the CGI. Sedation was the most frequently reported adverse effect.
Psychostimulants
Early reports showed little benefit from stimulants in pervasive developmental disorders, but more recent studies suggest a modest effect. The RUPP Autism Network is conducting a large controlled investigation of methylphenidate to better understand the role of stimulants in children and adolescents in this diagnostic cluster.
Quintana et al conducted a double-blind, crossover study of methylphenidate, 10 or 20 mg bid for 2 weeks, in 10 children ages 7 to 11 with autism.27 Although irritability and hyperactivity showed statistically significant improvement as determined by the ABC and Conners Teacher Questionnaire, the authors reported only modest clinical effects. More recently, use of methylphenidate, 0.3 and 0.6 mg/kg/dose, was associated with a 50% decrease on the Connors Hyperactivity Index in 8 of 13 autistic children ages 5 to 11.28 Adverse effects—most common with the 0.6 mg/kg/dose—included social withdrawal and irritability in this double-blind, placebo-controlled, crossover study.
Related resources
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice. New York: Oxford University Press, 2002.
- Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- National Institute of Mental Health. Autism booklet. www.nimh.nih.gov/publicat/autism.cfm
- Autism Society of America. www.autism-society.org
Drug brand names
- Clomipramine • Anafranil
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith
- Methylphenidate • Ritalin
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives grant support from and is a consultant to Eli Lilly & Co.
Dr. McDougle receives grant support from and is a consultant to Pfizer Inc., Eli Lilly & Co., and Janssen Pharmaceutica, and is a speaker for Pfizer Inc. and Janssen Pharmaceutica.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression Young Investigator Award (Dr. Posey), a Research Unit on Pediatric Psychopharmacology contract (N01MH70001) from the National Institute of Mental Health to Indiana University (Drs. McDougle and Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development grant (B-01-SP-IN-0200).
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed, text revision). Washington, DC: American Psychiatric Association, 2000.
2. McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55:633-41.
3. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
4. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: An open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
5. Malone RP, Cater J, Sheikh RM, Choudhury MS, Delaney MA. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
6. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
7. McDougle CJ, Kem DL, Posey DJ. Case series: use of ziprasidone for maladaptive symptoms in youths with autism. J Am Acad Child Adolesc Psychiatry 2002;41(8):921-7.
8. Zuddas A, Ledda MG, Fratta A, Muglia P, Cianchetti C. Clinical effects of clozapine on autistic disorder. Am J Psychiatry 1996;153(5):738.-
9. Chen NC, Bedair HS, McKay B, Bowers MB, Mazure C. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry 2001;62(6):479-80.
10. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psych Neurol 2001;26(4):340-1.
11. Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: A double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol 2001;21(4):440-4.
12. McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
13. Fatemi SH, Realmuto GM, Khan L, Thuras P. Fluoxetine in treatment of adolescent patients with autism: a longitudinal open trial. J Autism Dev Disord 1998;28(4):303-7.
14. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
15. Steingard RJ, Zimnitzky B, DeMaso DR, Bauman ML, Bucci JP. Sertraline treatment of transition-associated anxiety and agitation in children with autistic disorder. J Child Adolesc Psychopharmacol 1997;7(1):9-15.
16. McDougle CJ, Brodkin ES, Naylor ST, Carlson DC, Cohen DJ, Price LH. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
17. Davanzo PA, Belin TR, Widawski MH, King BH. Paroxetine treatment of aggression and self-injury in persons with mental retardation. Am J Ment Retard 1998;102(5):427-37.
18. Posey DJ, Guenin KD, Kohn AE, Swiezy NB, McDougle CJ. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol 2001;11(3):267-77.
19. Kerbeshian J, Burd L, Fisher W. Lithium carbonate in the treatment of two patients with infantile autism and atypical bipolar symptomatology. J Clin Psychopharmacol 1987;7(6):401-5.
20. Steingard R, Biederman J. Lithium-responsive manic-like symptoms in two individuals with autism and mental retardation. J Am Acad Child Adolesc Psychiatry 1987;26:932-5.
21. Epperson CN, McDougle CJ, Anand A, et al. Lithium augmentation of fluvoxamine in autistic disorder: a case report. J Child Adolesc Psychopharmacol 1994;4:201-7.
22. Hollander E, Dolgoff-Kaspar R, Cartwright C, Rawitt R, Novotny S. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62(7):530-4.
23. Belsito KM, Law PA, Kirk KS, Landa RJ, Zimmerman AW. Lamotrigine therapy for autistic disorder: a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord 2001;31(2):175-81.
24. Jaselskis CA, Cook EH, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
25. Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
26. Posey DJ, Decker J, Sasher TM, Kohburn A, Swiezy NB, McDougle CJ. A retrospective analysis of guanfacine in the treatment of autism. New Orleans: American Psychiatric Association annual meeting, 2001; new research abstracts no. 816.
27. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-95.
28. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed, text revision). Washington, DC: American Psychiatric Association, 2000.
2. McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55:633-41.
3. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
4. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: An open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
5. Malone RP, Cater J, Sheikh RM, Choudhury MS, Delaney MA. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
6. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
7. McDougle CJ, Kem DL, Posey DJ. Case series: use of ziprasidone for maladaptive symptoms in youths with autism. J Am Acad Child Adolesc Psychiatry 2002;41(8):921-7.
8. Zuddas A, Ledda MG, Fratta A, Muglia P, Cianchetti C. Clinical effects of clozapine on autistic disorder. Am J Psychiatry 1996;153(5):738.-
9. Chen NC, Bedair HS, McKay B, Bowers MB, Mazure C. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry 2001;62(6):479-80.
10. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psych Neurol 2001;26(4):340-1.
11. Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: A double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol 2001;21(4):440-4.
12. McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
13. Fatemi SH, Realmuto GM, Khan L, Thuras P. Fluoxetine in treatment of adolescent patients with autism: a longitudinal open trial. J Autism Dev Disord 1998;28(4):303-7.
14. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
15. Steingard RJ, Zimnitzky B, DeMaso DR, Bauman ML, Bucci JP. Sertraline treatment of transition-associated anxiety and agitation in children with autistic disorder. J Child Adolesc Psychopharmacol 1997;7(1):9-15.
16. McDougle CJ, Brodkin ES, Naylor ST, Carlson DC, Cohen DJ, Price LH. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
17. Davanzo PA, Belin TR, Widawski MH, King BH. Paroxetine treatment of aggression and self-injury in persons with mental retardation. Am J Ment Retard 1998;102(5):427-37.
18. Posey DJ, Guenin KD, Kohn AE, Swiezy NB, McDougle CJ. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol 2001;11(3):267-77.
19. Kerbeshian J, Burd L, Fisher W. Lithium carbonate in the treatment of two patients with infantile autism and atypical bipolar symptomatology. J Clin Psychopharmacol 1987;7(6):401-5.
20. Steingard R, Biederman J. Lithium-responsive manic-like symptoms in two individuals with autism and mental retardation. J Am Acad Child Adolesc Psychiatry 1987;26:932-5.
21. Epperson CN, McDougle CJ, Anand A, et al. Lithium augmentation of fluvoxamine in autistic disorder: a case report. J Child Adolesc Psychopharmacol 1994;4:201-7.
