User login
Trends in Thrombolytic Use for Stroke
Recombinant tissue plasminogen activator (tPA), approved for use in the United States for the treatment for acute ischemic stroke since 1996, improves overall outcomes from ischemic stroke when administered to selected patients.14 Several prominent guidelines, including the Brain Attack Coalition and the American Stroke Association, have recommended increasing the use of tPA for acute ischemic stroke.57 In addition, in 2003 the Joint Commission on Accreditation of Healthcare Organizations developed a disease‐specific certification program to designate certain institutions Primary Stroke Centers, with one of the performance measures being the availability of thrombolysis.8
Despite guidelines and regulatory agencies promoting the use of thrombolysis for ischemic stroke, previous studies have shown disappointingly low rates of use.912 The goals of this study were to assess whether national trends in the use of thrombolysis for acute ischemic stroke have increased in light of increased regulatory activity as well as to identify patient characteristics associated with thrombolytic administration.
Materials and Methods
Data for this study were obtained from the 2001 through 2006 National Hospital Discharge Survey (NHDS), a nationally representative sample of inpatient hospitalizations conducted annually by the National Center for Health Statistics.13 The NHDS collects data on approximately 300,000 patients from about 500 short‐stay nonfederal hospitals in the United States and uses a 3‐stage sampling strategy that allows for extrapolation to national level estimates. Response rates typically exceed 90% from participating hospitals. The survey collects demographic data, including age, sex, race, hospital geographic region, hospital bedsize and patient insurance status. In addition, up to 7 diagnostic and 4 procedural codes from the hospitalization are available, as is hospitalization length of stay and patient discharge disposition. No information on timing of symptoms, degree of neurologic compromise, or results of imaging tests were available in the NHDS.
We searched for all patients age 18 years or older with a primary diagnostic code of ischemic stroke using the International Classification of Diseases, 9th Edition, Clinical Modification (ICD‐9‐CM) codes 433, 434, 436, 437.0, and 437.1, excluding codes with a fifth digit of 0 (which indicated arterial occlusion without mention of cerebral infarction). We then searched for the presence of an ICD‐9‐CM procedure code for injection or infusion of thrombolytic agent (code 99.10). Specific comorbid conditions associated with ischemic stroke were identified by searching for specific ICD‐9‐CM codes, including for heart failure, coronary artery disease, hypertension, diabetes mellitus, and atrial fibrillation. To provide a general assessment of the severity of illness of the patients, we calculated an adapted Charlson comorbidity score for each patient using available secondary discharge diagnosis codes.14 We also searched for codes corresponding to intracranial hemorrhage, a complication associated with tPA administration.
Statistical Analysis
We defined thrombolytic utilization rates as the number of patients hospitalized with a primary diagnosis of ischemic stroke who had a procedure code for thrombolysis divided by the total number of patients hospitalized with ischemic stroke. To calculate nationally representative prevalence rates, we used the sample weights provided by the NHDS to account for the complex sampling design of the survey. Differences in thrombolytic administration rates by patient and hospital characteristics were tested using chi‐squared tests for categorical variables and t‐tests for continuous variables. Variables underwent a backwards selection process with a significance level of 0.05 to develop the final multivariable model of predictors of thrombolytic administration. Length of stay and hospital discharge status were not included in the variable selection process, as the focus was on predictors of initial administration of thrombolytics. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
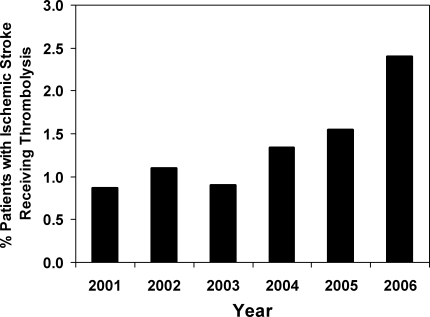
From years 2001 through 2006, we identified 22,842 patients with a primary diagnosis of ischemic stroke. Of these, 313 (1.37%, 95% confidence interval [CI], 1.22‐1.53%) had a procedure code for injection or infusion of thrombolysis. Using NHDS sample weights, these numbers corresponded to an estimated 2.55 million hospitalizations for ischemic stroke in the United States during the time period and to 35,082 patients receiving intravenous thrombolytics. Although the overall rate of thrombolysis administration was quite low overall, the administration rate increased over time, from 0.87% [95% CI, 0.61‐1.22%] of stroke patients in year 2001 to 2.40% [95% CI, 1.95‐2.93%] in year 2006 and with a particular increase especially noted after year 2003 (P <0.001 for trend, Figure 1).
