User login
Sacral Insufficiency Fracture After Partial Sacrectomy
Chordomas persist as one of the rarer malignancies, accounting for approximately 1% to 4% of primary bone cancers.1 When chordomas occur, these tumors localize predominantly in the sacrococcygeal region.2 In addition to the urgency for addressing a relatively fast-growing tumor, the anatomical complexity of this area complicates the potential treatments. Furthermore, because of the lack of definitive symptoms, diagnosis is often difficult and typically occurs later in the disease progression.3 An aggressive treatment approach is often warranted because of the biologically aggressive nature of this disease. Full or partial sacrectomy is often the only option that offers the possibility of a long-term cure.4 A sacrectomy is a destructive procedure that can lead to mechanical instability depending on the extent of the surgical resection. When the entire sacrum is removed, there is an obvious need for lumbar-pelvic fixation; however, traditionally, partial sacrectomy procedures have been successfully performed without the need for instrumentation.3,4
This report describes the case of a patient with a noninstrumented sacrectomy procedure distal to the S2 foramen that resulted in an insufficiency fracture. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman presented with severe lower back pain of a month’s duration. Her pain was localized to the coccyx area and did not radiate to the lower legs. Although the pain could not be elicited by palpation, pain occurred when sitting and increased when standing for prolonged periods. Three weeks prior to the patient’s initial office visit, she noticed transient constipation and urinary retention. She denied any fever, chills, nausea, vomiting, unexplained weight loss, weight gain, and abdominal pain. There were no motor deficits in the lower limbs. Sensation was intact in the lower limbs except for the posterior aspect of the left leg down to the popliteal fossa, where light touch perception was absent. She recalled the loss of sensation in this area 20 years earlier, and it had neither progressed nor abated since then. She had a history of osteoarthritis and had been diagnosed with degenerative disc disease 20 years ago.
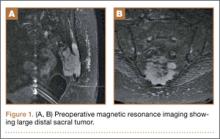
A radiographic review of her lumbar spine showed significant spinal stenosis and degenerative disease of the lumbar spine on non–contrast-enhanced magnetic resonance imaging (MRI). The MRI also revealed a large, soft-tissue mass at the S3-S4 level, eroding most of the S3 vertebral body and extending into the S4 vertebral body. The MRI images used for this analysis were insufficient in providing a complete portrayal of the entire mass. Because of these uncertainties, contrast-enhanced and non–contrast-enhanced pelvic MRIs were taken. The MRI analyses identified a mass density replacing the lower sacrum and upper coccyx that was bright in intensity on T2 and dim on T1 sequences. Sagittal imaging measurements were 5.9×2.5 cm and 4.4 cm right-to-left on coronal imaging. The mass extended beyond the involved sacrococcygeal segments and dorsally beyond the normal cortical margin of the sacrum and coccyx (Figures 1A, 1B). Next, a computer tomographic–guided needle biopsy through a posterior paraspinal approach was obtained. The biopsy consisted of fragments of a malignant neoplasm consistent with physaliferous cells. The specimen was positive for pankeratin, keratin AE1/AE3, epithelial membrane antigen, and S100 protein. This supported a diagnosis of a sacral chordoma. An en bloc sacrectomy at S2; lumbar laminectomy at L5, S1, and S2; and thecal sac transection at the S3 nerve roots were planned.
Surgical Procedure
The patient was placed in the prone position after a colostomy and harvesting of a rectus flap in the supine position. A midline incision was made from the spinous process of L5 down through the tip of the coccyx, and soft tissues were elevated while maintaining hemostasis. The most distal part of the coccyx was transected, and using a combination of electrocautery and paraspinal elevators, rectal and peritoneal tissues were elevated off the ventral component of the coccyx until a hand could easily reach the bifurcation of the iliac vessels. Electrocautery transected paraspinal muscles at the S1 and S2 levels while the more cranial paraspinal musculature was elevated to allow for a laminectomy. The spinous processes were removed from L5 and the sacrum with a Leksell rongeur. A high-speed burr thinned the dorsal lamina components of L5, S1, and the leading edge of S2. The L5, S1, and S2 nerve roots were identified. The gluteal muscles were elevated and the sacral coccygeal ligaments were transected. After identifying the sciatic notches, the S2 nerves exiting the foramen were identified, followed out through the sciatic notch, and a wire was passed through this region. Three 2-0 silk ties were applied to the exposed portion of the S3 and S4 nerve roots, and the nerves were transected because they were integrally involved with the tumor. Using a series of high-speed burrs and osteotomes, lateral cuts were made through the sciatic notch. The sacrum was osteotomized at the S2 sacral foramen through the anterior component with an osteotome, while a hand protected the ventral structures. The remaining parts of the S3 and S4 dorsal nerve roots were transected. An incision through the peritoneum was made to access the rectus flap, and a plastic surgeon closed the wounds and secured the flap.