22. Hollander E, Dolgoff-Kaspar R, Cartwright C, Rawitt R, Novotny S. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62(7):530-4.
23. Belsito KM, Law PA, Kirk KS, Landa RJ, Zimmerman AW. Lamotrigine therapy for autistic disorder: a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord 2001;31(2):175-81.
24. Jaselskis CA, Cook EH, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
25. Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
26. Posey DJ, Decker J, Sasher TM, Kohburn A, Swiezy NB, McDougle CJ. A retrospective analysis of guanfacine in the treatment of autism. New Orleans: American Psychiatric Association annual meeting, 2001; new research abstracts no. 816.
27. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-95.
28. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
Autism: A three-step practical approach to making the diagnosis
Autism and related pervasive developmental disorders (PDD) are increasingly being identified in children—and even in some adolescents and adults. As a result, psychiatry now recognizes that these debilitating disorders are more common than was once believed, with a prevalence as high as 1 in 250.
An accurate diagnosis can help families take advantage of the variety of treatments being offered and investigated for affected individuals (Box).1 As psychiatrists who primarily see patients with autism and PDD, we recommend a three-step diagnostic approach that includes:
- a comprehensive initial assessment to rule out medical or neurologic illnesses that can mimic or are associated with autism
- differentiating PDDs from other psychiatric disorders with similar symptoms
- distinguishing among the five PDD subtypes described in DSM-IV.2
Step 1: Comprehensive initial assessment
Assessment for possible PDD begins with a comprehensive history and examination. Most patients will be assessed in childhood, but milder symptoms of autism or Asperger’s disorder may go unrecognized initially and not be brought to a clinician’s attention until adolescence or even adulthood.
As PDDs are childhood-onset disorders, the logical approach emphasizes the developmental course and onset of symptoms. By definition, children with autism show evidence of the disorder by age 3. However, the diagnosis can often be made as early as age 18 to 24 months, when typically developing children exhibit a number of social and communicative milestones that are absent in autism.
History A thorough description of the mother’s pregnancy, labor, and delivery (if known) can help you determine whether intrauterine or perinatal events could be related to the patient’s presenting problem. These include infections and exposure to exogenous substances (e.g., alcohol) during the pregnancy, as well as complications during pregnancy and delivery (e.g., maternal bleeding, neonatal hypoxia).
A complete description of the child’s development including major milestones (e.g., sitting without support, walking, first words) can help distinguish among certain diagnoses and estimate the extent of developmental delay. Ask the parents what first concerned them about their child’s development, as children with autism most often present with delays in social or language milestones. Any developmental regression in acquired skills may implicate other neurologic processes and help distinguish among the subtypes of PDD.
Symptoms Review the symptoms of autism at length in all patients in whom you suspect a PDD. It is important to assess these symptoms in the context of the child’s overall developmental level. For example, a child with known mental retardation should be compared with peers of similar age and cognitive impairment.
Optimal treatment for autism and related pervasive developmental disorders (PDDs) involves the collaboration of many disciplines.
- All school-aged children who are diagnosed with a PDD should be evaluated by the local school system to determine eligibility for special education. For those who are eligible, an individualized educational plan (IEP) is established to outline specific educational objectives and how they will be met. This IEP will often recommend speech therapy, occupational therapy, and social skills training.
- Children with the disability of autism are guaranteed an appropriate education. Advocates are often available to assist parents in ensuring that their child’s educational needs are being met.
- Many families also make use of treatment offered outside the school, such as speech and occupational therapy. In addition, behavioral psychotherapy based on principles of applied behavioral analysis is often helpful. Certain specialty clinics offer social skills training and additional supports for families. Parent support groups can also be a crucial source of information and support (“Related resources”).
- Symptoms associated with autism such as aggression, irritability, hyperactivity, anxiety, and interfering repetitive phenomena may be reduced with appropriate psychopharmacologic treatment.2 In general, treatment is aimed at these associated target symptoms because no single drug treatment has been consistently shown to improve the core social and communication impairments.
Approximately 75% of persons with autism are diagnosed with mental retardation. A review of intellectual abilities and level of adaptive functioning can suggest the degree of mental retardation. A detailed family history is important because autism and other syndromes associated with mental retardation have varying degrees of heritability.
A thorough medical history, review of systems, and physical exam (with focus on the neurologic exam) can suggest the presence of medical conditions that could mimic or be associated with autism. The symptoms of autism are traditionally divided into three domains: social, communication, and repetitive behavior/narrow interests (Table 1):
- Social impairment includes problems with nonverbal behaviors such as eye contact, facial expressions, and “body language;” failure to develop peer relationships; lack of spontaneous seeking to share enjoyment, interests, or achievements with other people; and lack of social or emotional reciprocity.
- Communication impairment includes language delay, decreased communication with others, conversational impairment, echolalia, and lack of imaginative or social imitative play.
- Impairments in behavior, interests, and activity take the form of all-encompassing preoccupations, “need for sameness” and compulsive rituals, motor stereotypies, and preoccupation with parts of objects.
After the preliminary history and exam, you should have an initial impression as to whether a PDD may be present. Conditions that could mimic or co-exist with autism will then need to be evaluated (Table 2).
Hearing and vision testing Every child presenting with a language or cognitive delay should have an adequate hearing assessment, including an audiogram at the very least. If results are equivocal, then brainstem auditory-evoked responses are indicated to establish the auditory system’s integrity. Vision screening should be completed, and the child should be referred to an ophthalmologist if a problem is suspected.
IQ testing A child with cognitive delays, learning problems, or suspected PDD should be referred to an experienced psychologist for intelligence testing. Public school systems often provide this service. Intelligence testing can document mental retardation and offer important information about the child’s strengths and weaknesses in learning.
Speech and language assessment A speech and language pathologist should evaluate children with PDD and/or language problems for articulation, prosody, receptive and expressive language, and pragmatics.
Lab and genetic tests Laboratory investigation in a child with cognitive delays should include routine blood chemistries, CBC, thyroid function tests, and lead level. Screen for fragile X syndrome, as its symptoms overlap those of autism, and for disorders of amino acid and organic acid metabolism. Finally, consider a chromosome karyotype, especially in patients with dysmorphic features on physical exam.
Table 1
THE THREE DOMAINS OF IMPAIRMENT IN AUTISM
Impairment in social interaction
|
Impairment in communication
|
Restricted repetitive and stereotyped patterns of behavior, interests, and activities
|
| Adapted from DSM-IV-TR, American Psychiatric Association, 2000 |
EEG Obtain a sleep-deprived electroencephalogram (EEG) in children with a history of significant language regression, episodic symptoms, or other indicators of possible seizures. Ideally, the EEG should include monitoring during sleep to help rule out acquired epileptic aphasia (Landau-Kleffner syndrome), a rare disorder associated with late-onset language regression. MRI of the brain is not routine but should be considered if indicated by the history or neurologic exam.
Consultations Consider consulting colleagues in neurology and medical genetics, especially when patients present with definite neurologic signs and symptoms or obvious dysmorphic features. These medical specialists are trained to screen for complex and rare syndromes that are sometimes associated with features of PDD.
Step 2. Is it PDD or another psychiatric disorder?