On bivariate analysis, a lower proportion of African‐American patients received tPA compared to white patients (0.8% vs. 1.5%, P = 0.003), while a higher proportion of patients with atrial fibrillation received tPA (2.3% vs. 1.2%, P < 0.001). Older patients were less likely than younger patients to receive tPA (Table 1). The rate of intracranial hemorrhage was significantly higher in patients who received tPA (5.4% vs. 0.6%, P < 0.001) and the overall inpatient mortality in patients who received tPA was 9.0%. Mortality in patients receiving tPA continued to be higher than in patients who did not receive tPA even when patients with intracranial hemorrhage were excluded (8.1% vs. 5.3%, P < 0.001). Larger hospitals were more likely to administer tPA to patients with ischemic stroke, with a 1.79% administration rate in hospitals with 300 beds compared to 0.90% in hospitals with 100 to 199 beds and 0.52% in hospitals with 6 to 99 beds (P < 0.001).
| Thrombolysis, n (%) | No Thrombolysis, n (%) | P Value | |
|---|---|---|---|
| |||
| Age | 0.001 | ||
| <60 | 76 (24.3) | 4478 (19.9) | |
| 60‐69 | 73 (23.3) | 3942 (17.5) | |
| 70‐79 | 82 (26.2) | 6265 (27.8) | |
| 80+ | 82 (26.2) | 7844 (34.8) | |
| Female | 155 (49.5) | 12625 (56.0) | 0.02 |
| Race | 0.003 | ||
| White | 173 (55.3) | 11542 (51.2) | |
| African American | 29 (9.3) | 3774 (16.8) | |
| Other | 16 (5.1) | 814 (3.6) | |
| Not stated | 95 (30.4) | 6399 (28.4) | |
| Region | 0.05 | ||
| Northeast | 78 (24.9) | 4570 (20.3) | |
| Midwest | 84 (26.8) | 6924 (30.7) | |
| South | 104 (33.2) | 8284 (36.8) | |
| West | 47 (15.0) | 2751 (12.2) | |
| Type of admission | <0.001 | ||
| Emergent | 247 (78.9) | 14233 (63.2) | |
| Urgent | 33 (10.5) | 3703 (16.4) | |
| Elective | 5 (1.6) | 1346 (6.0) | |
| Unknown | 28 (9.0) | 3247 (14.4) | |
| Length of stay, days [95% CI] | 7.2 [6.6‐7.8] | 6.0 [5.9‐6.1] | <0.001 |
| Hospital bedsize | <0.001 | ||
| 6‐99 | 16 (5.1) | 3065 (13.6) | |
| 100‐199 | 48 (15.3) | 5289 (23.5) | |
| 200‐299 | 86 (27.5) | 5212 (23.1) | |
| 300+ | 163 (52.1) | 8963 (39.8) | |
| Payment type | <0.001 | ||
| Medicare | 176 (56.2) | 15197 (67.5) | |
| Medicaid | 21 (6.7) | 1245 (5.5) | |
| Private | 45 (14.4) | 2483 (11.0) | |
| HMO/PPO | 39 (12.5) | 2224 (9.9) | |
| Other/unknown | 32 (10.2) | 1380 (6.1) | |
| Discharge status | <0.001 | ||
| Home | 98 (31.3) | 9507 (42.2) | |
| Short term care facility | 25 (8.0) | 1271 (5.6) | |
| Long term care facility | 62 (19.8) | 5400 (24.0) | |
| Alive, status unknown | 89 (28.4) | 4514 (20.0) | |
| Death | 28 (9.0) | 1218 (5.4) | |
| Unknown | 11 (3.5) | 619 (2.8) | |
| Comorbid conditions | |||
| Congestive heart failure | 48 (15.3) | 2769 (12.3) | 0.10 |
| Coronary artery disease | 49 (15.7) | 4082 (18.1) | 0.26 |
| Hypertension | 164 (52.4) | 12480 (55.4) | 0.29 |
| Diabetes mellitus | 42 (13.4) | 4965 (22.0) | <0.001 |
| Atrial fibrillation | 96 (30.7) | 4096 (18.2) | <0.001 |
| Intracranial hemorrhage | 17 (5.4) | 139 (0.6) | <0.001 |
| Charlson score14 (mean) | 2.48 [2.32‐2.64] | 2.38 [2.36‐2.40] | 0.23 |
After adjusting for patient and hospital characteristics, the absolute rate of thrombolysis administration increased by an average of 0.19% per year (95% CI, 0.12‐0.26%). Factors that were significantly associated with administration of thrombolytics included being hospitalized in a larger hospital, having a history of atrial fibrillation, and a higher Charlson comorbidity index (Table 2). Patients aged 80 years or older, African American patients, and those with diabetes mellitus were significantly less likely to receive thrombolysis.