Postoperative Course
The patient’s final pathology confirmed a chordoma with negative margins. Postoperatively, the rectus flap became ischemic and a wound infection developed. It was irrigated, débrided, and treated with vacuum-assisted closure (VAC), in addition to perioperative antibiotic administration. An abdominal computed tomography (CT) scan did not show any fistula, and her wound remained healthy, pink, and viable as her VAC was changed every 3 days. Because the patient’s nutritional status was compromised, she started nutritional supplements in addition to a regular diet. Physical therapy was prescribed and the patient began bladder training with self-catheterization after a failed voiding trial attempt. After 2 months of convalescence, the patient had mobilized well and had progressed to walking without an ambulatory aide.
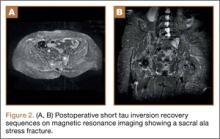
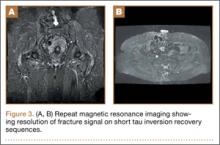
At her third postoperative month, the patient noted new onset of extreme pain in the groin and left thigh regions. The patient was examined and appeared to have a stable neurological exam. She had reproducible pain with a FABER (Flexion, Abduction, External Rotation, and Extension) test. MRI showed increased signal on short tau inversion recovery (STIR) sequences and T2-weighted images that was consistent with a left sacral ala stress fracture with a vertically oriented fracture line (Figures 2A, 2B). The patient was asked to begin utilizing a walker for ambulatory assistance, but her weight-bearing status was not changed. Over the course of 3 months, the patient noted a resolution of her pain. All postoperative MRI images confirmed the patient to be disease-free; and in addition, all of her follow-up radiographs showed a stable pelvic ring (Figures 3A, 3B). At her 2-year follow-up, the patient remained disease- and pain-free.
Discussion
Full discussions of the mechanical considerations of a partial sacrectomy have been described previously5-8; however, surgeons typically consider the need for lumbar-pelvic stabilization when the surgical resection requires a violation of the S1 body. Approximately two-thirds of sacral tumors occur at or below the level of the S2 body.8 These lesions of the caudal sacrum can sometimes be effectively resected with transverse partial sacrectomy. Great care is taken to resect only the portion of the sacrum necessary for local disease control, sparing as much of the sacroiliac joint and as many of the lumbosacral nerve roots as possible.
Under normal conditions, the sacroiliac articulation is stabilized by both its geometric interface and its extraordinarily strong ligaments. This spatial arrangement conveys stability primarily against caudal migration of the sacrum. The sacroiliac, sacrotuberous, sacrospinous, and lumbosacral ligaments, which are among the strongest ligaments in the body, primarily act to provide stability to the pelvic ring by preventing diastasis. The combination of these factors renders the spinopelvic segment especially stable. Previously, 2 biomechanical studies that specifically looked at extreme loading patterns to better understand the need for lumbar-pelvic instrumentation predicted a fracture pattern when there was an inability of the base of the sacral ala to resist shear.8,9 This is precisely where our patient’s insufficiency fracture occurred.