Psychiatric disorders that can be mistaken for PDD are listed in Table 3. The central feature of all PDDs is disturbance in social relatedness, and a diagnosis of PDD requires a history of significant social impairment.
Problems with social reciprocity in PDD are qualitatively different from the social impairment seen in other psychiatric disorders. For example, a child with attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) may have few friends because of a tendency to act impulsively and get into frequent conflicts with others. These social difficulties would not be considered indicative of PDD, as they typify those seen in children with ADHD and ODD.
Mental retardation Although mental retardation occurs in approximately 75% of persons with autism, most patients with mental retardation do not have autism. In assessing an individual with mental retardation for symptoms of PDD, consider the overall developmental level. It is not uncommon for individuals with mental retardation to have mild social problems, a history of language delay, and even motor stereotypies. These symptoms are considered indicative of PDD only when they are more severe than would be expected for the patient’s developmental level.
Table 2
TESTING OPTIONS FOR PATIENTS WITH PDD
| Hearing evaluation | Fragile X testing |
| Vision evaluation | Amino acids/organic acids |
| IQ testing | Chromosome karyotype |
| Speech/language evaluation | EEG |
| Chemistries, CBC, | Brain MRI |
| thyroid function tests | Neurology consultation |
| Lead level | Genetics consultation |
Table 3
DIFFERENTIAL DIAGNOSIS OF PDD
| Mental retardation |
| Reactive attachment disorder |
| Language disorders |
| Stereotypic movement disorder |
| Attention-deficit/hyperactivity disorder |
| Social phobia |
| Obsessive-compulsive disorder |
| Selective mutism |
| Schizophrenia |
| Personality disorders |
| Normality |
Other symptoms that are relatively specific to autism and PDD include lack of appropriate eye-to-eye gaze, abnormal speech prosody, echolalia, pronominal reversal, and narrow and circumscribed interests. The presence of these symptoms in excess should increase your suspicion of comorbid PDD.
RAD Reactive attachment disorder presents with abnormal social relatedness that can sometimes be confused with milder PDDs, especially in patients with comorbid mental retardation. In RAD, however, a history of severe neglect or abuse is thought to have caused the abnormal social relatedness. Placing the child in a caring and secure environment should improve many of the social deficits.
Language disorders are distinguished from PDDs by the absence of marked social impairment and lack of restricted interests and repetitive behaviors. In addition, children with primary language disorders often have intact nonverbal communication skills and make other attempts to communicate (e.g., through gesture, eye contact).
Stereotypic movement disorder can be seen in individuals with and without comorbid mental retardation. It is not diagnosed in the presence of autism, as these movements are thought to be part of the underlying disorder. The lack of social and communication impairments distinguishes stereotypic movement disorder from PDD.
ADHD Many children with autism and other PDDs have interfering symptoms of inattention, hyperactivity, and impulsivity. We usually do not give them an additional diagnosis of ADHD, as these symptoms are common in PDD. The pathophysiology of these symptoms may be different in ADHD and PDD, as evidenced by the frequent report of adverse effects following stimulant treatment of children with autism.2
Social phobia In higher functioning individuals with PDD, excessive social anxiety can sometimes be confused with social phobia. In social phobia, however, individuals usually do not exhibit marked problems with social relatedness and are able to interact normally with persons they know well and in some situations.
OCD Obsessive-compulsive disorder can occur in individuals with PDD but must be distinguished from the abnormal preoccupations and ritualistic behavior characteristic of autism. In autism, these activities often differ in quality from obsessions and compulsions.3 Furthermore, they usually are not associated with distress, and repetitive behaviors are not linked to a specific obsession.
Selective mutism is usually easy to distinguish from PDD because the affected child is typically able to talk in certain environments, such as at home. Also, the onset of selective mutism follows a period of normal social and communicative development.
Schizophrenia Autism was historically conceptualized as a type of childhood schizophrenia but is now thought to be distinct from the psychotic disorders. Schizophrenia with onset in childhood is much more rare than autism. Its onset usually occurs after several years of normal development, though some children with schizophrenia may have symptoms that resemble PDD early in their illness.4 Autistic persons may at times present with symptoms of a thought disorder. A diagnosis of schizophrenia usually is not made without evidence of prominent delusions and hallucinations.
Personality disorders PDDs are sometimes difficult to distinguish from personality disorders with similar features (e.g., schizotypal personality, schizoid personality). The social impairment in autistic and Asperger’s disorders is generally of earlier onset and greater severity than that seen in personality disorders. Those with personality disorders also typically lack stereotyped language or repetitive behaviors that are common in PDDs.
Table 4
CHARACTERISTIC FEATURES OF DSM-IV SUBTYPES OF PERVASIVE DEVELOPMENTAL DISORDERS
| Feature | Autistic disorder | Asperger’s disorder | Rett’s disorder | Childhood disintegrative disorder | Pervasive developmental disorder NOS* |
|---|---|---|---|---|---|
| Sex | Male:female ratio 4:1 | Male > female | Females only | Male > female | Male > female |
| Age of onset | < 3 years | Variable | 5-30 months | 2-10 years | Variable |
| Presence of regression | Mild regression in minority of patients | No | Yes | Yes | No |
| IQ | Most have mental retardation | Most have normal intellectual functioning | Severe mental retardation | Severe mental retardation | Variable |
| Social impairment | Yes | Yes | Yes | Yes | Yes |
| Communication impairment | Yes | No | Yes | Yes | Variable |
| Restricted interests/repetitive behaviors | Yes | Yes | Yes | Yes | Variable |
| Motor involvement | Usually not | Some have motor clumsiness | Gait and trunk ataxia; loss of purposeful hand movements | Loss of bowel/bladder control | Variable |
| *NOS: Not otherwise specified | |||||
Social awkwardness Finally, some of the PDDs that allow higher functioning (e.g., Asperger’s disorder and PDD not otherwise specified [NOS]) need to be distinguished from normal social awkwardness that can be common, especially in adolescence. The social impairment in PDD is marked and interferes with normal functioning and development.
Step 3. Which PDD is it?
DSM-IV describes five subtypes of PDD (autistic disorder, Asperger’s disorder, Rett’s disorder, childhood disintegrative disorder, and PDD NOS) that have in common problems with reciprocal social interaction (Table 4). For psychiatrists making the diagnosis, it is probably most difficult to differentiate the two most common types: autistic and Asperger’s disorders.
Autistic disorder is the prototypical PDD that is associated with abnormalities in reciprocal social interaction, qualitative impairments in communication, and narrow interests and repetitive behaviors (Table 1). By definition, symptoms of the disorder manifest by age 3.
Asperger’s disorder has several features that distinguish it from autistic disorder:
- Children with Asperger’s disorder do not have language delays. By definition, a child who has not developed single words by age 2 cannot be diagnosed with Asperger’s disorder.
- Early cognitive development in Asperger’s disorder is normal. Children with Asperger’s disorder are much more likely to have normal or above-average intellectual functioning than children with autistic disorder.
- Circumscribed interests and intense preoccupations are more common than motor stereotypies.
- Affected children may show verbal abilities that greatly exceed their visual-spatial skills. This may be apparent on individualized intelligence testing (i.e., verbal IQ > performance IQ) and clinically in the form of good language abilities but lagging fine-motor development (e.g., clumsiness).