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| |
| Year (per year, from 2001 to 2006) | 1.2 (1.1‐1.3) |
| Age, years | |
| <60 | Referent |
| 60‐69 | 1.0 (0.7‐1.4) |
| 70‐79 | 0.6 (0.5‐0.9) |
| 80+ | 0.4 (0.3‐0.6) |
| Race | |
| Not African American | Referent |
| African‐American | 0.4 (0.3‐0.7) |
| Unknown | 1.0 (0.8‐1.2) |
| Hospital bedsize | |
| 6‐99 | Referent |
| 100‐199 | 1.7 (1.0‐3.1) |
| 200‐299 | 3.2 (1.8‐5.4) |
| 300+ | 3.3 (2.0‐5.6) |
| Diabetes mellitus | 0.5 (0.3‐0.6) |
| Atrial fibrillation | 2.2 (1.7‐2.9) |
| Charlson comorbidity score14 (per point increase) | 1.1 (1.1‐1.2) |
Discussion
Despite strong recommendations from guidelines and regulatory agencies, national rates of intravenous thrombolysis for ischemic stroke continue to be quite low overall. However, tPA administration appears to have increased from previous years and particularly increased in years after the Joint Commission began to accredit institutions as Primary Stroke Centers.11 The oldest patients and African Americans were less likely to receive thrombolytics, while patients with atrial fibrillation were more likely to receive thrombolysis, potentially related to atrial fibrillation causing more severe strokes.15 A total of 5.4% of patients who received tPA were diagnosed with intracranial hemorrhage, and the inpatient mortality rate of patients with tPA was 9.0%.
The exact optimal rate of thrombolysis administration for the patients in our study is unknown, as the NHDS database lacked detailed information on factors that would preclude tPA administration such as late timing of presentation and mild stroke symptoms.3 Studies conducted in stroke registries and regional settings have found that only approximately 15% to 32% of patients presenting with ischemic stroke arrive within 3 hours of symptom onset, and of these, only about 40% to 50% are eligible for tPA clinically.9, 10, 1619 However, even among presumed eligible patients, tPA administration rates only range between 25% and 43%,17, 19, 20 and the ideal rate is likely to be higher than the very low rates we observed in our study. Newer evidence that extending the time window where tPA may be given safely may increase the number of eligible patients.21
Patients who received thrombolysis had higher mortality rates than patients who did not. Although we were unable to determine a causal association, prior observational studies of tPA administration for acute stroke have found that patients with more severe neurologic deficits were more likely to receive thrombolysis.17, 18 The 9.0% inpatient case‐fatality rate observed in our study compares favorably to the 13.4% mortality rate after tPA reported in a post‐approval meta‐analysis of safety outcomes22 and the rate of intracranial hemorrhage in our analysis was similar to those observed in other settings.9, 2225 We were unable to determine whether intracranial hemorrhages in our study were as a result of tPA administration or whether patients who received tPA were more likely to have intracranial hemorrhages detected, such as may be due to increased frequency of head imaging.
