User login
Broken Heart
Acute decompensated heart failure (ADHF) remains one of the most common reasons for hospitalization. ADHF patients who have co-morbid conditions present stubborn challenges for hospitalists. And ADHF is frequently observed in patients 65 and older. The neurohormonal activation that results as a consequence of myocardial dysfunction leads to progressive cardiac deterioration and hemodynamic disturbances that ultimately become manifest as acute decompensated heart failure.
ADHG management goals include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival.
In this article we present the case of a 26-year-old female with ADHF and highlight the management strategies that can result in stabilization and improved long-term outcome.
Introduction
Despite major advances in the treatment of heart disease, heart failure remains a growing public health problem of epidemic proportions in the United States. Approximately five million Americans have heart failure, and more than 550,000 patients are diagnosed with the disease each year.1 The annual number of hospitalizations for heart failure as a primary diagnosis has increased from approximately 810,000 in 1990 to more than 1 million in 1999, and it is the most common discharge diagnosis-related group for patients 65 and older.2 Medicare spent more dollars on the diagnosis and treatment of heart failure than on any other diagnosis—more than $27.9 billion in 2005.1
Patients presenting to the emergency department (ED) with ADHF are often hemodynamically unstable, with severe symptoms of dyspnea and fluid overload. Rapid assessment and prompt initiation of appropriate interventions are necessary to achieve clinical stability and prevent prolonged hospital stay if hospitalization is required. The in-hospital mortality rate for ADHF is 5%-8%; median duration of hospitalization is five days, and the six-month re-hospitalization rate is about 50%.1,3 Thus, it is clear that improved recognition and treatment are of paramount importance. With these goals in mind, we present a recent case that highlights many of the concerns about and treatment options for ADHF.
Case Presentation
Karen A. is a 26-year-old black female with stage III Hodgkin’s disease, diagnosed in 2000. She received chemotherapy (cisplatin, cytarabine, doxorubicin, rituxan, gemcitabine) and, as a result, in 2001 developed chemotherapy-induced cardiomyopathy with an ejection fraction of <20%. Her condition stabilized, and she remained in clinical remission until September 2002.
In 2003 she received an autologous stem cell transplant and subsequently presented to the ED with complaints of fatigue, progressive shortness of breath in the previous seven days, and lower extremity edema. She also reported right-sided pleuritic chest pain, but denied associated nausea, vomiting, or diaphoresis. In the three days preceding admission, she had gained 10 pounds. In the past six months, she had had multiple admissions for ADHF.
Her physical examination revealed an alert, obese female in moderate respiratory distress with dry mucous membranes. Her vital signs indicated a temperature of 36.5° C, a heart rate of 110 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure measuring 111/73 mm Hg, and an oxygen saturation of 92%. She had no scleral icterus, but did have jugular venous distention to the angle of the mandible at 45° upright. Her cardiac examination indicated tachycardia with distant heart sounds and an audible third heart sound (S3), as well as a grade 2/6 systolic ejection murmur at the left sternal border. Her lung examination demonstrated diffuse crackles present in both lung fields, but no wheezes. Abdominal examination was notable for tenderness to palpation at the right hypogastric region and for hepatomegaly, her skin was warm and dry with no cyanosis, and her extremities demonstrated significant bilateral pitting pre-tibial edema to her knees.
Her current medications included:
- Furosemide, 60 mg by mouth two times daily;
- Carvedilol, 12.5 mg by mouth in the morning, 6.25 mg in the evening;
- Amiodarone, 200 mg by mouth daily; and
- Digoxin, 0.125 mg by mouth daily.
Laboratory findings included normal electrolytes, blood urea nitrogen, and serum creatinine. Her hemoglobin was 11.3 gm/L, and her B-type natriuretic peptide (BNP) level was 4,837 pg/ml. Initial cardiac enzymes were negative (troponin I of <0. 03).
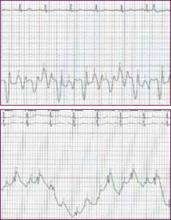
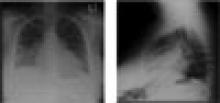
Her chest X-ray demonstrated moderate cardiac enlargement with bilateral thickening of subpleural septal lines and blurring of the pulmonary vasculature consistent with developing cardiogenic pulmonary edema. A 12-lead electrocardiogram indicated sinus tachycardia with a rate of 110 beats per minute and nonspecific ST-T wave changes in the inferior leads, but no Q waves were noted. Echocardiography showed severely reduced left ventricular systolic function with severe global hypokinesis of the left ventricle and a measured ejection fraction of 25%-30%. There was no pericardial effusion.
She was admitted to the telemetry floor with the diagnosis of ADHF. She was placed on supplemental oxygen and serial cardiac enzymes, and her electrocardiogram remained negative for injury or ischemia. Intravenous furosemide was initiated at 40 mg every 12 hours. After 24 hours, she was given a bolus of nesiritide (2 mcg/kg), followed by a continuous infusion at 0.01 mcg/kg/min. Cardiac medications were continued, and the dosage of carvedilol was reduced to 6.25 mg by mouth twice daily.
After a period of diuresis, the patient remained highly symptomatic; therefore, a right heart catheterization was done. The hemodynamics indicated a cardiac output of three liters per minute, a right atrial pressure of 20 mm Hg, a right ventricular pressure of 70/20 mm Hg, pulmonary artery pressure of 66/20 mm Hg with a mean pressure of 52 mm Hg, and a pulmonary capillary wedge pressure of 25 mm Hg. (See Figures 1 and 2, p. 23).
Over the next 24 hours, the patient had more than three liters of urine output and was significantly improved. The nesiritide infusion was discontinued after 48 hours of therapy, and the patient was weaned from supplemental oxygen. Lisinopril was started at 2.5 mg daily by mouth. On hospital day four, the patient walked around the nurses’ station without supplemental oxygen, and she was returned to the previous dose of furosemide, 60 mg twice daily.
The patient was enrolled in the Heart Success Program (HSP), a collaborative interdisciplinary program that is available in the institution for cancer patients with heart failure. She was provided with patient education materials that included educational videotapes on heart failure management, daily weight monitoring, diet, medications, exercise, and the emotional aspects of heart failure. Nurses with heart failure training were available to answer questions for the patient and to provide further instruction for follow-up after the patient’s discharge. The patient improved enough that she was able to enroll in a New York Heart Association (NYHA) class II and was discharged after five days, with a follow-up appointment to the outpatient clinic one week after hospital discharge.