Rett’s disorder occurs almost exclusively in girls, whereas autistic disorder is more common in boys. Following a brief period of normal development, affected girls experience deceleration of head growth, loss of previously acquired purposeful hand skills with subsequent development of stereotyped hand-wringing movements, loss of social engagement, onset of trunk and gait ataxia, and severe language and cognitive impairment. Genetic testing for mutations at MECP2 will be positive in most patients having all features of the classic phenotype.5
Childhood disintegrative disorder is thought to be more rare than autistic disorder. Following at least 2 years of normal development, affected children lose previously acquired skills. These can include play skills, language, social skills, bowel or bladder control, and motor skills. The children show impairments in social interaction, communication, and behavior of the type common to autistic disorder and often have severe mental retardation. The late onset of severe regression in development often prompts extensive neurologic evaluation, but a specific etiology is usually not found. The disorder is not diagnosed if full diagnostic criteria for autistic disorder are met (including onset of symptoms before age 3).
PDD NOS is diagnosed in many patients who are determined to have a significant impairment in social relatedness but do not meet full criteria for a specific PDD. Recent epidemiologic studies suggest that PDD NOS may be more common than autistic disorder.6 It may also be an appropriate designation for children with other proposed diagnostic constructs, such as nonverbal learning disabilities7 and multiple complex developmental disorders.8
Unfortunately, children diagnosed with PDD NOS instead of the better-recognized term “autism” may be denied appropriate social and financial services. When you inform others about the diagnosis of PDD NOS, it is important to emphasize to parents, schools, and funding agencies that PDD NOS is related to autism and should be considered one of the “autism spectrum disorders.” Children with PDDs or autism spectrum disorders will often benefit from similar treatment and educational interventions. In addition, their needs are equivalent for adequate insurance coverage and funding for specialized treatments.
- Cohen DJ, Volkmar FR (eds). Handbook of autism and pervasive developmental disorders (2nd ed). New York: Wiley, 1997.
- National Institute of Mental Health. Autism booklet. http://www.nimh.nih.gov/publicat/autism.cfm
- Autism Society of America http://www.autism-society.org Online Asperger Syndrome Information and Support (OASIS) http://www.udel.edu/bkirby/asperger
1. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harvard Rev Psychiatry 2000;8:45-63.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association, 2000.
3. McDougle CJ, Kresch LE, Goodman WK, et al. A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive-compulsive disorder. Am J Psychiatry 1995;152:772-7.
4. Kumra S. The diagnosis and treatment of children and adolescents with schizophrenia. “My mind is playing tricks on me.” Child Adolesc Psychiatr Clin N Am 2000;9:183-99.
5. Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet 1999;23:185-8.
6. Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA 2001;285:3093-9.
7. Rourke B. Nonverbal learning disabilities: The syndrome and the model. New York: Guilford Press, 1989.
8. Towbin KE, Dykens ED, Pearson GS, Cohen DJ. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993;32:775-82.
Autism and related pervasive developmental disorders (PDD) are increasingly being identified in children—and even in some adolescents and adults. As a result, psychiatry now recognizes that these debilitating disorders are more common than was once believed, with a prevalence as high as 1 in 250.
An accurate diagnosis can help families take advantage of the variety of treatments being offered and investigated for affected individuals (Box).1 As psychiatrists who primarily see patients with autism and PDD, we recommend a three-step diagnostic approach that includes:
- a comprehensive initial assessment to rule out medical or neurologic illnesses that can mimic or are associated with autism
- differentiating PDDs from other psychiatric disorders with similar symptoms
- distinguishing among the five PDD subtypes described in DSM-IV.2
Step 1: Comprehensive initial assessment
Assessment for possible PDD begins with a comprehensive history and examination. Most patients will be assessed in childhood, but milder symptoms of autism or Asperger’s disorder may go unrecognized initially and not be brought to a clinician’s attention until adolescence or even adulthood.
As PDDs are childhood-onset disorders, the logical approach emphasizes the developmental course and onset of symptoms. By definition, children with autism show evidence of the disorder by age 3. However, the diagnosis can often be made as early as age 18 to 24 months, when typically developing children exhibit a number of social and communicative milestones that are absent in autism.
History A thorough description of the mother’s pregnancy, labor, and delivery (if known) can help you determine whether intrauterine or perinatal events could be related to the patient’s presenting problem. These include infections and exposure to exogenous substances (e.g., alcohol) during the pregnancy, as well as complications during pregnancy and delivery (e.g., maternal bleeding, neonatal hypoxia).
A complete description of the child’s development including major milestones (e.g., sitting without support, walking, first words) can help distinguish among certain diagnoses and estimate the extent of developmental delay. Ask the parents what first concerned them about their child’s development, as children with autism most often present with delays in social or language milestones. Any developmental regression in acquired skills may implicate other neurologic processes and help distinguish among the subtypes of PDD.
Symptoms Review the symptoms of autism at length in all patients in whom you suspect a PDD. It is important to assess these symptoms in the context of the child’s overall developmental level. For example, a child with known mental retardation should be compared with peers of similar age and cognitive impairment.
Optimal treatment for autism and related pervasive developmental disorders (PDDs) involves the collaboration of many disciplines.
- All school-aged children who are diagnosed with a PDD should be evaluated by the local school system to determine eligibility for special education. For those who are eligible, an individualized educational plan (IEP) is established to outline specific educational objectives and how they will be met. This IEP will often recommend speech therapy, occupational therapy, and social skills training.
- Children with the disability of autism are guaranteed an appropriate education. Advocates are often available to assist parents in ensuring that their child’s educational needs are being met.
- Many families also make use of treatment offered outside the school, such as speech and occupational therapy. In addition, behavioral psychotherapy based on principles of applied behavioral analysis is often helpful. Certain specialty clinics offer social skills training and additional supports for families. Parent support groups can also be a crucial source of information and support (“Related resources”).
- Symptoms associated with autism such as aggression, irritability, hyperactivity, anxiety, and interfering repetitive phenomena may be reduced with appropriate psychopharmacologic treatment.2 In general, treatment is aimed at these associated target symptoms because no single drug treatment has been consistently shown to improve the core social and communication impairments.
Approximately 75% of persons with autism are diagnosed with mental retardation. A review of intellectual abilities and level of adaptive functioning can suggest the degree of mental retardation. A detailed family history is important because autism and other syndromes associated with mental retardation have varying degrees of heritability.
A thorough medical history, review of systems, and physical exam (with focus on the neurologic exam) can suggest the presence of medical conditions that could mimic or be associated with autism. The symptoms of autism are traditionally divided into three domains: social, communication, and repetitive behavior/narrow interests (Table 1):
- Social impairment includes problems with nonverbal behaviors such as eye contact, facial expressions, and “body language;” failure to develop peer relationships; lack of spontaneous seeking to share enjoyment, interests, or achievements with other people; and lack of social or emotional reciprocity.
- Communication impairment includes language delay, decreased communication with others, conversational impairment, echolalia, and lack of imaginative or social imitative play.
- Impairments in behavior, interests, and activity take the form of all-encompassing preoccupations, “need for sameness” and compulsive rituals, motor stereotypies, and preoccupation with parts of objects.