To our knowledge, this is the first reported in vivo evidence of this fracture pattern. While this patient’s potential history of osteoporosis may have elevated or contributed to her risk for fracture, her preoperative bone densitometry, with T scores of -1.0 on the left and right femur necks and 0.8 on her L1-L4 anteroposterior spine, would argue against this risk factor. None of these values represent a truly osteoporotic patient. It would appear that our patient sustained the fracture pattern predicted by Hugate and colleagues.8
The edema seen on the MRI most likely represents a fracture; however, sacroiliitis and infection are also potential diagnoses. Because there was no tumor in this region on the preoperative scans, we thought that a residual tumor was unlikely. The signal changes seen on T2 MRI sequences represent edema. The use of a bone scan that detects healing bone may have been a useful additional study to confirm this fracture as opposed to sacroiliitis. A CT scan would have been a potentially useful study to provide detail of fracture displacement and the overall fracture pattern. Standing plain radiographs are best for viewing fracture displacement with weight-bearing.
Surgeons contemplating performing partial sacrectomies should bear in mind that, even with preservation of the S1 body, a potential for fracture exists as evidenced by our patient. In our opinion, this patient did not require instrumentation but a more gradual rehabilitation program.
1. Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine. 2010;19(6):1039-1040.
2. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973; 32(2):410-420.
3. Varga PP, Bors I, Lazary A. Sacral tumors and management. Orthop Clin North Am. 2009;40(1):105-123.
4. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9(5):396-403.
5. Cheng L, Yu Y, Zhu R, et al. Structural stability of different reconstruction techniques following total sacrectomy: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26 (10):977-981.
6. Yu BS, Zhuang XM, Li ZM, et al. Biomechanical effects of the extent of sacrectomy on the stability of lumbo-iliac reconstruction using iliac screw techniques: What level of sacrectomy requires the bilateral dual iliac screw technique? Clin Biomech (Bristol, Avon). 2010;25(9):867-872.
7. Yu B, Zheng Z, Zhuang X, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009;34(13):1370-1375.
8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop. 2006;450:82-88.
9. Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976; 47(6):635-642.
Chordomas persist as one of the rarer malignancies, accounting for approximately 1% to 4% of primary bone cancers.1 When chordomas occur, these tumors localize predominantly in the sacrococcygeal region.2 In addition to the urgency for addressing a relatively fast-growing tumor, the anatomical complexity of this area complicates the potential treatments. Furthermore, because of the lack of definitive symptoms, diagnosis is often difficult and typically occurs later in the disease progression.3 An aggressive treatment approach is often warranted because of the biologically aggressive nature of this disease. Full or partial sacrectomy is often the only option that offers the possibility of a long-term cure.4 A sacrectomy is a destructive procedure that can lead to mechanical instability depending on the extent of the surgical resection. When the entire sacrum is removed, there is an obvious need for lumbar-pelvic fixation; however, traditionally, partial sacrectomy procedures have been successfully performed without the need for instrumentation.3,4
This report describes the case of a patient with a noninstrumented sacrectomy procedure distal to the S2 foramen that resulted in an insufficiency fracture. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman presented with severe lower back pain of a month’s duration. Her pain was localized to the coccyx area and did not radiate to the lower legs. Although the pain could not be elicited by palpation, pain occurred when sitting and increased when standing for prolonged periods. Three weeks prior to the patient’s initial office visit, she noticed transient constipation and urinary retention. She denied any fever, chills, nausea, vomiting, unexplained weight loss, weight gain, and abdominal pain. There were no motor deficits in the lower limbs. Sensation was intact in the lower limbs except for the posterior aspect of the left leg down to the popliteal fossa, where light touch perception was absent. She recalled the loss of sensation in this area 20 years earlier, and it had neither progressed nor abated since then. She had a history of osteoarthritis and had been diagnosed with degenerative disc disease 20 years ago.
A radiographic review of her lumbar spine showed significant spinal stenosis and degenerative disease of the lumbar spine on non–contrast-enhanced magnetic resonance imaging (MRI). The MRI also revealed a large, soft-tissue mass at the S3-S4 level, eroding most of the S3 vertebral body and extending into the S4 vertebral body. The MRI images used for this analysis were insufficient in providing a complete portrayal of the entire mass. Because of these uncertainties, contrast-enhanced and non–contrast-enhanced pelvic MRIs were taken. The MRI analyses identified a mass density replacing the lower sacrum and upper coccyx that was bright in intensity on T2 and dim on T1 sequences. Sagittal imaging measurements were 5.9×2.5 cm and 4.4 cm right-to-left on coronal imaging. The mass extended beyond the involved sacrococcygeal segments and dorsally beyond the normal cortical margin of the sacrum and coccyx (Figures 1A, 1B). Next, a computer tomographic–guided needle biopsy through a posterior paraspinal approach was obtained. The biopsy consisted of fragments of a malignant neoplasm consistent with physaliferous cells. The specimen was positive for pankeratin, keratin AE1/AE3, epithelial membrane antigen, and S100 protein. This supported a diagnosis of a sacral chordoma. An en bloc sacrectomy at S2; lumbar laminectomy at L5, S1, and S2; and thecal sac transection at the S3 nerve roots were planned.
