User login
Probiotics as a Tx resource in primary care
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
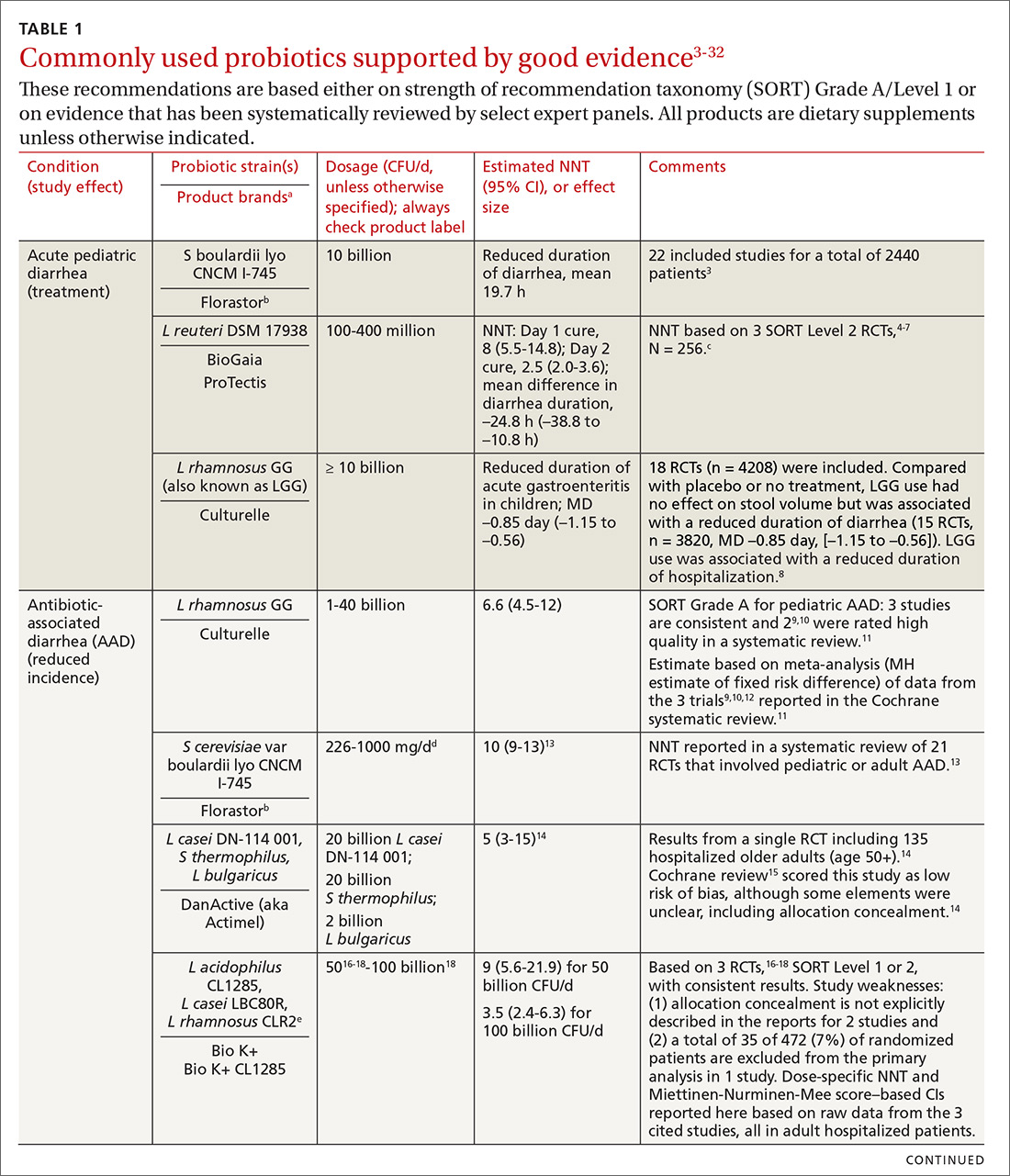
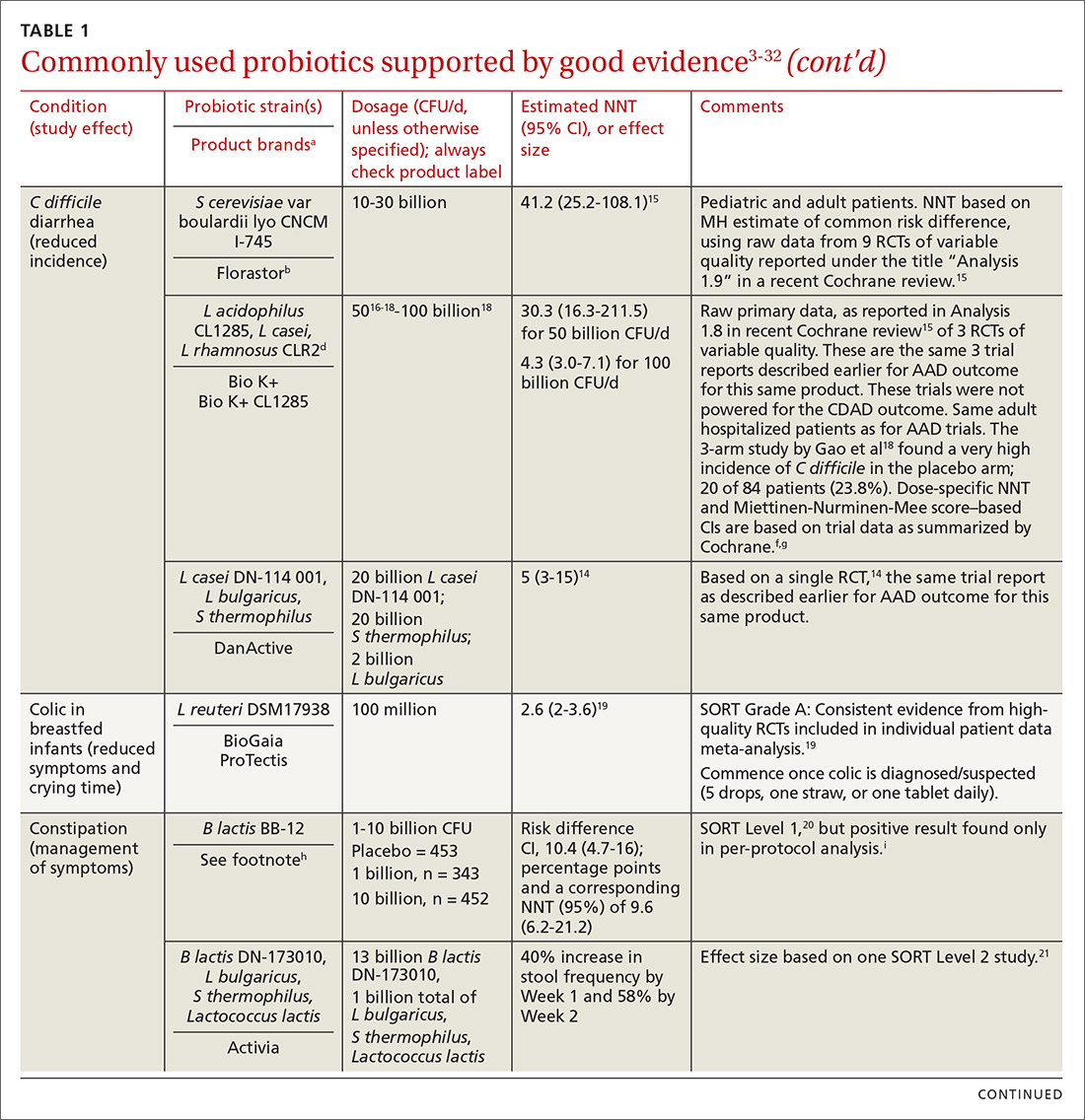
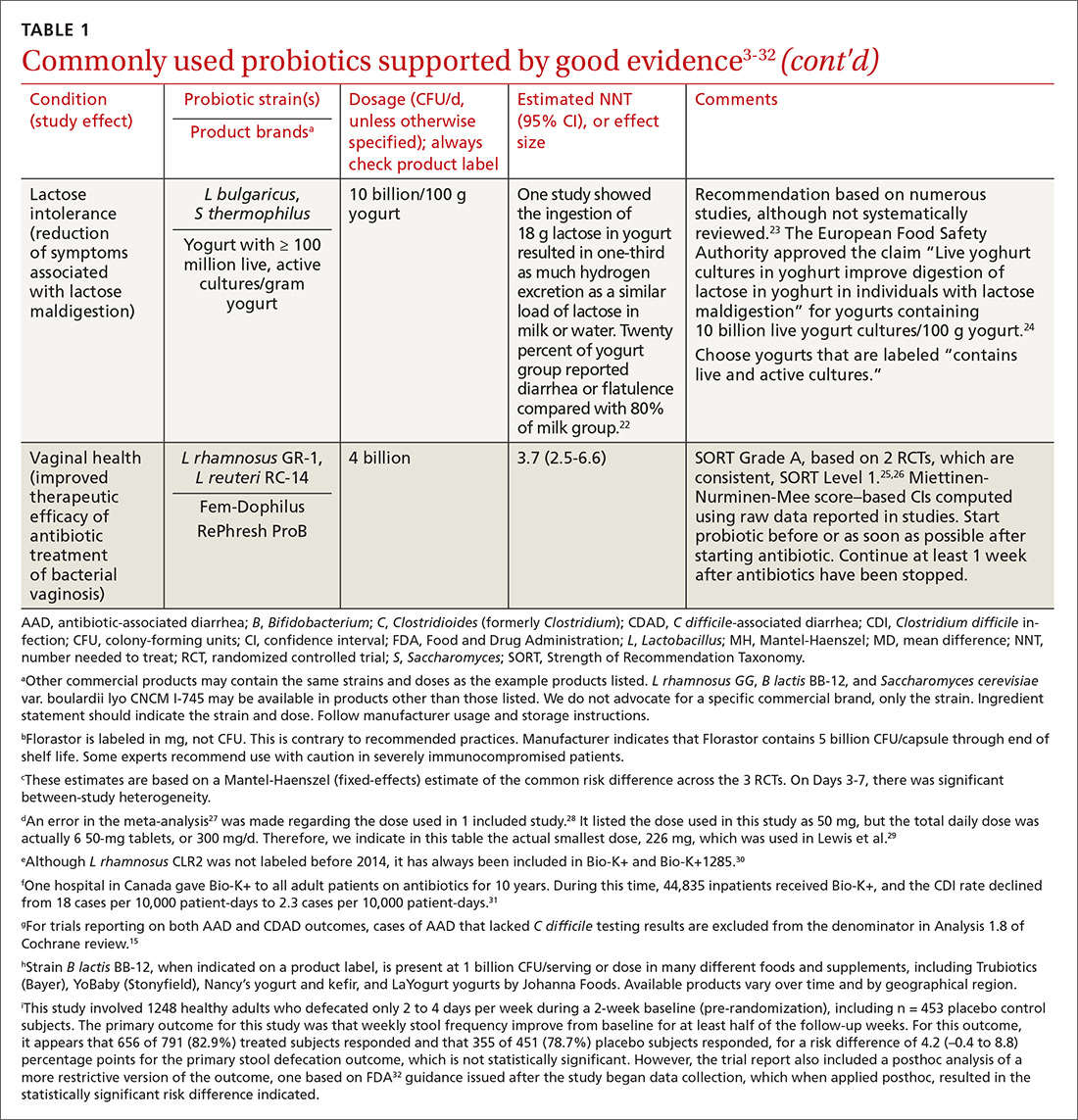
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
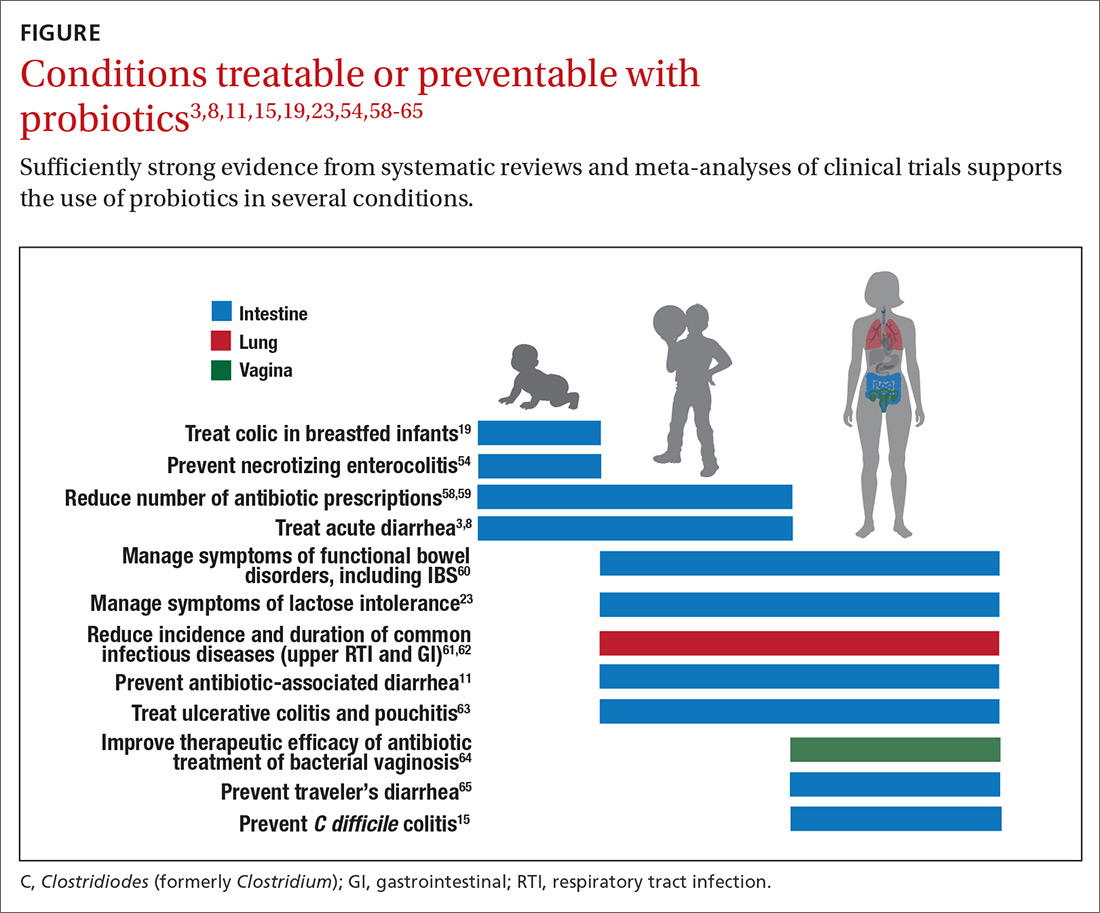
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.
The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; djm23@georgetown.edu.
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.
The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; djm23@georgetown.edu.
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.
The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; djm23@georgetown.edu.
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
PRACTICE RECOMMENDATIONS
› Consider specific probiotics to prevent antibioticassociated diarrhea, reduce crying time in colicky infants, and improve therapeutic effectiveness of antibiotics for bacterial vaginosis. A
› Consider specific probiotics to reduce the risk for Clostridioides (formerly Clostridium) difficile infections, to treat acute pediatric diarrhea, and to manage symptoms of constipation. B
› Check a product’s label to ensure that it includes the probiotic’s genus, species, and strains; the dose delivered in colony-forming units through the end of shelf life; and expected benefits C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Effects [FET1] of Influenza Vaccination of Health Care Workers on Mortality of Elderly People in Long-Term Care: A Randomized Controlled Trial
CLINICAL QUESTION: Does vaccination of health care providers working in long-term care facilities lower mortality and rates of influenza infection in patients?