After the preliminary history and exam, you should have an initial impression as to whether a PDD may be present. Conditions that could mimic or co-exist with autism will then need to be evaluated (Table 2).
Hearing and vision testing Every child presenting with a language or cognitive delay should have an adequate hearing assessment, including an audiogram at the very least. If results are equivocal, then brainstem auditory-evoked responses are indicated to establish the auditory system’s integrity. Vision screening should be completed, and the child should be referred to an ophthalmologist if a problem is suspected.
IQ testing A child with cognitive delays, learning problems, or suspected PDD should be referred to an experienced psychologist for intelligence testing. Public school systems often provide this service. Intelligence testing can document mental retardation and offer important information about the child’s strengths and weaknesses in learning.
Speech and language assessment A speech and language pathologist should evaluate children with PDD and/or language problems for articulation, prosody, receptive and expressive language, and pragmatics.
Lab and genetic tests Laboratory investigation in a child with cognitive delays should include routine blood chemistries, CBC, thyroid function tests, and lead level. Screen for fragile X syndrome, as its symptoms overlap those of autism, and for disorders of amino acid and organic acid metabolism. Finally, consider a chromosome karyotype, especially in patients with dysmorphic features on physical exam.
Table 1
THE THREE DOMAINS OF IMPAIRMENT IN AUTISM
Impairment in social interaction
|
Impairment in communication
|
Restricted repetitive and stereotyped patterns of behavior, interests, and activities
|
| Adapted from DSM-IV-TR, American Psychiatric Association, 2000 |
EEG Obtain a sleep-deprived electroencephalogram (EEG) in children with a history of significant language regression, episodic symptoms, or other indicators of possible seizures. Ideally, the EEG should include monitoring during sleep to help rule out acquired epileptic aphasia (Landau-Kleffner syndrome), a rare disorder associated with late-onset language regression. MRI of the brain is not routine but should be considered if indicated by the history or neurologic exam.
Consultations Consider consulting colleagues in neurology and medical genetics, especially when patients present with definite neurologic signs and symptoms or obvious dysmorphic features. These medical specialists are trained to screen for complex and rare syndromes that are sometimes associated with features of PDD.
Step 2. Is it PDD or another psychiatric disorder?
Psychiatric disorders that can be mistaken for PDD are listed in Table 3. The central feature of all PDDs is disturbance in social relatedness, and a diagnosis of PDD requires a history of significant social impairment.
Problems with social reciprocity in PDD are qualitatively different from the social impairment seen in other psychiatric disorders. For example, a child with attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) may have few friends because of a tendency to act impulsively and get into frequent conflicts with others. These social difficulties would not be considered indicative of PDD, as they typify those seen in children with ADHD and ODD.
Mental retardation Although mental retardation occurs in approximately 75% of persons with autism, most patients with mental retardation do not have autism. In assessing an individual with mental retardation for symptoms of PDD, consider the overall developmental level. It is not uncommon for individuals with mental retardation to have mild social problems, a history of language delay, and even motor stereotypies. These symptoms are considered indicative of PDD only when they are more severe than would be expected for the patient’s developmental level.
Table 2
TESTING OPTIONS FOR PATIENTS WITH PDD
| Hearing evaluation | Fragile X testing |
| Vision evaluation | Amino acids/organic acids |
| IQ testing | Chromosome karyotype |
| Speech/language evaluation | EEG |
| Chemistries, CBC, | Brain MRI |
| thyroid function tests | Neurology consultation |
| Lead level | Genetics consultation |
Table 3
DIFFERENTIAL DIAGNOSIS OF PDD
| Mental retardation |
| Reactive attachment disorder |
| Language disorders |
| Stereotypic movement disorder |
| Attention-deficit/hyperactivity disorder |
| Social phobia |
| Obsessive-compulsive disorder |
| Selective mutism |
| Schizophrenia |
| Personality disorders |
| Normality |
Other symptoms that are relatively specific to autism and PDD include lack of appropriate eye-to-eye gaze, abnormal speech prosody, echolalia, pronominal reversal, and narrow and circumscribed interests. The presence of these symptoms in excess should increase your suspicion of comorbid PDD.
RAD Reactive attachment disorder presents with abnormal social relatedness that can sometimes be confused with milder PDDs, especially in patients with comorbid mental retardation. In RAD, however, a history of severe neglect or abuse is thought to have caused the abnormal social relatedness. Placing the child in a caring and secure environment should improve many of the social deficits.
Language disorders are distinguished from PDDs by the absence of marked social impairment and lack of restricted interests and repetitive behaviors. In addition, children with primary language disorders often have intact nonverbal communication skills and make other attempts to communicate (e.g., through gesture, eye contact).
Stereotypic movement disorder can be seen in individuals with and without comorbid mental retardation. It is not diagnosed in the presence of autism, as these movements are thought to be part of the underlying disorder. The lack of social and communication impairments distinguishes stereotypic movement disorder from PDD.
ADHD Many children with autism and other PDDs have interfering symptoms of inattention, hyperactivity, and impulsivity. We usually do not give them an additional diagnosis of ADHD, as these symptoms are common in PDD. The pathophysiology of these symptoms may be different in ADHD and PDD, as evidenced by the frequent report of adverse effects following stimulant treatment of children with autism.2
Social phobia In higher functioning individuals with PDD, excessive social anxiety can sometimes be confused with social phobia. In social phobia, however, individuals usually do not exhibit marked problems with social relatedness and are able to interact normally with persons they know well and in some situations.
OCD Obsessive-compulsive disorder can occur in individuals with PDD but must be distinguished from the abnormal preoccupations and ritualistic behavior characteristic of autism. In autism, these activities often differ in quality from obsessions and compulsions.3 Furthermore, they usually are not associated with distress, and repetitive behaviors are not linked to a specific obsession.
Selective mutism is usually easy to distinguish from PDD because the affected child is typically able to talk in certain environments, such as at home. Also, the onset of selective mutism follows a period of normal social and communicative development.
Schizophrenia Autism was historically conceptualized as a type of childhood schizophrenia but is now thought to be distinct from the psychotic disorders. Schizophrenia with onset in childhood is much more rare than autism. Its onset usually occurs after several years of normal development, though some children with schizophrenia may have symptoms that resemble PDD early in their illness.4 Autistic persons may at times present with symptoms of a thought disorder. A diagnosis of schizophrenia usually is not made without evidence of prominent delusions and hallucinations.
Personality disorders PDDs are sometimes difficult to distinguish from personality disorders with similar features (e.g., schizotypal personality, schizoid personality). The social impairment in autistic and Asperger’s disorders is generally of earlier onset and greater severity than that seen in personality disorders. Those with personality disorders also typically lack stereotyped language or repetitive behaviors that are common in PDDs.