Discussion
This case illustrates the challenges inherent in the diagnosis and management of ADHF in a cancer patient with a known history of heart failure. Rapid assessment is critical in establishing a diagnosis and initiating appropriate intervention. The goals of managing ADHF remain the same regardless of etiology. These include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival. The same principles apply—even if the patient has a major comorbidity such as cancer, and suspicion for the diagnosis must remain high.
Initial Evaluation
Early diagnosis and effective management of ADHF are critically important, as these have been shown to reduce hospitalizations and intensive care unit admissions, to decrease length of stay, and to decrease cost of hospitalization.4 A comprehensive history and physical examination must be performed to identify signs and symptoms that lead to a heart failure diagnosis.
We must evaluate such potential risk factors as history of hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, valvular disease, peripheral vascular disease, a family history of cardiomyopathy, smoking, alcohol use, thyroid problems, sleep apnea, and any recent history of infection (particularly upper respiratory tract infection, which can cause viral cardiomyopathy).
As part of further investigation with cancer patients, include the patient’s possible past exposure to cardiotoxic agents (e.g., anthracyclines, trastuzumab, high-dose cyclophosphamide) or mediastinal irradiation. Chemotherapy-induced cardiomyopathy is increasingly becoming an issue in heart failure management as a result of the growing number of long-term cancer survivors who have received treatment with anthracycline-containing chemotherapy or other aggressive therapy.
Focus on volume and perfusion status during the physical assessment of each patient. Most patients presenting to the ED with acute decompensation are volume overloaded. Volume overload is manifested by symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea, as well as jugular venous distention, hepatojugular reflux, ascites, edema, and crackles in the lungs.5 Crackles are not always present in chronic heart failure patients, however, because of the continuous movement of fluid into the interstitium associated with increased lymphatic drainage, leaving the alveoli relatively dry.6
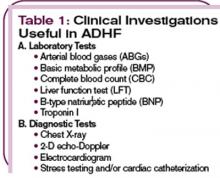
In addition to a comprehensive history and physical examination, several tests will help establish the diagnosis. (See Table 1, top left.) A measurement of a B-type natriuretic peptide (BNP) facilitates the diagnosis of ADHF. BNP is an endogenously generated natriuretic peptide that is activated in response to atrial or ventricular expansion due to volume overload and increased wall tension.7,8 Circulating levels of endogenous BNP are significantly elevated in ADHF patients and are a valuable tool for diagnosis of heart failure in the ED.9-11 In Karen A., the BNP level was 4,837 pg/mL, which is indicative of Stage D heart failure. This diagnosis was confirmed by chest X-ray findings of cardiogenic pulmonary edema in the setting of severe left ventricular hypokinesis and an ejection fraction of 25%-30%.
Although history and physical examination may provide important clues regarding the underlying cardiac abnormality, both invasive and noninvasive testing are necessary to provide a definitive diagnosis of heart failure and to evaluate potential exacerbating conditions. A two-dimensional echocardiogram with Doppler flow study is an essential diagnostic test for evaluating myocardial contractility or ejection fraction. Echocardiogram can also evaluate other structural components such as the pericardium, valvular status, and hemodynamic parameters that may contribute to the development of ADHF. In patients with cardiac risk factors, a myocardial perfusion stress test or catheterization may identify the presence of coronary artery disease as a contributor.
A 12-lead electrocardiogram is necessary to establish the rhythm and to show evidence of acute or prior myocardial infarction, pericarditis, conduction abnormalities, or left ventricular hypertrophy as a result of prolonged uncontrolled hypertension. It is known that rhythm disturbances such as atrial fibrillation can be a precipitating factor for ADHF.
A chest X-ray is needed to uncover pulmonary edema in cases of fluid overload and to show an enlarged cardiac silhouette in cases of dilated cardiomyopathy. Identification of a definitive cause or causes that precipitate the occurrence of ADHF is crucial in devising a management plan and initiating appropriate intervention. (See Table 2, p. 22.)
Immediate Management of the ADHF
Newly developed clinical practice guidelines for the management of ADHF exist, but management is still based largely on empirical evidence.12 Begin ADHF treatment in the ED with intravenous diuretics (unless contraindicated). A majority of ADHF patients will respond to diuretics alone.13 If the patient responds poorly to diuretics, the use of nesiritide in conjunction with diuretics has proven beneficial, as shown in the analysis of data from the ADHERE registry indicating that patients treated with intravenous nesiritide had a lower hospital mortality rate than patients treated with milrinone or dobutamine.14 Other options include ultrafiltration, an intervention that has been noted to reduce lengths of stay and rehospitalization rates in patients with ADHF.
Nesiritide is a recombinant form of BNP without direct inotropic effects but with venous, arterial, and coronary vasodilatory properties that can improve symptoms in ADHF.15 The recommended dosage for nesiritide is an IV bolus of 2 mcg per kg, followed by a continuous infusion of 0.01 mcg/kg/min. In the setting of hypotension with a systolic blood pressure less than 100 mm Hg, however, an initial IV bolus dose is not recommended; instead, the patient may start with a continuous infusion of 0.01 mcg/kg/min, or consider other therapies.
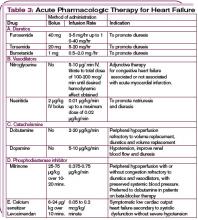
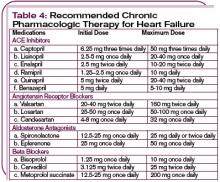
In hemodynamically unstable patients with a systolic blood pressure less than 90 mm Hg, or in those with evidence of end organ hypoperfusion (cardiogenic shock), inotropic support may be considered until the patient is stabilized. (See Table 3, p. 22.) Recognize that inotropic agents have adverse effects on the neurohormonal system and are not recommended routinely but may be essential for temporary stabilization. (For more on pharmacologic management of ADHF, see Table 4, p. 24.)
Subacute Management of ADHF
Once acute decompensation has been reversed and an euvolemic state has been achieved, shift therapy to a combination of three classes of medications: diuretics, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers, unless contraindicated. The benefits of these drugs have been established by evidence from numerous large-scale clinical trials.3,12
Start ACE inhibitors in all patients with heart failure due to left ventricular systolic dysfunction (unless contraindicated or if the patient is intolerant).3 Give ACE-I to patients who have experienced a recent episode of ADHF, along with diuretics to maintain sodium balance and to prevent peripheral and pulmonary edema. ACE inhibitors are contraindicated for patients who are pregnant and for those with childbearing potential, as well as for individuals with a prior history of angioedema or renal failure after receiving the drug. Instruct patients to avoid any sudden change of position, as they may experience orthostatic hypotension while taking ACE inhibitors.