Larger hospitals were more likely to administer tPA. This may reflect regionalization of stroke care, particularly in those designated as stroke centers of excellence. As well, there is some evidence that there is a learning curve with thrombolysis administration, where guideline‐recommended practice and use of tPA increases with additional experience with the drug.9, 26 Promoting systems that allow for rapid triage and diagnosis of acute stroke should be encouraged and hospital leaders should develop strategies that allow for early recognition of potential tPA candidates.
There are several limitations to our analysis. The NHDS does not collect detailed data on clinical or presenting features of stroke, and so we lacked information on stroke severity and eligibility for administration of thrombolysis. Our study may have underestimated the overall rates of thrombolysis, as it was dependent on diagnostic codes. A previous study of 34 patients who received tPA found that although the 99.10 code was 100% specific, the code identified only 17 patients who actually received tPA (sensitivity of 50%).20 Another study comparing Medicare administrative claims data to actual pharmacy billing charges for tPA found that administrative data underestimated the rate of tPA administration by approximately 25% to 30%.12 If a diagnostic code sensitivity of 50% was assumed, rates of tPA administration in our study may have been as high as 4.8% (95% CI, 4.1‐5.5%) by year 2006.
Conclusion
In conclusion, the use of intravenous thrombolysis in patients admitted with acute ischemic stroke in the United States has risen over time, but overall use remains very low. Further efforts to improve appropriate administration rates should be encouraged, particularly as the acceptable time‐window for using tPA widens.
Acknowledgements
The authors thank Mr. Loren Yglecias for his assistance with manuscript text and references.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rT‐PA stroke trials.Lancet.2004;363:768–774.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): An observational study.Lancet.2007;369:275–282.
- ,,, et al.Recommendations for the establishment of primary stroke centers.JAMA.2000;283:3102–3109.
- ,.Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative.Lancet Neurol.2003;2:698–701.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Circulation.2007;115:e478–534.
- The Joint Commission Primary Stroke Center Certification. Available at: http://www.jointcommission.org/CertificationPrograms/PrimaryStrokeCenters. Accessed February 2010.
- ,,, et al.Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience.JAMA.2000;283:1151–1158.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- ,,, et al.Thrombolysis for ischemic stroke in the united states: Data from National Hospital Discharge Survey 1999–2001.Neurosurgery.2005;57:647–654; discussion647–654.
- ,,,,.National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate.Stroke.2008;39:924–928.
- US Department of Health and Human Services Public Health Service and National Center for Health Statistics.National Hospital Discharge Durvey 1991–2006. Multi‐year public‐use data file documentation.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.Stroke severity in atrial fibrillation. The Framingham study.Stroke.1996;27:1760–1764.
- ,,,,.Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility.Neurology.2001;57:1739–1740.
- ,,,,,.Utilization of intravenous tissue plasminogen activator for acute ischemic stroke.Arch Neurol.2004;61:346–350.
- ,,, et al.Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study.Stroke.2004;35:27–29.
- ,,, et al.Tpa use for stroke in the registry of the Canadian stroke network.Can J Neurol Sci.2005;32:433–439.
- ,,, et al.Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity.Stroke.2001;32:1061–1068.
- ,,, et al.Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3‐ to 4.5‐hour window: Joint outcome table analysis of the ECASS 3 trial.Stroke.2009;40:2433–2437.
- .Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta‐analysis of safety data.Stroke.2003;34:2847–2850.
- ,,, et al.Early intravenous thrombolysis for acute ischemic stroke in a community‐based approach.Stroke.1998;29:1544–1549.