Table 4
CHARACTERISTIC FEATURES OF DSM-IV SUBTYPES OF PERVASIVE DEVELOPMENTAL DISORDERS
| Feature | Autistic disorder | Asperger’s disorder | Rett’s disorder | Childhood disintegrative disorder | Pervasive developmental disorder NOS* |
|---|---|---|---|---|---|
| Sex | Male:female ratio 4:1 | Male > female | Females only | Male > female | Male > female |
| Age of onset | < 3 years | Variable | 5-30 months | 2-10 years | Variable |
| Presence of regression | Mild regression in minority of patients | No | Yes | Yes | No |
| IQ | Most have mental retardation | Most have normal intellectual functioning | Severe mental retardation | Severe mental retardation | Variable |
| Social impairment | Yes | Yes | Yes | Yes | Yes |
| Communication impairment | Yes | No | Yes | Yes | Variable |
| Restricted interests/repetitive behaviors | Yes | Yes | Yes | Yes | Variable |
| Motor involvement | Usually not | Some have motor clumsiness | Gait and trunk ataxia; loss of purposeful hand movements | Loss of bowel/bladder control | Variable |
| *NOS: Not otherwise specified | |||||
Social awkwardness Finally, some of the PDDs that allow higher functioning (e.g., Asperger’s disorder and PDD not otherwise specified [NOS]) need to be distinguished from normal social awkwardness that can be common, especially in adolescence. The social impairment in PDD is marked and interferes with normal functioning and development.
Step 3. Which PDD is it?
DSM-IV describes five subtypes of PDD (autistic disorder, Asperger’s disorder, Rett’s disorder, childhood disintegrative disorder, and PDD NOS) that have in common problems with reciprocal social interaction (Table 4). For psychiatrists making the diagnosis, it is probably most difficult to differentiate the two most common types: autistic and Asperger’s disorders.
Autistic disorder is the prototypical PDD that is associated with abnormalities in reciprocal social interaction, qualitative impairments in communication, and narrow interests and repetitive behaviors (Table 1). By definition, symptoms of the disorder manifest by age 3.
Asperger’s disorder has several features that distinguish it from autistic disorder:
- Children with Asperger’s disorder do not have language delays. By definition, a child who has not developed single words by age 2 cannot be diagnosed with Asperger’s disorder.
- Early cognitive development in Asperger’s disorder is normal. Children with Asperger’s disorder are much more likely to have normal or above-average intellectual functioning than children with autistic disorder.
- Circumscribed interests and intense preoccupations are more common than motor stereotypies.
- Affected children may show verbal abilities that greatly exceed their visual-spatial skills. This may be apparent on individualized intelligence testing (i.e., verbal IQ > performance IQ) and clinically in the form of good language abilities but lagging fine-motor development (e.g., clumsiness).
Rett’s disorder occurs almost exclusively in girls, whereas autistic disorder is more common in boys. Following a brief period of normal development, affected girls experience deceleration of head growth, loss of previously acquired purposeful hand skills with subsequent development of stereotyped hand-wringing movements, loss of social engagement, onset of trunk and gait ataxia, and severe language and cognitive impairment. Genetic testing for mutations at MECP2 will be positive in most patients having all features of the classic phenotype.5
Childhood disintegrative disorder is thought to be more rare than autistic disorder. Following at least 2 years of normal development, affected children lose previously acquired skills. These can include play skills, language, social skills, bowel or bladder control, and motor skills. The children show impairments in social interaction, communication, and behavior of the type common to autistic disorder and often have severe mental retardation. The late onset of severe regression in development often prompts extensive neurologic evaluation, but a specific etiology is usually not found. The disorder is not diagnosed if full diagnostic criteria for autistic disorder are met (including onset of symptoms before age 3).
PDD NOS is diagnosed in many patients who are determined to have a significant impairment in social relatedness but do not meet full criteria for a specific PDD. Recent epidemiologic studies suggest that PDD NOS may be more common than autistic disorder.6 It may also be an appropriate designation for children with other proposed diagnostic constructs, such as nonverbal learning disabilities7 and multiple complex developmental disorders.8
Unfortunately, children diagnosed with PDD NOS instead of the better-recognized term “autism” may be denied appropriate social and financial services. When you inform others about the diagnosis of PDD NOS, it is important to emphasize to parents, schools, and funding agencies that PDD NOS is related to autism and should be considered one of the “autism spectrum disorders.” Children with PDDs or autism spectrum disorders will often benefit from similar treatment and educational interventions. In addition, their needs are equivalent for adequate insurance coverage and funding for specialized treatments.
- Cohen DJ, Volkmar FR (eds). Handbook of autism and pervasive developmental disorders (2nd ed). New York: Wiley, 1997.
- National Institute of Mental Health. Autism booklet. http://www.nimh.nih.gov/publicat/autism.cfm
- Autism Society of America http://www.autism-society.org Online Asperger Syndrome Information and Support (OASIS) http://www.udel.edu/bkirby/asperger
Autism and related pervasive developmental disorders (PDD) are increasingly being identified in children—and even in some adolescents and adults. As a result, psychiatry now recognizes that these debilitating disorders are more common than was once believed, with a prevalence as high as 1 in 250.
An accurate diagnosis can help families take advantage of the variety of treatments being offered and investigated for affected individuals (Box).1 As psychiatrists who primarily see patients with autism and PDD, we recommend a three-step diagnostic approach that includes:
- a comprehensive initial assessment to rule out medical or neurologic illnesses that can mimic or are associated with autism
- differentiating PDDs from other psychiatric disorders with similar symptoms
- distinguishing among the five PDD subtypes described in DSM-IV.2
Step 1: Comprehensive initial assessment
Assessment for possible PDD begins with a comprehensive history and examination. Most patients will be assessed in childhood, but milder symptoms of autism or Asperger’s disorder may go unrecognized initially and not be brought to a clinician’s attention until adolescence or even adulthood.
As PDDs are childhood-onset disorders, the logical approach emphasizes the developmental course and onset of symptoms. By definition, children with autism show evidence of the disorder by age 3. However, the diagnosis can often be made as early as age 18 to 24 months, when typically developing children exhibit a number of social and communicative milestones that are absent in autism.
History A thorough description of the mother’s pregnancy, labor, and delivery (if known) can help you determine whether intrauterine or perinatal events could be related to the patient’s presenting problem. These include infections and exposure to exogenous substances (e.g., alcohol) during the pregnancy, as well as complications during pregnancy and delivery (e.g., maternal bleeding, neonatal hypoxia).
A complete description of the child’s development including major milestones (e.g., sitting without support, walking, first words) can help distinguish among certain diagnoses and estimate the extent of developmental delay. Ask the parents what first concerned them about their child’s development, as children with autism most often present with delays in social or language milestones. Any developmental regression in acquired skills may implicate other neurologic processes and help distinguish among the subtypes of PDD.
Symptoms Review the symptoms of autism at length in all patients in whom you suspect a PDD. It is important to assess these symptoms in the context of the child’s overall developmental level. For example, a child with known mental retardation should be compared with peers of similar age and cognitive impairment.
Optimal treatment for autism and related pervasive developmental disorders (PDDs) involves the collaboration of many disciplines.
- All school-aged children who are diagnosed with a PDD should be evaluated by the local school system to determine eligibility for special education. For those who are eligible, an individualized educational plan (IEP) is established to outline specific educational objectives and how they will be met. This IEP will often recommend speech therapy, occupational therapy, and social skills training.
- Children with the disability of autism are guaranteed an appropriate education. Advocates are often available to assist parents in ensuring that their child’s educational needs are being met.
- Many families also make use of treatment offered outside the school, such as speech and occupational therapy. In addition, behavioral psychotherapy based on principles of applied behavioral analysis is often helpful. Certain specialty clinics offer social skills training and additional supports for families. Parent support groups can also be a crucial source of information and support (“Related resources”).