Some patients are intolerant of ACE inhibitors due to a persistent cough that occurs in approximately 5% to 10% of Caucasian patients and in up to 50% of Chinese patients.16 Angiotensin receptor blockers (ARBs) are an established alternative.17 Two ARBs (candesartan and valsartan) are recommended for treatment of heart failure based on evidence from controlled clinical trials.17,18 These drugs have demonstrated a reduction in hospitalizations, and candesartan, when used as an alternative to ACE-I, has been shown to reduce mortality. Additionally, in patients with evidence of left ventricular dysfunction after myocardial infarction, valsartan provided a benefit that was not inferior to ACE inhibitors.17
Initiate beta-blockers at very low doses and gradually increased as tolerated. Monitor patients closely for symptoms of hypotension, significant weight gain, fluid retention, bradycardia, and heart block. In addition, inform patients that they may experience generalized fatigue or weakness with the initiation of beta-blockers. In a cancer patient it is difficult to differentiate between fatigue caused by the disease and the side effects of therapy. Fatigue associated with beta-blocker therapy usually resolves spontaneously within a few days. Make every effort to achieve optimum target beta-blocker dose.
An aldosterone antagonist, such as spironolactone or eplerenone, given at a daily dose of 12.5 to 25 mg in addition to standard therapy, effectively blocks the effects of aldosterone (RALES study) to achieve comprehensive neurohormonal blockade.19 When prescribing an aldosterone antagonist, especially in combination with ACE inhibitors and loop diuretics, it is important to monitor serum potassium levels because this combination can result in hyperkalemia.
Disease Management Programs
Comprehensive management of heart failure is not only limited to hospital care during an episode of ADHF. In order to prevent repeated hospitalizations, implement additional measures through formal disease management programs. These disease management programs are often directed or coordinated by advanced practice nurses who address the comprehensive care of heart failure patients with emphasis on patient education and counseling to improve patient compliance.20
The non-pharmacologic treatment strategies emphasized in disease management programs have proven effective in achieving positive outcomes. These include counseling patients on dietary management, including encouraging a two-gram sodium diet, alcohol restriction, and adequate supplementation of electrolyte loss from diuretics. Keeping a diary of the patient’s daily weight at home and bringing it to office visits will help both the patient and the clinician monitor fluid retention efficiently.
Hypotension is a common side effect from the pharmacologic therapy for heart failure. Employ comprehensive education with both patient and family to avoid unnecessary discontinuation of the medications. A systolic blood pressure of 90 mm Hg is acceptable as long as there are no associated symptoms of dizziness or syncope.
Encourage activity guidelines, including participation in exercise programs. Attendance at support group meetings will provide a venue in which patients can share common problems and concerns with others in similar situations. One to two weeks after hospital discharge, schedule an outpatient follow-up in a heart failure clinic, where heart failure education is reinforced to prevent another episode of ADHF admission.
Summary
Despite the added challenges, managing ADHF in a patient with a serious comorbidity such as cancer involves the same goals as the treatment of ADHF in any other patient. With rapid assessment and appropriate intervention, the patient is given the best possible chance of survival. TH
The authors work at the University of Texas M.D. Anderson Cancer Center, Department of Cardiology, Houston.
References
- American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas.: American Heart Association; 2005. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Last accessed August 20, 2006.
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004 Jan;147(1):74-78.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154-235.Epub 2005 Sep 13.
- Peacock WF IV, Emerman CL, Wynne J, for the ADHERE Scientific Advisory Committee and Investigators and the ADHERE Study Group. Early use of nesiritide in the emergency department is associated with improved outcome: an ADHERE registry analysis. Ann Emerg Med. 2004;44:S78.
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail. 1999 Aug;1(3):251-257.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888.
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995 Sep;96(3):1280-1287.
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825-832.
- Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005 Jan-Feb;11(1):30-38.
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul;106(4):416-422.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161-167.
- Adams KF, Lindenfeld J, Arnold JMO, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10-38.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol. 2005 Jul;46(1):65-67.
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul;46(1):57-64.
- Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002 Dec;144(6):1102-1108.
- Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995 Aug;40(2):141-144.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003 Nov 13;349(20):1893-1906. Epub 2003 Nov 10.
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-776.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-717.
- Albert NM, Eastwood CA, Edwards ML. Evidence-based practice for acute decompensated heart failure. Crit Care Nurse. 2004 Dec;24(6):14-16, 18-24, 26-29; quiz 30-31.
Acute decompensated heart failure (ADHF) remains one of the most common reasons for hospitalization. ADHF patients who have co-morbid conditions present stubborn challenges for hospitalists. And ADHF is frequently observed in patients 65 and older. The neurohormonal activation that results as a consequence of myocardial dysfunction leads to progressive cardiac deterioration and hemodynamic disturbances that ultimately become manifest as acute decompensated heart failure.
ADHG management goals include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival.
In this article we present the case of a 26-year-old female with ADHF and highlight the management strategies that can result in stabilization and improved long-term outcome.
Introduction
Despite major advances in the treatment of heart disease, heart failure remains a growing public health problem of epidemic proportions in the United States. Approximately five million Americans have heart failure, and more than 550,000 patients are diagnosed with the disease each year.1 The annual number of hospitalizations for heart failure as a primary diagnosis has increased from approximately 810,000 in 1990 to more than 1 million in 1999, and it is the most common discharge diagnosis-related group for patients 65 and older.2 Medicare spent more dollars on the diagnosis and treatment of heart failure than on any other diagnosis—more than $27.9 billion in 2005.1
Patients presenting to the emergency department (ED) with ADHF are often hemodynamically unstable, with severe symptoms of dyspnea and fluid overload. Rapid assessment and prompt initiation of appropriate interventions are necessary to achieve clinical stability and prevent prolonged hospital stay if hospitalization is required. The in-hospital mortality rate for ADHF is 5%-8%; median duration of hospitalization is five days, and the six-month re-hospitalization rate is about 50%.1,3 Thus, it is clear that improved recognition and treatment are of paramount importance. With these goals in mind, we present a recent case that highlights many of the concerns about and treatment options for ADHF.
