Article
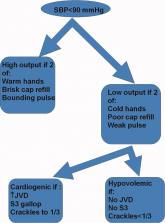
Accuracy of bedside physical examination in distinguishing categories of shock
- Author:
- Rodrigo Vazquez, MD
- Cristina Gheorghe, MD
- David Kaufman, MD
- Constantine A. Manthous, MD
Article
Enhanced end‐of‐life care associated with deploying a rapid response team: A pilot study
- Author:
- Rodrigo Vazquez, MD
- Cristina Gheorghe, MD
- Artur Grigoriyan, MD
- Tatsiana Palvinskaya, MD
- Yaw Amoateng‐Adjepong, MD, PhD
- Constantine A. Manthous, MD
