Article
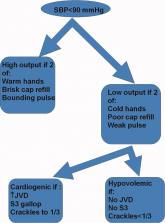
Accuracy of bedside physical examination in distinguishing categories of shock
- Author:
- Rodrigo Vazquez, MD
- Cristina Gheorghe, MD
- David Kaufman, MD
- Constantine A. Manthous, MD
Article
Communications Bluefish A Newly Discovered Cause of Scombroid Poisoning
- Author:
- Perry A. Pugno, MD
- David Kaufman, MD
- Henry M. Feder Jr, MD