Case Presentation
Karen A. is a 26-year-old black female with stage III Hodgkin’s disease, diagnosed in 2000. She received chemotherapy (cisplatin, cytarabine, doxorubicin, rituxan, gemcitabine) and, as a result, in 2001 developed chemotherapy-induced cardiomyopathy with an ejection fraction of <20%. Her condition stabilized, and she remained in clinical remission until September 2002.
In 2003 she received an autologous stem cell transplant and subsequently presented to the ED with complaints of fatigue, progressive shortness of breath in the previous seven days, and lower extremity edema. She also reported right-sided pleuritic chest pain, but denied associated nausea, vomiting, or diaphoresis. In the three days preceding admission, she had gained 10 pounds. In the past six months, she had had multiple admissions for ADHF.
Her physical examination revealed an alert, obese female in moderate respiratory distress with dry mucous membranes. Her vital signs indicated a temperature of 36.5° C, a heart rate of 110 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure measuring 111/73 mm Hg, and an oxygen saturation of 92%. She had no scleral icterus, but did have jugular venous distention to the angle of the mandible at 45° upright. Her cardiac examination indicated tachycardia with distant heart sounds and an audible third heart sound (S3), as well as a grade 2/6 systolic ejection murmur at the left sternal border. Her lung examination demonstrated diffuse crackles present in both lung fields, but no wheezes. Abdominal examination was notable for tenderness to palpation at the right hypogastric region and for hepatomegaly, her skin was warm and dry with no cyanosis, and her extremities demonstrated significant bilateral pitting pre-tibial edema to her knees.
Her current medications included:
- Furosemide, 60 mg by mouth two times daily;
- Carvedilol, 12.5 mg by mouth in the morning, 6.25 mg in the evening;
- Amiodarone, 200 mg by mouth daily; and
- Digoxin, 0.125 mg by mouth daily.
Laboratory findings included normal electrolytes, blood urea nitrogen, and serum creatinine. Her hemoglobin was 11.3 gm/L, and her B-type natriuretic peptide (BNP) level was 4,837 pg/ml. Initial cardiac enzymes were negative (troponin I of <0. 03).
Her chest X-ray demonstrated moderate cardiac enlargement with bilateral thickening of subpleural septal lines and blurring of the pulmonary vasculature consistent with developing cardiogenic pulmonary edema. A 12-lead electrocardiogram indicated sinus tachycardia with a rate of 110 beats per minute and nonspecific ST-T wave changes in the inferior leads, but no Q waves were noted. Echocardiography showed severely reduced left ventricular systolic function with severe global hypokinesis of the left ventricle and a measured ejection fraction of 25%-30%. There was no pericardial effusion.
She was admitted to the telemetry floor with the diagnosis of ADHF. She was placed on supplemental oxygen and serial cardiac enzymes, and her electrocardiogram remained negative for injury or ischemia. Intravenous furosemide was initiated at 40 mg every 12 hours. After 24 hours, she was given a bolus of nesiritide (2 mcg/kg), followed by a continuous infusion at 0.01 mcg/kg/min. Cardiac medications were continued, and the dosage of carvedilol was reduced to 6.25 mg by mouth twice daily.
After a period of diuresis, the patient remained highly symptomatic; therefore, a right heart catheterization was done. The hemodynamics indicated a cardiac output of three liters per minute, a right atrial pressure of 20 mm Hg, a right ventricular pressure of 70/20 mm Hg, pulmonary artery pressure of 66/20 mm Hg with a mean pressure of 52 mm Hg, and a pulmonary capillary wedge pressure of 25 mm Hg. (See Figures 1 and 2, p. 23).
Over the next 24 hours, the patient had more than three liters of urine output and was significantly improved. The nesiritide infusion was discontinued after 48 hours of therapy, and the patient was weaned from supplemental oxygen. Lisinopril was started at 2.5 mg daily by mouth. On hospital day four, the patient walked around the nurses’ station without supplemental oxygen, and she was returned to the previous dose of furosemide, 60 mg twice daily.
The patient was enrolled in the Heart Success Program (HSP), a collaborative interdisciplinary program that is available in the institution for cancer patients with heart failure. She was provided with patient education materials that included educational videotapes on heart failure management, daily weight monitoring, diet, medications, exercise, and the emotional aspects of heart failure. Nurses with heart failure training were available to answer questions for the patient and to provide further instruction for follow-up after the patient’s discharge. The patient improved enough that she was able to enroll in a New York Heart Association (NYHA) class II and was discharged after five days, with a follow-up appointment to the outpatient clinic one week after hospital discharge.
Discussion
This case illustrates the challenges inherent in the diagnosis and management of ADHF in a cancer patient with a known history of heart failure. Rapid assessment is critical in establishing a diagnosis and initiating appropriate intervention. The goals of managing ADHF remain the same regardless of etiology. These include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival. The same principles apply—even if the patient has a major comorbidity such as cancer, and suspicion for the diagnosis must remain high.
Initial Evaluation
Early diagnosis and effective management of ADHF are critically important, as these have been shown to reduce hospitalizations and intensive care unit admissions, to decrease length of stay, and to decrease cost of hospitalization.4 A comprehensive history and physical examination must be performed to identify signs and symptoms that lead to a heart failure diagnosis.
We must evaluate such potential risk factors as history of hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, valvular disease, peripheral vascular disease, a family history of cardiomyopathy, smoking, alcohol use, thyroid problems, sleep apnea, and any recent history of infection (particularly upper respiratory tract infection, which can cause viral cardiomyopathy).
As part of further investigation with cancer patients, include the patient’s possible past exposure to cardiotoxic agents (e.g., anthracyclines, trastuzumab, high-dose cyclophosphamide) or mediastinal irradiation. Chemotherapy-induced cardiomyopathy is increasingly becoming an issue in heart failure management as a result of the growing number of long-term cancer survivors who have received treatment with anthracycline-containing chemotherapy or other aggressive therapy.