BACKGROUND: The Centers for Disease Control and Prevention (CDC) recommend influenza vaccination of all patients in long-term care facilities and of health care workers employed there. Several studies have demonstrated the effectiveness of vaccinating elderly patients, and other studies have shown decreased infection rates in vaccinated health care workers.1,2 The effectiveness of vaccinating health care workers for preventing the spread of infection from worker to patient is not as well documented. The authors of this study evaluated whether vaccinating the health care workers at long-term care facilities reduced the nosocomial infection rate and the mortality of the patients in the facilities.
POPULATION STUDIED: A total of 1217 health care workers from 20 long-term care geriatric facilities in Scotland and the 1437 patients for whom they cared during a 6-month period participated in the study. Patients’ age, sex, and degree of disability based on a modified Barthel index were recorded.
STUDY DESIGN AND VALIDITY: Long-term care facilities were matched according to the number of beds and the vaccination policy. Employees randomly selected from half of these facilities were offered an influenza vaccination. Approximately half of the health care workers who were offered a vaccination received it, compared with less than 5% of workers in the control group. A random sample of 50% of the patients at each facility underwent prospective influenza monitoring by nasal and throat swab. Because patient demographics were not well defined, it is difficult to determine if the patients and long-term care facilities in the study are similar to those in other countries. Vaccinations are not routine for the elderly population of the United Kingdom. Consequently, vaccinating a transmission source such as health care workers could be more beneficial in the United Kingdom than in the United States. Also, all-cause mortality rates were very high (13.6%-22.4%) during the 6 months of the study, denoting a higher-risk population than that encountered in many other facilities.
OUTCOMES MEASURED: The outcomes measured included the mortality rate of patients during the winter months and the number of confirmed cases of influenza A and B.
RESULTS: Influenza rates were similar (5.4% vs 6.7%). Overall, the vaccination program was associated with lower mortality (13.6% vs 22.4%, P=.014) among residents. This benefit remained even after adjusting for the higher vaccination rate of residents in the facilities in which the health care workers were not vaccinated. However, after accounting for differences in age, sex, vaccination rate, and disability between the 2 groups, the reduction in the adjusted mortality rate was not statistically significant (adjusted odds ratio=0.6; 95% confidence interval, 0.36-1.04; P=.09).
Vaccination of health care providers working in geriatric inpatient facilities was associated with a decreased mortality among residents, despite equal rates of influenza infection. However, after adjusting for the baseline health of the patients, this benefit disappeared. Practitioners should continue to strive to meet CDC guidelines for vaccination of elderly adults and health care workers, but this study provides only a small impetus to do so.
CLINICAL QUESTION: Does vaccination of health care providers working in long-term care facilities lower mortality and rates of influenza infection in patients?
BACKGROUND: The Centers for Disease Control and Prevention (CDC) recommend influenza vaccination of all patients in long-term care facilities and of health care workers employed there. Several studies have demonstrated the effectiveness of vaccinating elderly patients, and other studies have shown decreased infection rates in vaccinated health care workers.1,2 The effectiveness of vaccinating health care workers for preventing the spread of infection from worker to patient is not as well documented. The authors of this study evaluated whether vaccinating the health care workers at long-term care facilities reduced the nosocomial infection rate and the mortality of the patients in the facilities.
POPULATION STUDIED: A total of 1217 health care workers from 20 long-term care geriatric facilities in Scotland and the 1437 patients for whom they cared during a 6-month period participated in the study. Patients’ age, sex, and degree of disability based on a modified Barthel index were recorded.
STUDY DESIGN AND VALIDITY: Long-term care facilities were matched according to the number of beds and the vaccination policy. Employees randomly selected from half of these facilities were offered an influenza vaccination. Approximately half of the health care workers who were offered a vaccination received it, compared with less than 5% of workers in the control group. A random sample of 50% of the patients at each facility underwent prospective influenza monitoring by nasal and throat swab. Because patient demographics were not well defined, it is difficult to determine if the patients and long-term care facilities in the study are similar to those in other countries. Vaccinations are not routine for the elderly population of the United Kingdom. Consequently, vaccinating a transmission source such as health care workers could be more beneficial in the United Kingdom than in the United States. Also, all-cause mortality rates were very high (13.6%-22.4%) during the 6 months of the study, denoting a higher-risk population than that encountered in many other facilities.