- ,,, et al.Factors associated with in‐hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the Nationwide Inpatient Sample 1999 to 2002.Stroke.2006;37:440–446.
- ,,, et al.Intravenous t‐PA for acute ischemic stroke: therapeutic yield of a stroke code system.Neurology.1998;50:501–503.
- ,,, et al.Intravenous tissue‐type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000.Arch Neurol.2001;58:2009–2013.
Recombinant tissue plasminogen activator (tPA), approved for use in the United States for the treatment for acute ischemic stroke since 1996, improves overall outcomes from ischemic stroke when administered to selected patients.14 Several prominent guidelines, including the Brain Attack Coalition and the American Stroke Association, have recommended increasing the use of tPA for acute ischemic stroke.57 In addition, in 2003 the Joint Commission on Accreditation of Healthcare Organizations developed a disease‐specific certification program to designate certain institutions Primary Stroke Centers, with one of the performance measures being the availability of thrombolysis.8
Despite guidelines and regulatory agencies promoting the use of thrombolysis for ischemic stroke, previous studies have shown disappointingly low rates of use.912 The goals of this study were to assess whether national trends in the use of thrombolysis for acute ischemic stroke have increased in light of increased regulatory activity as well as to identify patient characteristics associated with thrombolytic administration.
Materials and Methods
Data for this study were obtained from the 2001 through 2006 National Hospital Discharge Survey (NHDS), a nationally representative sample of inpatient hospitalizations conducted annually by the National Center for Health Statistics.13 The NHDS collects data on approximately 300,000 patients from about 500 short‐stay nonfederal hospitals in the United States and uses a 3‐stage sampling strategy that allows for extrapolation to national level estimates. Response rates typically exceed 90% from participating hospitals. The survey collects demographic data, including age, sex, race, hospital geographic region, hospital bedsize and patient insurance status. In addition, up to 7 diagnostic and 4 procedural codes from the hospitalization are available, as is hospitalization length of stay and patient discharge disposition. No information on timing of symptoms, degree of neurologic compromise, or results of imaging tests were available in the NHDS.
We searched for all patients age 18 years or older with a primary diagnostic code of ischemic stroke using the International Classification of Diseases, 9th Edition, Clinical Modification (ICD‐9‐CM) codes 433, 434, 436, 437.0, and 437.1, excluding codes with a fifth digit of 0 (which indicated arterial occlusion without mention of cerebral infarction). We then searched for the presence of an ICD‐9‐CM procedure code for injection or infusion of thrombolytic agent (code 99.10). Specific comorbid conditions associated with ischemic stroke were identified by searching for specific ICD‐9‐CM codes, including for heart failure, coronary artery disease, hypertension, diabetes mellitus, and atrial fibrillation. To provide a general assessment of the severity of illness of the patients, we calculated an adapted Charlson comorbidity score for each patient using available secondary discharge diagnosis codes.14 We also searched for codes corresponding to intracranial hemorrhage, a complication associated with tPA administration.
Statistical Analysis
We defined thrombolytic utilization rates as the number of patients hospitalized with a primary diagnosis of ischemic stroke who had a procedure code for thrombolysis divided by the total number of patients hospitalized with ischemic stroke. To calculate nationally representative prevalence rates, we used the sample weights provided by the NHDS to account for the complex sampling design of the survey. Differences in thrombolytic administration rates by patient and hospital characteristics were tested using chi‐squared tests for categorical variables and t‐tests for continuous variables. Variables underwent a backwards selection process with a significance level of 0.05 to develop the final multivariable model of predictors of thrombolytic administration. Length of stay and hospital discharge status were not included in the variable selection process, as the focus was on predictors of initial administration of thrombolytics. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
From years 2001 through 2006, we identified 22,842 patients with a primary diagnosis of ischemic stroke. Of these, 313 (1.37%, 95% confidence interval [CI], 1.22‐1.53%) had a procedure code for injection or infusion of thrombolysis. Using NHDS sample weights, these numbers corresponded to an estimated 2.55 million hospitalizations for ischemic stroke in the United States during the time period and to 35,082 patients receiving intravenous thrombolytics. Although the overall rate of thrombolysis administration was quite low overall, the administration rate increased over time, from 0.87% [95% CI, 0.61‐1.22%] of stroke patients in year 2001 to 2.40% [95% CI, 1.95‐2.93%] in year 2006 and with a particular increase especially noted after year 2003 (P <0.001 for trend, Figure 1).

