Article
Risk Factors Predicting Cellulitis Diagnosis in a Prospective Cohort Undergoing Dermatology Consultation in the Emergency Department
- Author:
- Sidharth Chand, MD
- Renajd Rrapi, MD
- Lauren N. Ko, MD, MEd
- Colleen K. Gabel, MD
- Anna Cristina Garza-Mayers, MD, PhD
- Leslie W. Milne, MD
- Emily D. Nguyen, MD
- Blair Alden Parry, BA
- Radhika Shah, MD, PharmD
- Jessica St. John, MD, MBA, MPH
- Lauren Strazzula, MD
- Priyanka C. Vedak, MD
- Daniela Kroshinsky, MD, MPH
Cellulitis is an infection of the skin and skin-associated structures with many clinical mimickers known collectively as pseudocellulitis.
Article
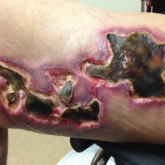
Update on Calciphylaxis Etiopathogenesis, Diagnosis, and Management
- Author:
- Urmi Khanna, MD
- Arturo Dominguez, MD
- Jesse Keller, MD
- Daniela Kroshinsky, MD, MPH
- Alex G. Ortega-Loayza, MD
- Lindsay Strowd, MD
- Robert G. Micheletti, MD
Calciphylaxis is associated with potentially severe complications and high mortality rates. This article highlights the challenges faced in...