Focus on volume and perfusion status during the physical assessment of each patient. Most patients presenting to the ED with acute decompensation are volume overloaded. Volume overload is manifested by symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea, as well as jugular venous distention, hepatojugular reflux, ascites, edema, and crackles in the lungs.5 Crackles are not always present in chronic heart failure patients, however, because of the continuous movement of fluid into the interstitium associated with increased lymphatic drainage, leaving the alveoli relatively dry.6
In addition to a comprehensive history and physical examination, several tests will help establish the diagnosis. (See Table 1, top left.) A measurement of a B-type natriuretic peptide (BNP) facilitates the diagnosis of ADHF. BNP is an endogenously generated natriuretic peptide that is activated in response to atrial or ventricular expansion due to volume overload and increased wall tension.7,8 Circulating levels of endogenous BNP are significantly elevated in ADHF patients and are a valuable tool for diagnosis of heart failure in the ED.9-11 In Karen A., the BNP level was 4,837 pg/mL, which is indicative of Stage D heart failure. This diagnosis was confirmed by chest X-ray findings of cardiogenic pulmonary edema in the setting of severe left ventricular hypokinesis and an ejection fraction of 25%-30%.
Although history and physical examination may provide important clues regarding the underlying cardiac abnormality, both invasive and noninvasive testing are necessary to provide a definitive diagnosis of heart failure and to evaluate potential exacerbating conditions. A two-dimensional echocardiogram with Doppler flow study is an essential diagnostic test for evaluating myocardial contractility or ejection fraction. Echocardiogram can also evaluate other structural components such as the pericardium, valvular status, and hemodynamic parameters that may contribute to the development of ADHF. In patients with cardiac risk factors, a myocardial perfusion stress test or catheterization may identify the presence of coronary artery disease as a contributor.
A 12-lead electrocardiogram is necessary to establish the rhythm and to show evidence of acute or prior myocardial infarction, pericarditis, conduction abnormalities, or left ventricular hypertrophy as a result of prolonged uncontrolled hypertension. It is known that rhythm disturbances such as atrial fibrillation can be a precipitating factor for ADHF.
A chest X-ray is needed to uncover pulmonary edema in cases of fluid overload and to show an enlarged cardiac silhouette in cases of dilated cardiomyopathy. Identification of a definitive cause or causes that precipitate the occurrence of ADHF is crucial in devising a management plan and initiating appropriate intervention. (See Table 2, p. 22.)
Immediate Management of the ADHF
Newly developed clinical practice guidelines for the management of ADHF exist, but management is still based largely on empirical evidence.12 Begin ADHF treatment in the ED with intravenous diuretics (unless contraindicated). A majority of ADHF patients will respond to diuretics alone.13 If the patient responds poorly to diuretics, the use of nesiritide in conjunction with diuretics has proven beneficial, as shown in the analysis of data from the ADHERE registry indicating that patients treated with intravenous nesiritide had a lower hospital mortality rate than patients treated with milrinone or dobutamine.14 Other options include ultrafiltration, an intervention that has been noted to reduce lengths of stay and rehospitalization rates in patients with ADHF.
Nesiritide is a recombinant form of BNP without direct inotropic effects but with venous, arterial, and coronary vasodilatory properties that can improve symptoms in ADHF.15 The recommended dosage for nesiritide is an IV bolus of 2 mcg per kg, followed by a continuous infusion of 0.01 mcg/kg/min. In the setting of hypotension with a systolic blood pressure less than 100 mm Hg, however, an initial IV bolus dose is not recommended; instead, the patient may start with a continuous infusion of 0.01 mcg/kg/min, or consider other therapies.
In hemodynamically unstable patients with a systolic blood pressure less than 90 mm Hg, or in those with evidence of end organ hypoperfusion (cardiogenic shock), inotropic support may be considered until the patient is stabilized. (See Table 3, p. 22.) Recognize that inotropic agents have adverse effects on the neurohormonal system and are not recommended routinely but may be essential for temporary stabilization. (For more on pharmacologic management of ADHF, see Table 4, p. 24.)
Subacute Management of ADHF
Once acute decompensation has been reversed and an euvolemic state has been achieved, shift therapy to a combination of three classes of medications: diuretics, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers, unless contraindicated. The benefits of these drugs have been established by evidence from numerous large-scale clinical trials.3,12
Start ACE inhibitors in all patients with heart failure due to left ventricular systolic dysfunction (unless contraindicated or if the patient is intolerant).3 Give ACE-I to patients who have experienced a recent episode of ADHF, along with diuretics to maintain sodium balance and to prevent peripheral and pulmonary edema. ACE inhibitors are contraindicated for patients who are pregnant and for those with childbearing potential, as well as for individuals with a prior history of angioedema or renal failure after receiving the drug. Instruct patients to avoid any sudden change of position, as they may experience orthostatic hypotension while taking ACE inhibitors.
Some patients are intolerant of ACE inhibitors due to a persistent cough that occurs in approximately 5% to 10% of Caucasian patients and in up to 50% of Chinese patients.16 Angiotensin receptor blockers (ARBs) are an established alternative.17 Two ARBs (candesartan and valsartan) are recommended for treatment of heart failure based on evidence from controlled clinical trials.17,18 These drugs have demonstrated a reduction in hospitalizations, and candesartan, when used as an alternative to ACE-I, has been shown to reduce mortality. Additionally, in patients with evidence of left ventricular dysfunction after myocardial infarction, valsartan provided a benefit that was not inferior to ACE inhibitors.17
Initiate beta-blockers at very low doses and gradually increased as tolerated. Monitor patients closely for symptoms of hypotension, significant weight gain, fluid retention, bradycardia, and heart block. In addition, inform patients that they may experience generalized fatigue or weakness with the initiation of beta-blockers. In a cancer patient it is difficult to differentiate between fatigue caused by the disease and the side effects of therapy. Fatigue associated with beta-blocker therapy usually resolves spontaneously within a few days. Make every effort to achieve optimum target beta-blocker dose.
An aldosterone antagonist, such as spironolactone or eplerenone, given at a daily dose of 12.5 to 25 mg in addition to standard therapy, effectively blocks the effects of aldosterone (RALES study) to achieve comprehensive neurohormonal blockade.19 When prescribing an aldosterone antagonist, especially in combination with ACE inhibitors and loop diuretics, it is important to monitor serum potassium levels because this combination can result in hyperkalemia.
Disease Management Programs
Comprehensive management of heart failure is not only limited to hospital care during an episode of ADHF. In order to prevent repeated hospitalizations, implement additional measures through formal disease management programs. These disease management programs are often directed or coordinated by advanced practice nurses who address the comprehensive care of heart failure patients with emphasis on patient education and counseling to improve patient compliance.20
The non-pharmacologic treatment strategies emphasized in disease management programs have proven effective in achieving positive outcomes. These include counseling patients on dietary management, including encouraging a two-gram sodium diet, alcohol restriction, and adequate supplementation of electrolyte loss from diuretics. Keeping a diary of the patient’s daily weight at home and bringing it to office visits will help both the patient and the clinician monitor fluid retention efficiently.