- Symptoms associated with autism such as aggression, irritability, hyperactivity, anxiety, and interfering repetitive phenomena may be reduced with appropriate psychopharmacologic treatment.2 In general, treatment is aimed at these associated target symptoms because no single drug treatment has been consistently shown to improve the core social and communication impairments.
Approximately 75% of persons with autism are diagnosed with mental retardation. A review of intellectual abilities and level of adaptive functioning can suggest the degree of mental retardation. A detailed family history is important because autism and other syndromes associated with mental retardation have varying degrees of heritability.
A thorough medical history, review of systems, and physical exam (with focus on the neurologic exam) can suggest the presence of medical conditions that could mimic or be associated with autism. The symptoms of autism are traditionally divided into three domains: social, communication, and repetitive behavior/narrow interests (Table 1):
- Social impairment includes problems with nonverbal behaviors such as eye contact, facial expressions, and “body language;” failure to develop peer relationships; lack of spontaneous seeking to share enjoyment, interests, or achievements with other people; and lack of social or emotional reciprocity.
- Communication impairment includes language delay, decreased communication with others, conversational impairment, echolalia, and lack of imaginative or social imitative play.
- Impairments in behavior, interests, and activity take the form of all-encompassing preoccupations, “need for sameness” and compulsive rituals, motor stereotypies, and preoccupation with parts of objects.
After the preliminary history and exam, you should have an initial impression as to whether a PDD may be present. Conditions that could mimic or co-exist with autism will then need to be evaluated (Table 2).
Hearing and vision testing Every child presenting with a language or cognitive delay should have an adequate hearing assessment, including an audiogram at the very least. If results are equivocal, then brainstem auditory-evoked responses are indicated to establish the auditory system’s integrity. Vision screening should be completed, and the child should be referred to an ophthalmologist if a problem is suspected.
IQ testing A child with cognitive delays, learning problems, or suspected PDD should be referred to an experienced psychologist for intelligence testing. Public school systems often provide this service. Intelligence testing can document mental retardation and offer important information about the child’s strengths and weaknesses in learning.
Speech and language assessment A speech and language pathologist should evaluate children with PDD and/or language problems for articulation, prosody, receptive and expressive language, and pragmatics.
Lab and genetic tests Laboratory investigation in a child with cognitive delays should include routine blood chemistries, CBC, thyroid function tests, and lead level. Screen for fragile X syndrome, as its symptoms overlap those of autism, and for disorders of amino acid and organic acid metabolism. Finally, consider a chromosome karyotype, especially in patients with dysmorphic features on physical exam.
Table 1
THE THREE DOMAINS OF IMPAIRMENT IN AUTISM
Impairment in social interaction
|
Impairment in communication
|
Restricted repetitive and stereotyped patterns of behavior, interests, and activities
|
| Adapted from DSM-IV-TR, American Psychiatric Association, 2000 |
EEG Obtain a sleep-deprived electroencephalogram (EEG) in children with a history of significant language regression, episodic symptoms, or other indicators of possible seizures. Ideally, the EEG should include monitoring during sleep to help rule out acquired epileptic aphasia (Landau-Kleffner syndrome), a rare disorder associated with late-onset language regression. MRI of the brain is not routine but should be considered if indicated by the history or neurologic exam.
Consultations Consider consulting colleagues in neurology and medical genetics, especially when patients present with definite neurologic signs and symptoms or obvious dysmorphic features. These medical specialists are trained to screen for complex and rare syndromes that are sometimes associated with features of PDD.
Step 2. Is it PDD or another psychiatric disorder?
Psychiatric disorders that can be mistaken for PDD are listed in Table 3. The central feature of all PDDs is disturbance in social relatedness, and a diagnosis of PDD requires a history of significant social impairment.
Problems with social reciprocity in PDD are qualitatively different from the social impairment seen in other psychiatric disorders. For example, a child with attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) may have few friends because of a tendency to act impulsively and get into frequent conflicts with others. These social difficulties would not be considered indicative of PDD, as they typify those seen in children with ADHD and ODD.
Mental retardation Although mental retardation occurs in approximately 75% of persons with autism, most patients with mental retardation do not have autism. In assessing an individual with mental retardation for symptoms of PDD, consider the overall developmental level. It is not uncommon for individuals with mental retardation to have mild social problems, a history of language delay, and even motor stereotypies. These symptoms are considered indicative of PDD only when they are more severe than would be expected for the patient’s developmental level.
Table 2
TESTING OPTIONS FOR PATIENTS WITH PDD
| Hearing evaluation | Fragile X testing |
| Vision evaluation | Amino acids/organic acids |
| IQ testing | Chromosome karyotype |
| Speech/language evaluation | EEG |
| Chemistries, CBC, | Brain MRI |
| thyroid function tests | Neurology consultation |
| Lead level | Genetics consultation |
Table 3
DIFFERENTIAL DIAGNOSIS OF PDD
| Mental retardation |
| Reactive attachment disorder |
| Language disorders |
| Stereotypic movement disorder |
| Attention-deficit/hyperactivity disorder |
| Social phobia |
| Obsessive-compulsive disorder |
| Selective mutism |
| Schizophrenia |
| Personality disorders |
| Normality |
Other symptoms that are relatively specific to autism and PDD include lack of appropriate eye-to-eye gaze, abnormal speech prosody, echolalia, pronominal reversal, and narrow and circumscribed interests. The presence of these symptoms in excess should increase your suspicion of comorbid PDD.
RAD Reactive attachment disorder presents with abnormal social relatedness that can sometimes be confused with milder PDDs, especially in patients with comorbid mental retardation. In RAD, however, a history of severe neglect or abuse is thought to have caused the abnormal social relatedness. Placing the child in a caring and secure environment should improve many of the social deficits.
Language disorders are distinguished from PDDs by the absence of marked social impairment and lack of restricted interests and repetitive behaviors. In addition, children with primary language disorders often have intact nonverbal communication skills and make other attempts to communicate (e.g., through gesture, eye contact).
Stereotypic movement disorder can be seen in individuals with and without comorbid mental retardation. It is not diagnosed in the presence of autism, as these movements are thought to be part of the underlying disorder. The lack of social and communication impairments distinguishes stereotypic movement disorder from PDD.
ADHD Many children with autism and other PDDs have interfering symptoms of inattention, hyperactivity, and impulsivity. We usually do not give them an additional diagnosis of ADHD, as these symptoms are common in PDD. The pathophysiology of these symptoms may be different in ADHD and PDD, as evidenced by the frequent report of adverse effects following stimulant treatment of children with autism.2
Social phobia In higher functioning individuals with PDD, excessive social anxiety can sometimes be confused with social phobia. In social phobia, however, individuals usually do not exhibit marked problems with social relatedness and are able to interact normally with persons they know well and in some situations.
OCD Obsessive-compulsive disorder can occur in individuals with PDD but must be distinguished from the abnormal preoccupations and ritualistic behavior characteristic of autism. In autism, these activities often differ in quality from obsessions and compulsions.3 Furthermore, they usually are not associated with distress, and repetitive behaviors are not linked to a specific obsession.
