Article
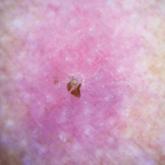
Punked By the Punctum: Domestically Acquired Cutaneous Myiasis
- Author:
- Jeffrey Globerson, DO
- Danielle Yee, MD
- Stephen Olsen, MD
- Brett Bender, DO
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on...
Article

25-year-old woman • abdominal pain • urticarial rash • recent influenza immunization • Dx?
- Author:
- Danielle Yee, MD
- Adam Skrynski, MD
- Kathleen Dass, MD
► Abdominal pain
► Urticarial rash
► Recent influenza immunization