On bivariate analysis, a lower proportion of African‐American patients received tPA compared to white patients (0.8% vs. 1.5%, P = 0.003), while a higher proportion of patients with atrial fibrillation received tPA (2.3% vs. 1.2%, P < 0.001). Older patients were less likely than younger patients to receive tPA (Table 1). The rate of intracranial hemorrhage was significantly higher in patients who received tPA (5.4% vs. 0.6%, P < 0.001) and the overall inpatient mortality in patients who received tPA was 9.0%. Mortality in patients receiving tPA continued to be higher than in patients who did not receive tPA even when patients with intracranial hemorrhage were excluded (8.1% vs. 5.3%, P < 0.001). Larger hospitals were more likely to administer tPA to patients with ischemic stroke, with a 1.79% administration rate in hospitals with 300 beds compared to 0.90% in hospitals with 100 to 199 beds and 0.52% in hospitals with 6 to 99 beds (P < 0.001).
| Thrombolysis, n (%) | No Thrombolysis, n (%) | P Value | |
|---|---|---|---|
| |||
| Age | 0.001 | ||
| <60 | 76 (24.3) | 4478 (19.9) | |
| 60‐69 | 73 (23.3) | 3942 (17.5) | |
| 70‐79 | 82 (26.2) | 6265 (27.8) | |
| 80+ | 82 (26.2) | 7844 (34.8) | |
| Female | 155 (49.5) | 12625 (56.0) | 0.02 |
| Race | 0.003 | ||
| White | 173 (55.3) | 11542 (51.2) | |
| African American | 29 (9.3) | 3774 (16.8) | |
| Other | 16 (5.1) | 814 (3.6) | |
| Not stated | 95 (30.4) | 6399 (28.4) | |
| Region | 0.05 | ||
| Northeast | 78 (24.9) | 4570 (20.3) | |
| Midwest | 84 (26.8) | 6924 (30.7) | |
| South | 104 (33.2) | 8284 (36.8) | |
| West | 47 (15.0) | 2751 (12.2) | |
| Type of admission | <0.001 | ||
| Emergent | 247 (78.9) | 14233 (63.2) | |
| Urgent | 33 (10.5) | 3703 (16.4) | |
| Elective | 5 (1.6) | 1346 (6.0) | |
| Unknown | 28 (9.0) | 3247 (14.4) | |
| Length of stay, days [95% CI] | 7.2 [6.6‐7.8] | 6.0 [5.9‐6.1] | <0.001 |
| Hospital bedsize | <0.001 | ||
| 6‐99 | 16 (5.1) | 3065 (13.6) | |
| 100‐199 | 48 (15.3) | 5289 (23.5) | |
| 200‐299 | 86 (27.5) | 5212 (23.1) | |
| 300+ | 163 (52.1) | 8963 (39.8) | |
| Payment type | <0.001 | ||
| Medicare | 176 (56.2) | 15197 (67.5) | |
| Medicaid | 21 (6.7) | 1245 (5.5) | |
| Private | 45 (14.4) | 2483 (11.0) | |
| HMO/PPO | 39 (12.5) | 2224 (9.9) | |
| Other/unknown | 32 (10.2) | 1380 (6.1) | |
| Discharge status | <0.001 | ||
| Home | 98 (31.3) | 9507 (42.2) | |
| Short term care facility | 25 (8.0) | 1271 (5.6) | |
| Long term care facility | 62 (19.8) | 5400 (24.0) | |
| Alive, status unknown | 89 (28.4) | 4514 (20.0) | |
| Death | 28 (9.0) | 1218 (5.4) | |
| Unknown | 11 (3.5) | 619 (2.8) | |
| Comorbid conditions | |||
| Congestive heart failure | 48 (15.3) | 2769 (12.3) | 0.10 |
| Coronary artery disease | 49 (15.7) | 4082 (18.1) | 0.26 |
| Hypertension | 164 (52.4) | 12480 (55.4) | 0.29 |
| Diabetes mellitus | 42 (13.4) | 4965 (22.0) | <0.001 |
| Atrial fibrillation | 96 (30.7) | 4096 (18.2) | <0.001 |
| Intracranial hemorrhage | 17 (5.4) | 139 (0.6) | <0.001 |
| Charlson score14 (mean) | 2.48 [2.32‐2.64] | 2.38 [2.36‐2.40] | 0.23 |
After adjusting for patient and hospital characteristics, the absolute rate of thrombolysis administration increased by an average of 0.19% per year (95% CI, 0.12‐0.26%). Factors that were significantly associated with administration of thrombolytics included being hospitalized in a larger hospital, having a history of atrial fibrillation, and a higher Charlson comorbidity index (Table 2). Patients aged 80 years or older, African American patients, and those with diabetes mellitus were significantly less likely to receive thrombolysis.