Surgical Procedure
The patient was placed in the prone position after a colostomy and harvesting of a rectus flap in the supine position. A midline incision was made from the spinous process of L5 down through the tip of the coccyx, and soft tissues were elevated while maintaining hemostasis. The most distal part of the coccyx was transected, and using a combination of electrocautery and paraspinal elevators, rectal and peritoneal tissues were elevated off the ventral component of the coccyx until a hand could easily reach the bifurcation of the iliac vessels. Electrocautery transected paraspinal muscles at the S1 and S2 levels while the more cranial paraspinal musculature was elevated to allow for a laminectomy. The spinous processes were removed from L5 and the sacrum with a Leksell rongeur. A high-speed burr thinned the dorsal lamina components of L5, S1, and the leading edge of S2. The L5, S1, and S2 nerve roots were identified. The gluteal muscles were elevated and the sacral coccygeal ligaments were transected. After identifying the sciatic notches, the S2 nerves exiting the foramen were identified, followed out through the sciatic notch, and a wire was passed through this region. Three 2-0 silk ties were applied to the exposed portion of the S3 and S4 nerve roots, and the nerves were transected because they were integrally involved with the tumor. Using a series of high-speed burrs and osteotomes, lateral cuts were made through the sciatic notch. The sacrum was osteotomized at the S2 sacral foramen through the anterior component with an osteotome, while a hand protected the ventral structures. The remaining parts of the S3 and S4 dorsal nerve roots were transected. An incision through the peritoneum was made to access the rectus flap, and a plastic surgeon closed the wounds and secured the flap.
Postoperative Course
The patient’s final pathology confirmed a chordoma with negative margins. Postoperatively, the rectus flap became ischemic and a wound infection developed. It was irrigated, débrided, and treated with vacuum-assisted closure (VAC), in addition to perioperative antibiotic administration. An abdominal computed tomography (CT) scan did not show any fistula, and her wound remained healthy, pink, and viable as her VAC was changed every 3 days. Because the patient’s nutritional status was compromised, she started nutritional supplements in addition to a regular diet. Physical therapy was prescribed and the patient began bladder training with self-catheterization after a failed voiding trial attempt. After 2 months of convalescence, the patient had mobilized well and had progressed to walking without an ambulatory aide.
At her third postoperative month, the patient noted new onset of extreme pain in the groin and left thigh regions. The patient was examined and appeared to have a stable neurological exam. She had reproducible pain with a FABER (Flexion, Abduction, External Rotation, and Extension) test. MRI showed increased signal on short tau inversion recovery (STIR) sequences and T2-weighted images that was consistent with a left sacral ala stress fracture with a vertically oriented fracture line (Figures 2A, 2B). The patient was asked to begin utilizing a walker for ambulatory assistance, but her weight-bearing status was not changed. Over the course of 3 months, the patient noted a resolution of her pain. All postoperative MRI images confirmed the patient to be disease-free; and in addition, all of her follow-up radiographs showed a stable pelvic ring (Figures 3A, 3B). At her 2-year follow-up, the patient remained disease- and pain-free.
Discussion
Full discussions of the mechanical considerations of a partial sacrectomy have been described previously5-8; however, surgeons typically consider the need for lumbar-pelvic stabilization when the surgical resection requires a violation of the S1 body. Approximately two-thirds of sacral tumors occur at or below the level of the S2 body.8 These lesions of the caudal sacrum can sometimes be effectively resected with transverse partial sacrectomy. Great care is taken to resect only the portion of the sacrum necessary for local disease control, sparing as much of the sacroiliac joint and as many of the lumbosacral nerve roots as possible.