Hypotension is a common side effect from the pharmacologic therapy for heart failure. Employ comprehensive education with both patient and family to avoid unnecessary discontinuation of the medications. A systolic blood pressure of 90 mm Hg is acceptable as long as there are no associated symptoms of dizziness or syncope.
Encourage activity guidelines, including participation in exercise programs. Attendance at support group meetings will provide a venue in which patients can share common problems and concerns with others in similar situations. One to two weeks after hospital discharge, schedule an outpatient follow-up in a heart failure clinic, where heart failure education is reinforced to prevent another episode of ADHF admission.
Summary
Despite the added challenges, managing ADHF in a patient with a serious comorbidity such as cancer involves the same goals as the treatment of ADHF in any other patient. With rapid assessment and appropriate intervention, the patient is given the best possible chance of survival. TH
The authors work at the University of Texas M.D. Anderson Cancer Center, Department of Cardiology, Houston.
References
- American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas.: American Heart Association; 2005. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Last accessed August 20, 2006.
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004 Jan;147(1):74-78.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154-235.Epub 2005 Sep 13.
- Peacock WF IV, Emerman CL, Wynne J, for the ADHERE Scientific Advisory Committee and Investigators and the ADHERE Study Group. Early use of nesiritide in the emergency department is associated with improved outcome: an ADHERE registry analysis. Ann Emerg Med. 2004;44:S78.
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail. 1999 Aug;1(3):251-257.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888.
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995 Sep;96(3):1280-1287.
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825-832.
- Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005 Jan-Feb;11(1):30-38.
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul;106(4):416-422.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161-167.
- Adams KF, Lindenfeld J, Arnold JMO, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10-38.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol. 2005 Jul;46(1):65-67.
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul;46(1):57-64.
- Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002 Dec;144(6):1102-1108.
- Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995 Aug;40(2):141-144.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003 Nov 13;349(20):1893-1906. Epub 2003 Nov 10.
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-776.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-717.
- Albert NM, Eastwood CA, Edwards ML. Evidence-based practice for acute decompensated heart failure. Crit Care Nurse. 2004 Dec;24(6):14-16, 18-24, 26-29; quiz 30-31.
Acute decompensated heart failure (ADHF) remains one of the most common reasons for hospitalization. ADHF patients who have co-morbid conditions present stubborn challenges for hospitalists. And ADHF is frequently observed in patients 65 and older. The neurohormonal activation that results as a consequence of myocardial dysfunction leads to progressive cardiac deterioration and hemodynamic disturbances that ultimately become manifest as acute decompensated heart failure.
ADHG management goals include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival.
In this article we present the case of a 26-year-old female with ADHF and highlight the management strategies that can result in stabilization and improved long-term outcome.
Introduction
Despite major advances in the treatment of heart disease, heart failure remains a growing public health problem of epidemic proportions in the United States. Approximately five million Americans have heart failure, and more than 550,000 patients are diagnosed with the disease each year.1 The annual number of hospitalizations for heart failure as a primary diagnosis has increased from approximately 810,000 in 1990 to more than 1 million in 1999, and it is the most common discharge diagnosis-related group for patients 65 and older.2 Medicare spent more dollars on the diagnosis and treatment of heart failure than on any other diagnosis—more than $27.9 billion in 2005.1
Patients presenting to the emergency department (ED) with ADHF are often hemodynamically unstable, with severe symptoms of dyspnea and fluid overload. Rapid assessment and prompt initiation of appropriate interventions are necessary to achieve clinical stability and prevent prolonged hospital stay if hospitalization is required. The in-hospital mortality rate for ADHF is 5%-8%; median duration of hospitalization is five days, and the six-month re-hospitalization rate is about 50%.1,3 Thus, it is clear that improved recognition and treatment are of paramount importance. With these goals in mind, we present a recent case that highlights many of the concerns about and treatment options for ADHF.
Case Presentation
Karen A. is a 26-year-old black female with stage III Hodgkin’s disease, diagnosed in 2000. She received chemotherapy (cisplatin, cytarabine, doxorubicin, rituxan, gemcitabine) and, as a result, in 2001 developed chemotherapy-induced cardiomyopathy with an ejection fraction of <20%. Her condition stabilized, and she remained in clinical remission until September 2002.
In 2003 she received an autologous stem cell transplant and subsequently presented to the ED with complaints of fatigue, progressive shortness of breath in the previous seven days, and lower extremity edema. She also reported right-sided pleuritic chest pain, but denied associated nausea, vomiting, or diaphoresis. In the three days preceding admission, she had gained 10 pounds. In the past six months, she had had multiple admissions for ADHF.
Her physical examination revealed an alert, obese female in moderate respiratory distress with dry mucous membranes. Her vital signs indicated a temperature of 36.5° C, a heart rate of 110 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure measuring 111/73 mm Hg, and an oxygen saturation of 92%. She had no scleral icterus, but did have jugular venous distention to the angle of the mandible at 45° upright. Her cardiac examination indicated tachycardia with distant heart sounds and an audible third heart sound (S3), as well as a grade 2/6 systolic ejection murmur at the left sternal border. Her lung examination demonstrated diffuse crackles present in both lung fields, but no wheezes. Abdominal examination was notable for tenderness to palpation at the right hypogastric region and for hepatomegaly, her skin was warm and dry with no cyanosis, and her extremities demonstrated significant bilateral pitting pre-tibial edema to her knees.
Her current medications included:
- Furosemide, 60 mg by mouth two times daily;
- Carvedilol, 12.5 mg by mouth in the morning, 6.25 mg in the evening;
- Amiodarone, 200 mg by mouth daily; and
- Digoxin, 0.125 mg by mouth daily.
Laboratory findings included normal electrolytes, blood urea nitrogen, and serum creatinine. Her hemoglobin was 11.3 gm/L, and her B-type natriuretic peptide (BNP) level was 4,837 pg/ml. Initial cardiac enzymes were negative (troponin I of <0. 03).