Selective mutism is usually easy to distinguish from PDD because the affected child is typically able to talk in certain environments, such as at home. Also, the onset of selective mutism follows a period of normal social and communicative development.
Schizophrenia Autism was historically conceptualized as a type of childhood schizophrenia but is now thought to be distinct from the psychotic disorders. Schizophrenia with onset in childhood is much more rare than autism. Its onset usually occurs after several years of normal development, though some children with schizophrenia may have symptoms that resemble PDD early in their illness.4 Autistic persons may at times present with symptoms of a thought disorder. A diagnosis of schizophrenia usually is not made without evidence of prominent delusions and hallucinations.
Personality disorders PDDs are sometimes difficult to distinguish from personality disorders with similar features (e.g., schizotypal personality, schizoid personality). The social impairment in autistic and Asperger’s disorders is generally of earlier onset and greater severity than that seen in personality disorders. Those with personality disorders also typically lack stereotyped language or repetitive behaviors that are common in PDDs.
Table 4
CHARACTERISTIC FEATURES OF DSM-IV SUBTYPES OF PERVASIVE DEVELOPMENTAL DISORDERS
| Feature | Autistic disorder | Asperger’s disorder | Rett’s disorder | Childhood disintegrative disorder | Pervasive developmental disorder NOS* |
|---|---|---|---|---|---|
| Sex | Male:female ratio 4:1 | Male > female | Females only | Male > female | Male > female |
| Age of onset | < 3 years | Variable | 5-30 months | 2-10 years | Variable |
| Presence of regression | Mild regression in minority of patients | No | Yes | Yes | No |
| IQ | Most have mental retardation | Most have normal intellectual functioning | Severe mental retardation | Severe mental retardation | Variable |
| Social impairment | Yes | Yes | Yes | Yes | Yes |
| Communication impairment | Yes | No | Yes | Yes | Variable |
| Restricted interests/repetitive behaviors | Yes | Yes | Yes | Yes | Variable |
| Motor involvement | Usually not | Some have motor clumsiness | Gait and trunk ataxia; loss of purposeful hand movements | Loss of bowel/bladder control | Variable |
| *NOS: Not otherwise specified | |||||
Social awkwardness Finally, some of the PDDs that allow higher functioning (e.g., Asperger’s disorder and PDD not otherwise specified [NOS]) need to be distinguished from normal social awkwardness that can be common, especially in adolescence. The social impairment in PDD is marked and interferes with normal functioning and development.
Step 3. Which PDD is it?
DSM-IV describes five subtypes of PDD (autistic disorder, Asperger’s disorder, Rett’s disorder, childhood disintegrative disorder, and PDD NOS) that have in common problems with reciprocal social interaction (Table 4). For psychiatrists making the diagnosis, it is probably most difficult to differentiate the two most common types: autistic and Asperger’s disorders.
Autistic disorder is the prototypical PDD that is associated with abnormalities in reciprocal social interaction, qualitative impairments in communication, and narrow interests and repetitive behaviors (Table 1). By definition, symptoms of the disorder manifest by age 3.
Asperger’s disorder has several features that distinguish it from autistic disorder:
- Children with Asperger’s disorder do not have language delays. By definition, a child who has not developed single words by age 2 cannot be diagnosed with Asperger’s disorder.
- Early cognitive development in Asperger’s disorder is normal. Children with Asperger’s disorder are much more likely to have normal or above-average intellectual functioning than children with autistic disorder.
- Circumscribed interests and intense preoccupations are more common than motor stereotypies.
- Affected children may show verbal abilities that greatly exceed their visual-spatial skills. This may be apparent on individualized intelligence testing (i.e., verbal IQ > performance IQ) and clinically in the form of good language abilities but lagging fine-motor development (e.g., clumsiness).
Rett’s disorder occurs almost exclusively in girls, whereas autistic disorder is more common in boys. Following a brief period of normal development, affected girls experience deceleration of head growth, loss of previously acquired purposeful hand skills with subsequent development of stereotyped hand-wringing movements, loss of social engagement, onset of trunk and gait ataxia, and severe language and cognitive impairment. Genetic testing for mutations at MECP2 will be positive in most patients having all features of the classic phenotype.5
Childhood disintegrative disorder is thought to be more rare than autistic disorder. Following at least 2 years of normal development, affected children lose previously acquired skills. These can include play skills, language, social skills, bowel or bladder control, and motor skills. The children show impairments in social interaction, communication, and behavior of the type common to autistic disorder and often have severe mental retardation. The late onset of severe regression in development often prompts extensive neurologic evaluation, but a specific etiology is usually not found. The disorder is not diagnosed if full diagnostic criteria for autistic disorder are met (including onset of symptoms before age 3).
PDD NOS is diagnosed in many patients who are determined to have a significant impairment in social relatedness but do not meet full criteria for a specific PDD. Recent epidemiologic studies suggest that PDD NOS may be more common than autistic disorder.6 It may also be an appropriate designation for children with other proposed diagnostic constructs, such as nonverbal learning disabilities7 and multiple complex developmental disorders.8
Unfortunately, children diagnosed with PDD NOS instead of the better-recognized term “autism” may be denied appropriate social and financial services. When you inform others about the diagnosis of PDD NOS, it is important to emphasize to parents, schools, and funding agencies that PDD NOS is related to autism and should be considered one of the “autism spectrum disorders.” Children with PDDs or autism spectrum disorders will often benefit from similar treatment and educational interventions. In addition, their needs are equivalent for adequate insurance coverage and funding for specialized treatments.
- Cohen DJ, Volkmar FR (eds). Handbook of autism and pervasive developmental disorders (2nd ed). New York: Wiley, 1997.
- National Institute of Mental Health. Autism booklet. http://www.nimh.nih.gov/publicat/autism.cfm
- Autism Society of America http://www.autism-society.org Online Asperger Syndrome Information and Support (OASIS) http://www.udel.edu/bkirby/asperger
1. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harvard Rev Psychiatry 2000;8:45-63.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association, 2000.
3. McDougle CJ, Kresch LE, Goodman WK, et al. A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive-compulsive disorder. Am J Psychiatry 1995;152:772-7.
4. Kumra S. The diagnosis and treatment of children and adolescents with schizophrenia. “My mind is playing tricks on me.” Child Adolesc Psychiatr Clin N Am 2000;9:183-99.
5. Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet 1999;23:185-8.
6. Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA 2001;285:3093-9.
7. Rourke B. Nonverbal learning disabilities: The syndrome and the model. New York: Guilford Press, 1989.
8. Towbin KE, Dykens ED, Pearson GS, Cohen DJ. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993;32:775-82.
1. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harvard Rev Psychiatry 2000;8:45-63.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association, 2000.
3. McDougle CJ, Kresch LE, Goodman WK, et al. A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive-compulsive disorder. Am J Psychiatry 1995;152:772-7.
4. Kumra S. The diagnosis and treatment of children and adolescents with schizophrenia. “My mind is playing tricks on me.” Child Adolesc Psychiatr Clin N Am 2000;9:183-99.
5. Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet 1999;23:185-8.
6. Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA 2001;285:3093-9.
7. Rourke B. Nonverbal learning disabilities: The syndrome and the model. New York: Guilford Press, 1989.
8. Towbin KE, Dykens ED, Pearson GS, Cohen DJ. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993;32:775-82.