Under normal conditions, the sacroiliac articulation is stabilized by both its geometric interface and its extraordinarily strong ligaments. This spatial arrangement conveys stability primarily against caudal migration of the sacrum. The sacroiliac, sacrotuberous, sacrospinous, and lumbosacral ligaments, which are among the strongest ligaments in the body, primarily act to provide stability to the pelvic ring by preventing diastasis. The combination of these factors renders the spinopelvic segment especially stable. Previously, 2 biomechanical studies that specifically looked at extreme loading patterns to better understand the need for lumbar-pelvic instrumentation predicted a fracture pattern when there was an inability of the base of the sacral ala to resist shear.8,9 This is precisely where our patient’s insufficiency fracture occurred.
To our knowledge, this is the first reported in vivo evidence of this fracture pattern. While this patient’s potential history of osteoporosis may have elevated or contributed to her risk for fracture, her preoperative bone densitometry, with T scores of -1.0 on the left and right femur necks and 0.8 on her L1-L4 anteroposterior spine, would argue against this risk factor. None of these values represent a truly osteoporotic patient. It would appear that our patient sustained the fracture pattern predicted by Hugate and colleagues.8
The edema seen on the MRI most likely represents a fracture; however, sacroiliitis and infection are also potential diagnoses. Because there was no tumor in this region on the preoperative scans, we thought that a residual tumor was unlikely. The signal changes seen on T2 MRI sequences represent edema. The use of a bone scan that detects healing bone may have been a useful additional study to confirm this fracture as opposed to sacroiliitis. A CT scan would have been a potentially useful study to provide detail of fracture displacement and the overall fracture pattern. Standing plain radiographs are best for viewing fracture displacement with weight-bearing.
Surgeons contemplating performing partial sacrectomies should bear in mind that, even with preservation of the S1 body, a potential for fracture exists as evidenced by our patient. In our opinion, this patient did not require instrumentation but a more gradual rehabilitation program.
Chordomas persist as one of the rarer malignancies, accounting for approximately 1% to 4% of primary bone cancers.1 When chordomas occur, these tumors localize predominantly in the sacrococcygeal region.2 In addition to the urgency for addressing a relatively fast-growing tumor, the anatomical complexity of this area complicates the potential treatments. Furthermore, because of the lack of definitive symptoms, diagnosis is often difficult and typically occurs later in the disease progression.3 An aggressive treatment approach is often warranted because of the biologically aggressive nature of this disease. Full or partial sacrectomy is often the only option that offers the possibility of a long-term cure.4 A sacrectomy is a destructive procedure that can lead to mechanical instability depending on the extent of the surgical resection. When the entire sacrum is removed, there is an obvious need for lumbar-pelvic fixation; however, traditionally, partial sacrectomy procedures have been successfully performed without the need for instrumentation.3,4
This report describes the case of a patient with a noninstrumented sacrectomy procedure distal to the S2 foramen that resulted in an insufficiency fracture. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman presented with severe lower back pain of a month’s duration. Her pain was localized to the coccyx area and did not radiate to the lower legs. Although the pain could not be elicited by palpation, pain occurred when sitting and increased when standing for prolonged periods. Three weeks prior to the patient’s initial office visit, she noticed transient constipation and urinary retention. She denied any fever, chills, nausea, vomiting, unexplained weight loss, weight gain, and abdominal pain. There were no motor deficits in the lower limbs. Sensation was intact in the lower limbs except for the posterior aspect of the left leg down to the popliteal fossa, where light touch perception was absent. She recalled the loss of sensation in this area 20 years earlier, and it had neither progressed nor abated since then. She had a history of osteoarthritis and had been diagnosed with degenerative disc disease 20 years ago.