Her chest X-ray demonstrated moderate cardiac enlargement with bilateral thickening of subpleural septal lines and blurring of the pulmonary vasculature consistent with developing cardiogenic pulmonary edema. A 12-lead electrocardiogram indicated sinus tachycardia with a rate of 110 beats per minute and nonspecific ST-T wave changes in the inferior leads, but no Q waves were noted. Echocardiography showed severely reduced left ventricular systolic function with severe global hypokinesis of the left ventricle and a measured ejection fraction of 25%-30%. There was no pericardial effusion.
She was admitted to the telemetry floor with the diagnosis of ADHF. She was placed on supplemental oxygen and serial cardiac enzymes, and her electrocardiogram remained negative for injury or ischemia. Intravenous furosemide was initiated at 40 mg every 12 hours. After 24 hours, she was given a bolus of nesiritide (2 mcg/kg), followed by a continuous infusion at 0.01 mcg/kg/min. Cardiac medications were continued, and the dosage of carvedilol was reduced to 6.25 mg by mouth twice daily.
After a period of diuresis, the patient remained highly symptomatic; therefore, a right heart catheterization was done. The hemodynamics indicated a cardiac output of three liters per minute, a right atrial pressure of 20 mm Hg, a right ventricular pressure of 70/20 mm Hg, pulmonary artery pressure of 66/20 mm Hg with a mean pressure of 52 mm Hg, and a pulmonary capillary wedge pressure of 25 mm Hg. (See Figures 1 and 2, p. 23).
Over the next 24 hours, the patient had more than three liters of urine output and was significantly improved. The nesiritide infusion was discontinued after 48 hours of therapy, and the patient was weaned from supplemental oxygen. Lisinopril was started at 2.5 mg daily by mouth. On hospital day four, the patient walked around the nurses’ station without supplemental oxygen, and she was returned to the previous dose of furosemide, 60 mg twice daily.
The patient was enrolled in the Heart Success Program (HSP), a collaborative interdisciplinary program that is available in the institution for cancer patients with heart failure. She was provided with patient education materials that included educational videotapes on heart failure management, daily weight monitoring, diet, medications, exercise, and the emotional aspects of heart failure. Nurses with heart failure training were available to answer questions for the patient and to provide further instruction for follow-up after the patient’s discharge. The patient improved enough that she was able to enroll in a New York Heart Association (NYHA) class II and was discharged after five days, with a follow-up appointment to the outpatient clinic one week after hospital discharge.
Discussion
This case illustrates the challenges inherent in the diagnosis and management of ADHF in a cancer patient with a known history of heart failure. Rapid assessment is critical in establishing a diagnosis and initiating appropriate intervention. The goals of managing ADHF remain the same regardless of etiology. These include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival. The same principles apply—even if the patient has a major comorbidity such as cancer, and suspicion for the diagnosis must remain high.
Initial Evaluation
Early diagnosis and effective management of ADHF are critically important, as these have been shown to reduce hospitalizations and intensive care unit admissions, to decrease length of stay, and to decrease cost of hospitalization.4 A comprehensive history and physical examination must be performed to identify signs and symptoms that lead to a heart failure diagnosis.
We must evaluate such potential risk factors as history of hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, valvular disease, peripheral vascular disease, a family history of cardiomyopathy, smoking, alcohol use, thyroid problems, sleep apnea, and any recent history of infection (particularly upper respiratory tract infection, which can cause viral cardiomyopathy).
As part of further investigation with cancer patients, include the patient’s possible past exposure to cardiotoxic agents (e.g., anthracyclines, trastuzumab, high-dose cyclophosphamide) or mediastinal irradiation. Chemotherapy-induced cardiomyopathy is increasingly becoming an issue in heart failure management as a result of the growing number of long-term cancer survivors who have received treatment with anthracycline-containing chemotherapy or other aggressive therapy.
Focus on volume and perfusion status during the physical assessment of each patient. Most patients presenting to the ED with acute decompensation are volume overloaded. Volume overload is manifested by symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea, as well as jugular venous distention, hepatojugular reflux, ascites, edema, and crackles in the lungs.5 Crackles are not always present in chronic heart failure patients, however, because of the continuous movement of fluid into the interstitium associated with increased lymphatic drainage, leaving the alveoli relatively dry.6
In addition to a comprehensive history and physical examination, several tests will help establish the diagnosis. (See Table 1, top left.) A measurement of a B-type natriuretic peptide (BNP) facilitates the diagnosis of ADHF. BNP is an endogenously generated natriuretic peptide that is activated in response to atrial or ventricular expansion due to volume overload and increased wall tension.7,8 Circulating levels of endogenous BNP are significantly elevated in ADHF patients and are a valuable tool for diagnosis of heart failure in the ED.9-11 In Karen A., the BNP level was 4,837 pg/mL, which is indicative of Stage D heart failure. This diagnosis was confirmed by chest X-ray findings of cardiogenic pulmonary edema in the setting of severe left ventricular hypokinesis and an ejection fraction of 25%-30%.
Although history and physical examination may provide important clues regarding the underlying cardiac abnormality, both invasive and noninvasive testing are necessary to provide a definitive diagnosis of heart failure and to evaluate potential exacerbating conditions. A two-dimensional echocardiogram with Doppler flow study is an essential diagnostic test for evaluating myocardial contractility or ejection fraction. Echocardiogram can also evaluate other structural components such as the pericardium, valvular status, and hemodynamic parameters that may contribute to the development of ADHF. In patients with cardiac risk factors, a myocardial perfusion stress test or catheterization may identify the presence of coronary artery disease as a contributor.
A 12-lead electrocardiogram is necessary to establish the rhythm and to show evidence of acute or prior myocardial infarction, pericarditis, conduction abnormalities, or left ventricular hypertrophy as a result of prolonged uncontrolled hypertension. It is known that rhythm disturbances such as atrial fibrillation can be a precipitating factor for ADHF.
A chest X-ray is needed to uncover pulmonary edema in cases of fluid overload and to show an enlarged cardiac silhouette in cases of dilated cardiomyopathy. Identification of a definitive cause or causes that precipitate the occurrence of ADHF is crucial in devising a management plan and initiating appropriate intervention. (See Table 2, p. 22.)
Immediate Management of the ADHF
Newly developed clinical practice guidelines for the management of ADHF exist, but management is still based largely on empirical evidence.12 Begin ADHF treatment in the ED with intravenous diuretics (unless contraindicated). A majority of ADHF patients will respond to diuretics alone.13 If the patient responds poorly to diuretics, the use of nesiritide in conjunction with diuretics has proven beneficial, as shown in the analysis of data from the ADHERE registry indicating that patients treated with intravenous nesiritide had a lower hospital mortality rate than patients treated with milrinone or dobutamine.14 Other options include ultrafiltration, an intervention that has been noted to reduce lengths of stay and rehospitalization rates in patients with ADHF.