OUTCOMES MEASURED: The outcomes measured included the mortality rate of patients during the winter months and the number of confirmed cases of influenza A and B.
RESULTS: Influenza rates were similar (5.4% vs 6.7%). Overall, the vaccination program was associated with lower mortality (13.6% vs 22.4%, P=.014) among residents. This benefit remained even after adjusting for the higher vaccination rate of residents in the facilities in which the health care workers were not vaccinated. However, after accounting for differences in age, sex, vaccination rate, and disability between the 2 groups, the reduction in the adjusted mortality rate was not statistically significant (adjusted odds ratio=0.6; 95% confidence interval, 0.36-1.04; P=.09).
Vaccination of health care providers working in geriatric inpatient facilities was associated with a decreased mortality among residents, despite equal rates of influenza infection. However, after adjusting for the baseline health of the patients, this benefit disappeared. Practitioners should continue to strive to meet CDC guidelines for vaccination of elderly adults and health care workers, but this study provides only a small impetus to do so.
CLINICAL QUESTION: Does vaccination of health care providers working in long-term care facilities lower mortality and rates of influenza infection in patients?
BACKGROUND: The Centers for Disease Control and Prevention (CDC) recommend influenza vaccination of all patients in long-term care facilities and of health care workers employed there. Several studies have demonstrated the effectiveness of vaccinating elderly patients, and other studies have shown decreased infection rates in vaccinated health care workers.1,2 The effectiveness of vaccinating health care workers for preventing the spread of infection from worker to patient is not as well documented. The authors of this study evaluated whether vaccinating the health care workers at long-term care facilities reduced the nosocomial infection rate and the mortality of the patients in the facilities.
POPULATION STUDIED: A total of 1217 health care workers from 20 long-term care geriatric facilities in Scotland and the 1437 patients for whom they cared during a 6-month period participated in the study. Patients’ age, sex, and degree of disability based on a modified Barthel index were recorded.
STUDY DESIGN AND VALIDITY: Long-term care facilities were matched according to the number of beds and the vaccination policy. Employees randomly selected from half of these facilities were offered an influenza vaccination. Approximately half of the health care workers who were offered a vaccination received it, compared with less than 5% of workers in the control group. A random sample of 50% of the patients at each facility underwent prospective influenza monitoring by nasal and throat swab. Because patient demographics were not well defined, it is difficult to determine if the patients and long-term care facilities in the study are similar to those in other countries. Vaccinations are not routine for the elderly population of the United Kingdom. Consequently, vaccinating a transmission source such as health care workers could be more beneficial in the United Kingdom than in the United States. Also, all-cause mortality rates were very high (13.6%-22.4%) during the 6 months of the study, denoting a higher-risk population than that encountered in many other facilities.
OUTCOMES MEASURED: The outcomes measured included the mortality rate of patients during the winter months and the number of confirmed cases of influenza A and B.
RESULTS: Influenza rates were similar (5.4% vs 6.7%). Overall, the vaccination program was associated with lower mortality (13.6% vs 22.4%, P=.014) among residents. This benefit remained even after adjusting for the higher vaccination rate of residents in the facilities in which the health care workers were not vaccinated. However, after accounting for differences in age, sex, vaccination rate, and disability between the 2 groups, the reduction in the adjusted mortality rate was not statistically significant (adjusted odds ratio=0.6; 95% confidence interval, 0.36-1.04; P=.09).
Vaccination of health care providers working in geriatric inpatient facilities was associated with a decreased mortality among residents, despite equal rates of influenza infection. However, after adjusting for the baseline health of the patients, this benefit disappeared. Practitioners should continue to strive to meet CDC guidelines for vaccination of elderly adults and health care workers, but this study provides only a small impetus to do so.