A radiographic review of her lumbar spine showed significant spinal stenosis and degenerative disease of the lumbar spine on non–contrast-enhanced magnetic resonance imaging (MRI). The MRI also revealed a large, soft-tissue mass at the S3-S4 level, eroding most of the S3 vertebral body and extending into the S4 vertebral body. The MRI images used for this analysis were insufficient in providing a complete portrayal of the entire mass. Because of these uncertainties, contrast-enhanced and non–contrast-enhanced pelvic MRIs were taken. The MRI analyses identified a mass density replacing the lower sacrum and upper coccyx that was bright in intensity on T2 and dim on T1 sequences. Sagittal imaging measurements were 5.9×2.5 cm and 4.4 cm right-to-left on coronal imaging. The mass extended beyond the involved sacrococcygeal segments and dorsally beyond the normal cortical margin of the sacrum and coccyx (Figures 1A, 1B). Next, a computer tomographic–guided needle biopsy through a posterior paraspinal approach was obtained. The biopsy consisted of fragments of a malignant neoplasm consistent with physaliferous cells. The specimen was positive for pankeratin, keratin AE1/AE3, epithelial membrane antigen, and S100 protein. This supported a diagnosis of a sacral chordoma. An en bloc sacrectomy at S2; lumbar laminectomy at L5, S1, and S2; and thecal sac transection at the S3 nerve roots were planned.
Surgical Procedure
The patient was placed in the prone position after a colostomy and harvesting of a rectus flap in the supine position. A midline incision was made from the spinous process of L5 down through the tip of the coccyx, and soft tissues were elevated while maintaining hemostasis. The most distal part of the coccyx was transected, and using a combination of electrocautery and paraspinal elevators, rectal and peritoneal tissues were elevated off the ventral component of the coccyx until a hand could easily reach the bifurcation of the iliac vessels. Electrocautery transected paraspinal muscles at the S1 and S2 levels while the more cranial paraspinal musculature was elevated to allow for a laminectomy. The spinous processes were removed from L5 and the sacrum with a Leksell rongeur. A high-speed burr thinned the dorsal lamina components of L5, S1, and the leading edge of S2. The L5, S1, and S2 nerve roots were identified. The gluteal muscles were elevated and the sacral coccygeal ligaments were transected. After identifying the sciatic notches, the S2 nerves exiting the foramen were identified, followed out through the sciatic notch, and a wire was passed through this region. Three 2-0 silk ties were applied to the exposed portion of the S3 and S4 nerve roots, and the nerves were transected because they were integrally involved with the tumor. Using a series of high-speed burrs and osteotomes, lateral cuts were made through the sciatic notch. The sacrum was osteotomized at the S2 sacral foramen through the anterior component with an osteotome, while a hand protected the ventral structures. The remaining parts of the S3 and S4 dorsal nerve roots were transected. An incision through the peritoneum was made to access the rectus flap, and a plastic surgeon closed the wounds and secured the flap.
Postoperative Course
The patient’s final pathology confirmed a chordoma with negative margins. Postoperatively, the rectus flap became ischemic and a wound infection developed. It was irrigated, débrided, and treated with vacuum-assisted closure (VAC), in addition to perioperative antibiotic administration. An abdominal computed tomography (CT) scan did not show any fistula, and her wound remained healthy, pink, and viable as her VAC was changed every 3 days. Because the patient’s nutritional status was compromised, she started nutritional supplements in addition to a regular diet. Physical therapy was prescribed and the patient began bladder training with self-catheterization after a failed voiding trial attempt. After 2 months of convalescence, the patient had mobilized well and had progressed to walking without an ambulatory aide.
At her third postoperative month, the patient noted new onset of extreme pain in the groin and left thigh regions. The patient was examined and appeared to have a stable neurological exam. She had reproducible pain with a FABER (Flexion, Abduction, External Rotation, and Extension) test. MRI showed increased signal on short tau inversion recovery (STIR) sequences and T2-weighted images that was consistent with a left sacral ala stress fracture with a vertically oriented fracture line (Figures 2A, 2B). The patient was asked to begin utilizing a walker for ambulatory assistance, but her weight-bearing status was not changed. Over the course of 3 months, the patient noted a resolution of her pain. All postoperative MRI images confirmed the patient to be disease-free; and in addition, all of her follow-up radiographs showed a stable pelvic ring (Figures 3A, 3B). At her 2-year follow-up, the patient remained disease- and pain-free.