Nesiritide is a recombinant form of BNP without direct inotropic effects but with venous, arterial, and coronary vasodilatory properties that can improve symptoms in ADHF.15 The recommended dosage for nesiritide is an IV bolus of 2 mcg per kg, followed by a continuous infusion of 0.01 mcg/kg/min. In the setting of hypotension with a systolic blood pressure less than 100 mm Hg, however, an initial IV bolus dose is not recommended; instead, the patient may start with a continuous infusion of 0.01 mcg/kg/min, or consider other therapies.
In hemodynamically unstable patients with a systolic blood pressure less than 90 mm Hg, or in those with evidence of end organ hypoperfusion (cardiogenic shock), inotropic support may be considered until the patient is stabilized. (See Table 3, p. 22.) Recognize that inotropic agents have adverse effects on the neurohormonal system and are not recommended routinely but may be essential for temporary stabilization. (For more on pharmacologic management of ADHF, see Table 4, p. 24.)
Subacute Management of ADHF
Once acute decompensation has been reversed and an euvolemic state has been achieved, shift therapy to a combination of three classes of medications: diuretics, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers, unless contraindicated. The benefits of these drugs have been established by evidence from numerous large-scale clinical trials.3,12
Start ACE inhibitors in all patients with heart failure due to left ventricular systolic dysfunction (unless contraindicated or if the patient is intolerant).3 Give ACE-I to patients who have experienced a recent episode of ADHF, along with diuretics to maintain sodium balance and to prevent peripheral and pulmonary edema. ACE inhibitors are contraindicated for patients who are pregnant and for those with childbearing potential, as well as for individuals with a prior history of angioedema or renal failure after receiving the drug. Instruct patients to avoid any sudden change of position, as they may experience orthostatic hypotension while taking ACE inhibitors.
Some patients are intolerant of ACE inhibitors due to a persistent cough that occurs in approximately 5% to 10% of Caucasian patients and in up to 50% of Chinese patients.16 Angiotensin receptor blockers (ARBs) are an established alternative.17 Two ARBs (candesartan and valsartan) are recommended for treatment of heart failure based on evidence from controlled clinical trials.17,18 These drugs have demonstrated a reduction in hospitalizations, and candesartan, when used as an alternative to ACE-I, has been shown to reduce mortality. Additionally, in patients with evidence of left ventricular dysfunction after myocardial infarction, valsartan provided a benefit that was not inferior to ACE inhibitors.17
Initiate beta-blockers at very low doses and gradually increased as tolerated. Monitor patients closely for symptoms of hypotension, significant weight gain, fluid retention, bradycardia, and heart block. In addition, inform patients that they may experience generalized fatigue or weakness with the initiation of beta-blockers. In a cancer patient it is difficult to differentiate between fatigue caused by the disease and the side effects of therapy. Fatigue associated with beta-blocker therapy usually resolves spontaneously within a few days. Make every effort to achieve optimum target beta-blocker dose.
An aldosterone antagonist, such as spironolactone or eplerenone, given at a daily dose of 12.5 to 25 mg in addition to standard therapy, effectively blocks the effects of aldosterone (RALES study) to achieve comprehensive neurohormonal blockade.19 When prescribing an aldosterone antagonist, especially in combination with ACE inhibitors and loop diuretics, it is important to monitor serum potassium levels because this combination can result in hyperkalemia.
Disease Management Programs
Comprehensive management of heart failure is not only limited to hospital care during an episode of ADHF. In order to prevent repeated hospitalizations, implement additional measures through formal disease management programs. These disease management programs are often directed or coordinated by advanced practice nurses who address the comprehensive care of heart failure patients with emphasis on patient education and counseling to improve patient compliance.20
The non-pharmacologic treatment strategies emphasized in disease management programs have proven effective in achieving positive outcomes. These include counseling patients on dietary management, including encouraging a two-gram sodium diet, alcohol restriction, and adequate supplementation of electrolyte loss from diuretics. Keeping a diary of the patient’s daily weight at home and bringing it to office visits will help both the patient and the clinician monitor fluid retention efficiently.
Hypotension is a common side effect from the pharmacologic therapy for heart failure. Employ comprehensive education with both patient and family to avoid unnecessary discontinuation of the medications. A systolic blood pressure of 90 mm Hg is acceptable as long as there are no associated symptoms of dizziness or syncope.
Encourage activity guidelines, including participation in exercise programs. Attendance at support group meetings will provide a venue in which patients can share common problems and concerns with others in similar situations. One to two weeks after hospital discharge, schedule an outpatient follow-up in a heart failure clinic, where heart failure education is reinforced to prevent another episode of ADHF admission.
Summary
Despite the added challenges, managing ADHF in a patient with a serious comorbidity such as cancer involves the same goals as the treatment of ADHF in any other patient. With rapid assessment and appropriate intervention, the patient is given the best possible chance of survival. TH
The authors work at the University of Texas M.D. Anderson Cancer Center, Department of Cardiology, Houston.
References
- American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas.: American Heart Association; 2005. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Last accessed August 20, 2006.
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004 Jan;147(1):74-78.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154-235.Epub 2005 Sep 13.
- Peacock WF IV, Emerman CL, Wynne J, for the ADHERE Scientific Advisory Committee and Investigators and the ADHERE Study Group. Early use of nesiritide in the emergency department is associated with improved outcome: an ADHERE registry analysis. Ann Emerg Med. 2004;44:S78.
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail. 1999 Aug;1(3):251-257.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888.
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995 Sep;96(3):1280-1287.
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825-832.
- Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005 Jan-Feb;11(1):30-38.
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul;106(4):416-422.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161-167.
- Adams KF, Lindenfeld J, Arnold JMO, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10-38.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol. 2005 Jul;46(1):65-67.
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul;46(1):57-64.
- Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002 Dec;144(6):1102-1108.
- Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995 Aug;40(2):141-144.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003 Nov 13;349(20):1893-1906. Epub 2003 Nov 10.
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-776.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-717.
- Albert NM, Eastwood CA, Edwards ML. Evidence-based practice for acute decompensated heart failure. Crit Care Nurse. 2004 Dec;24(6):14-16, 18-24, 26-29; quiz 30-31.