User login
Eccrine Porocarcinoma in 2 Patients
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.
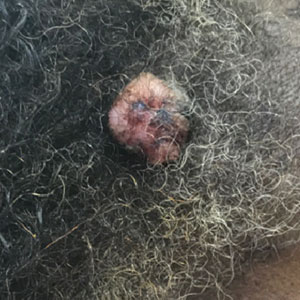
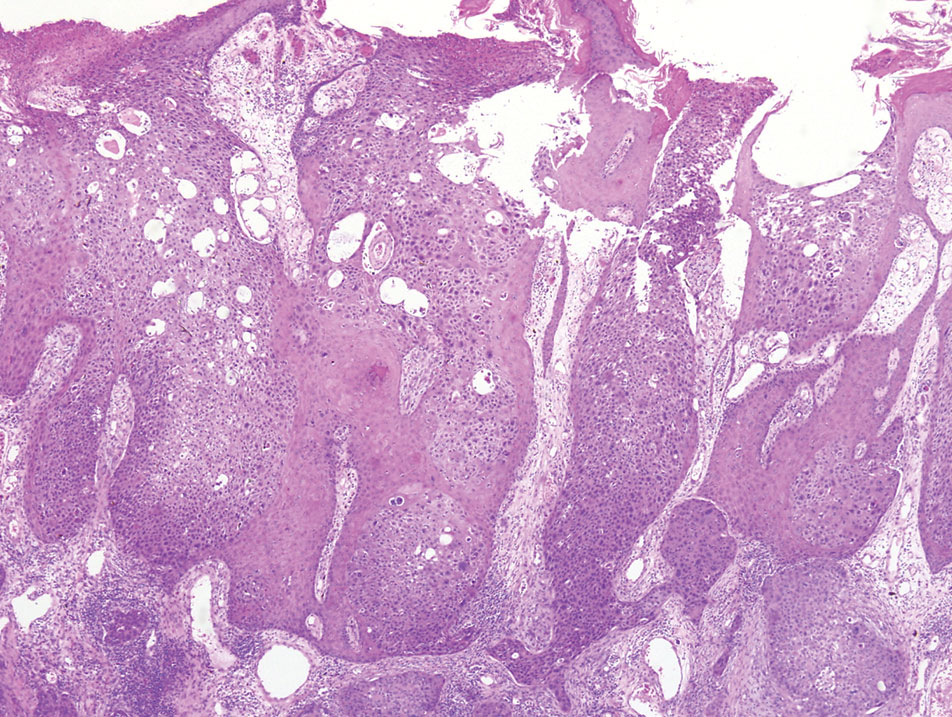
A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.
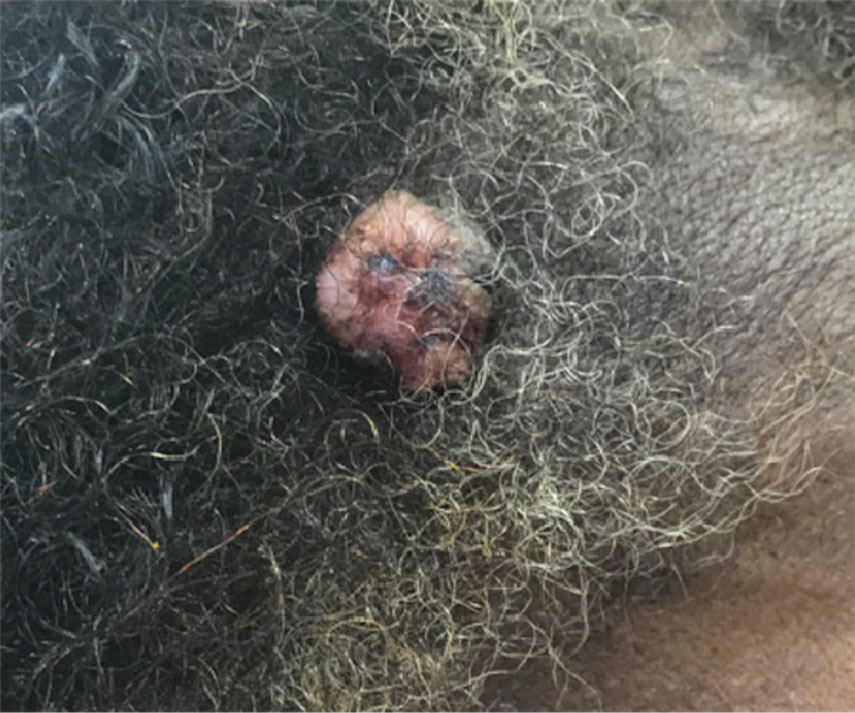
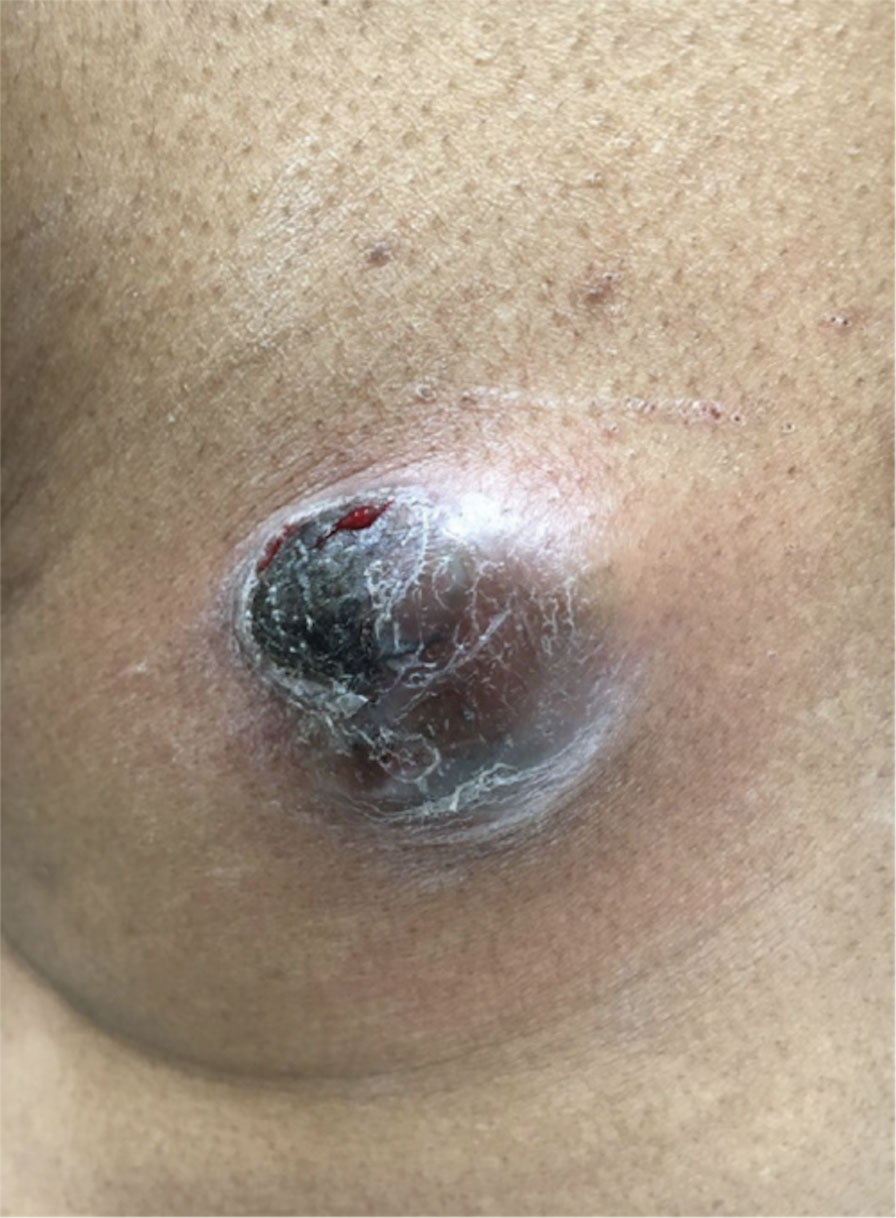
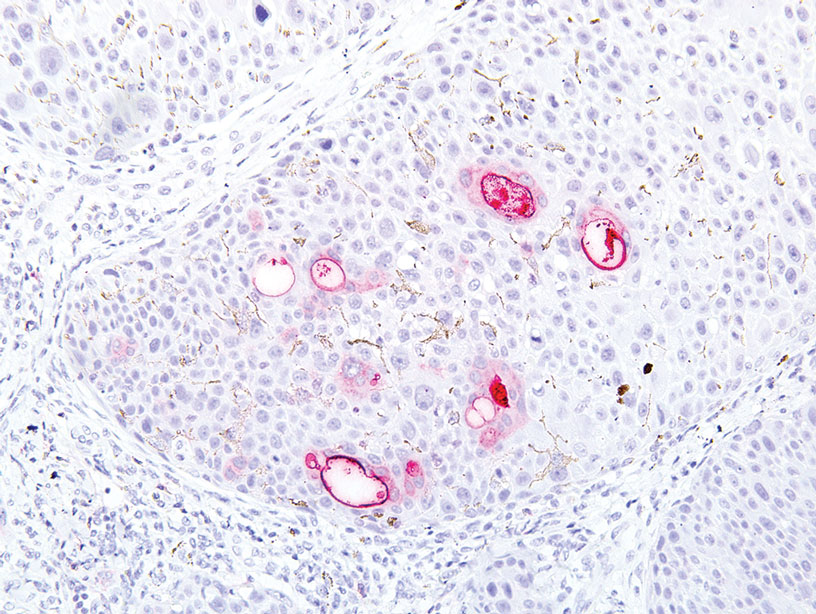
A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.
Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.
Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.

A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.

A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.

Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.

Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.

A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.

A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.

Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.

Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
Practice Points
- Eccrine porocarcinoma is a rare malignancy that clinically mimics other cutaneous malignancies.
- Early histologic diagnosis is essential, as lymphatic metastasis is common and carries a 65% to 67% mortality rate.