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| |
| Year (per year, from 2001 to 2006) | 1.2 (1.1‐1.3) |
| Age, years | |
| <60 | Referent |
| 60‐69 | 1.0 (0.7‐1.4) |
| 70‐79 | 0.6 (0.5‐0.9) |
| 80+ | 0.4 (0.3‐0.6) |
| Race | |
| Not African American | Referent |
| African‐American | 0.4 (0.3‐0.7) |
| Unknown | 1.0 (0.8‐1.2) |
| Hospital bedsize | |
| 6‐99 | Referent |
| 100‐199 | 1.7 (1.0‐3.1) |
| 200‐299 | 3.2 (1.8‐5.4) |
| 300+ | 3.3 (2.0‐5.6) |
| Diabetes mellitus | 0.5 (0.3‐0.6) |
| Atrial fibrillation | 2.2 (1.7‐2.9) |
| Charlson comorbidity score14 (per point increase) | 1.1 (1.1‐1.2) |
Discussion
Despite strong recommendations from guidelines and regulatory agencies, national rates of intravenous thrombolysis for ischemic stroke continue to be quite low overall. However, tPA administration appears to have increased from previous years and particularly increased in years after the Joint Commission began to accredit institutions as Primary Stroke Centers.11 The oldest patients and African Americans were less likely to receive thrombolytics, while patients with atrial fibrillation were more likely to receive thrombolysis, potentially related to atrial fibrillation causing more severe strokes.15 A total of 5.4% of patients who received tPA were diagnosed with intracranial hemorrhage, and the inpatient mortality rate of patients with tPA was 9.0%.
The exact optimal rate of thrombolysis administration for the patients in our study is unknown, as the NHDS database lacked detailed information on factors that would preclude tPA administration such as late timing of presentation and mild stroke symptoms.3 Studies conducted in stroke registries and regional settings have found that only approximately 15% to 32% of patients presenting with ischemic stroke arrive within 3 hours of symptom onset, and of these, only about 40% to 50% are eligible for tPA clinically.9, 10, 1619 However, even among presumed eligible patients, tPA administration rates only range between 25% and 43%,17, 19, 20 and the ideal rate is likely to be higher than the very low rates we observed in our study. Newer evidence that extending the time window where tPA may be given safely may increase the number of eligible patients.21
Patients who received thrombolysis had higher mortality rates than patients who did not. Although we were unable to determine a causal association, prior observational studies of tPA administration for acute stroke have found that patients with more severe neurologic deficits were more likely to receive thrombolysis.17, 18 The 9.0% inpatient case‐fatality rate observed in our study compares favorably to the 13.4% mortality rate after tPA reported in a post‐approval meta‐analysis of safety outcomes22 and the rate of intracranial hemorrhage in our analysis was similar to those observed in other settings.9, 2225 We were unable to determine whether intracranial hemorrhages in our study were as a result of tPA administration or whether patients who received tPA were more likely to have intracranial hemorrhages detected, such as may be due to increased frequency of head imaging.
