Damian McNamara is a journalist for Medscape Medical News and MDedge. He worked full-time for MDedge as the Miami Bureau covering a dozen medical specialties during 2001-2012, then as a freelancer for Medscape and MDedge, before being hired on staff by Medscape in 2018. Now the two companies are one. He uses what he learned in school – Damian has a BS in chemistry and an MS in science, health and environmental reporting/journalism. He works out of a home office in Miami, with a 100-pound chocolate lab known to snore under his desk during work hours.
News
Mirikizumab Shows Promise for Moderate to Severe Crohn’s Disease
- Author:
- Damian McNamara
Safety outcomes during the 52-week study were “consistent with the known safety profile” of mirikizumab.
News
Measles Control So Far in 2024: ‘Not Off to a Great Start’
- Author:
- Damian McNamara
- MA
The recent rise in cases across the U.S. is linked to unvaccinated travelers, lower than ideal vaccination rates, and misinformation.
News
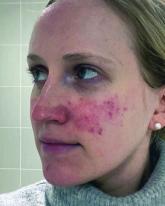
A Neurotoxin, an Antidepressant, and More Emerging Options for Treating Rosacea
- Author:
- Damian McNamara
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, so paroxetine "may be beneficial in...
News
Treating Acne Scars Can Improve Aesthetics, Quality of Life
- Author:
- Damian McNamara
Different approaches may be required even for the same patient because some people present with all three types of acne scars, said Robyn...
News
Think Outside the Traditional Toolbox to Treat Itch
- Author:
- Damian McNamara
Itch is a common symptom that may be debilitating for patients.
News

Cutaneous lupus, dermatomyositis: Excitement growing around emerging therapies
- Author:
- Damian McNamara
Anticipation is growing for emerging therapies and their potential to provide relief to patients, said Anthony Fernandez, MD, PhD.
News
Hair Loss in Children: How to Spot and Treat Different Causes
- Author:
- Damian McNamara
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid...
News

A Look at the Evidence Linking Diet to Skin Conditions
- Author:
- Damian McNamara
Milk and other dairy products can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
News
More Evidence Suggests That ‘Long Flu’ Is a Thing
- Author:
- Damian McNamara
‘Long flu,’ or symptoms lasting 4 weeks or more after hospitalization, can have long-term impact.
News
GLP-1s Face Off Against Each Other, Weight-Loss Surgery in New GI Studies
- Author:
- Damian McNamara
“While clinical trials have been conducted to assess the weight-loss efficacy of GLP-1 agonists, there has been limited head-to-head comparisons,...
News
Rise in number of unclaimed dead bodies used in medical schools
- Author:
- Damian McNamara
The number of unclaimed bodies accepted by medical schools in Texas rose by almost seven times between 2017 and 2021.
News
Hold Ozempic before surgery to optimize patient safety?
- Author:
- Damian McNamara
“It’s a really hot issue now. We are getting emails from our members looking for guidance.”
News
Nearly one in three patients with IBD affected by skin lesions
- Author:
- Damian McNamara
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it...
News
AI-assisted colonoscopy in IBD: Not all it’s cut out to be?
- Author:
- Damian McNamara
The lower ADR rate in AI-assisted procedures could be the result of an overreliance on the AI technology and shorter procedure times, which may...
News
Mental health risks higher among young people with IBD
- Author:
- Damian McNamara
“Anxiety and depression will not be a surprise to most of us. But we also saw changes for eating disorders, PTSD, and sleep changes.”