Discussion
Full discussions of the mechanical considerations of a partial sacrectomy have been described previously5-8; however, surgeons typically consider the need for lumbar-pelvic stabilization when the surgical resection requires a violation of the S1 body. Approximately two-thirds of sacral tumors occur at or below the level of the S2 body.8 These lesions of the caudal sacrum can sometimes be effectively resected with transverse partial sacrectomy. Great care is taken to resect only the portion of the sacrum necessary for local disease control, sparing as much of the sacroiliac joint and as many of the lumbosacral nerve roots as possible.
Under normal conditions, the sacroiliac articulation is stabilized by both its geometric interface and its extraordinarily strong ligaments. This spatial arrangement conveys stability primarily against caudal migration of the sacrum. The sacroiliac, sacrotuberous, sacrospinous, and lumbosacral ligaments, which are among the strongest ligaments in the body, primarily act to provide stability to the pelvic ring by preventing diastasis. The combination of these factors renders the spinopelvic segment especially stable. Previously, 2 biomechanical studies that specifically looked at extreme loading patterns to better understand the need for lumbar-pelvic instrumentation predicted a fracture pattern when there was an inability of the base of the sacral ala to resist shear.8,9 This is precisely where our patient’s insufficiency fracture occurred.
To our knowledge, this is the first reported in vivo evidence of this fracture pattern. While this patient’s potential history of osteoporosis may have elevated or contributed to her risk for fracture, her preoperative bone densitometry, with T scores of -1.0 on the left and right femur necks and 0.8 on her L1-L4 anteroposterior spine, would argue against this risk factor. None of these values represent a truly osteoporotic patient. It would appear that our patient sustained the fracture pattern predicted by Hugate and colleagues.8
The edema seen on the MRI most likely represents a fracture; however, sacroiliitis and infection are also potential diagnoses. Because there was no tumor in this region on the preoperative scans, we thought that a residual tumor was unlikely. The signal changes seen on T2 MRI sequences represent edema. The use of a bone scan that detects healing bone may have been a useful additional study to confirm this fracture as opposed to sacroiliitis. A CT scan would have been a potentially useful study to provide detail of fracture displacement and the overall fracture pattern. Standing plain radiographs are best for viewing fracture displacement with weight-bearing.
Surgeons contemplating performing partial sacrectomies should bear in mind that, even with preservation of the S1 body, a potential for fracture exists as evidenced by our patient. In our opinion, this patient did not require instrumentation but a more gradual rehabilitation program.
1. Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine. 2010;19(6):1039-1040.
2. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973; 32(2):410-420.
3. Varga PP, Bors I, Lazary A. Sacral tumors and management. Orthop Clin North Am. 2009;40(1):105-123.
4. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9(5):396-403.
5. Cheng L, Yu Y, Zhu R, et al. Structural stability of different reconstruction techniques following total sacrectomy: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26 (10):977-981.
6. Yu BS, Zhuang XM, Li ZM, et al. Biomechanical effects of the extent of sacrectomy on the stability of lumbo-iliac reconstruction using iliac screw techniques: What level of sacrectomy requires the bilateral dual iliac screw technique? Clin Biomech (Bristol, Avon). 2010;25(9):867-872.
7. Yu B, Zheng Z, Zhuang X, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009;34(13):1370-1375.
8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop. 2006;450:82-88.
9. Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976; 47(6):635-642.
1. Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine. 2010;19(6):1039-1040.
2. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973; 32(2):410-420.
3. Varga PP, Bors I, Lazary A. Sacral tumors and management. Orthop Clin North Am. 2009;40(1):105-123.
4. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9(5):396-403.
5. Cheng L, Yu Y, Zhu R, et al. Structural stability of different reconstruction techniques following total sacrectomy: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26 (10):977-981.
6. Yu BS, Zhuang XM, Li ZM, et al. Biomechanical effects of the extent of sacrectomy on the stability of lumbo-iliac reconstruction using iliac screw techniques: What level of sacrectomy requires the bilateral dual iliac screw technique? Clin Biomech (Bristol, Avon). 2010;25(9):867-872.
7. Yu B, Zheng Z, Zhuang X, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009;34(13):1370-1375.
8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop. 2006;450:82-88.
9. Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976; 47(6):635-642.