Larger hospitals were more likely to administer tPA. This may reflect regionalization of stroke care, particularly in those designated as stroke centers of excellence. As well, there is some evidence that there is a learning curve with thrombolysis administration, where guideline‐recommended practice and use of tPA increases with additional experience with the drug.9, 26 Promoting systems that allow for rapid triage and diagnosis of acute stroke should be encouraged and hospital leaders should develop strategies that allow for early recognition of potential tPA candidates.
There are several limitations to our analysis. The NHDS does not collect detailed data on clinical or presenting features of stroke, and so we lacked information on stroke severity and eligibility for administration of thrombolysis. Our study may have underestimated the overall rates of thrombolysis, as it was dependent on diagnostic codes. A previous study of 34 patients who received tPA found that although the 99.10 code was 100% specific, the code identified only 17 patients who actually received tPA (sensitivity of 50%).20 Another study comparing Medicare administrative claims data to actual pharmacy billing charges for tPA found that administrative data underestimated the rate of tPA administration by approximately 25% to 30%.12 If a diagnostic code sensitivity of 50% was assumed, rates of tPA administration in our study may have been as high as 4.8% (95% CI, 4.1‐5.5%) by year 2006.
Conclusion
In conclusion, the use of intravenous thrombolysis in patients admitted with acute ischemic stroke in the United States has risen over time, but overall use remains very low. Further efforts to improve appropriate administration rates should be encouraged, particularly as the acceptable time‐window for using tPA widens.
Acknowledgements
The authors thank Mr. Loren Yglecias for his assistance with manuscript text and references.
Recombinant tissue plasminogen activator (tPA), approved for use in the United States for the treatment for acute ischemic stroke since 1996, improves overall outcomes from ischemic stroke when administered to selected patients.14 Several prominent guidelines, including the Brain Attack Coalition and the American Stroke Association, have recommended increasing the use of tPA for acute ischemic stroke.57 In addition, in 2003 the Joint Commission on Accreditation of Healthcare Organizations developed a disease‐specific certification program to designate certain institutions Primary Stroke Centers, with one of the performance measures being the availability of thrombolysis.8
Despite guidelines and regulatory agencies promoting the use of thrombolysis for ischemic stroke, previous studies have shown disappointingly low rates of use.912 The goals of this study were to assess whether national trends in the use of thrombolysis for acute ischemic stroke have increased in light of increased regulatory activity as well as to identify patient characteristics associated with thrombolytic administration.
Materials and Methods
Data for this study were obtained from the 2001 through 2006 National Hospital Discharge Survey (NHDS), a nationally representative sample of inpatient hospitalizations conducted annually by the National Center for Health Statistics.13 The NHDS collects data on approximately 300,000 patients from about 500 short‐stay nonfederal hospitals in the United States and uses a 3‐stage sampling strategy that allows for extrapolation to national level estimates. Response rates typically exceed 90% from participating hospitals. The survey collects demographic data, including age, sex, race, hospital geographic region, hospital bedsize and patient insurance status. In addition, up to 7 diagnostic and 4 procedural codes from the hospitalization are available, as is hospitalization length of stay and patient discharge disposition. No information on timing of symptoms, degree of neurologic compromise, or results of imaging tests were available in the NHDS.
We searched for all patients age 18 years or older with a primary diagnostic code of ischemic stroke using the International Classification of Diseases, 9th Edition, Clinical Modification (ICD‐9‐CM) codes 433, 434, 436, 437.0, and 437.1, excluding codes with a fifth digit of 0 (which indicated arterial occlusion without mention of cerebral infarction). We then searched for the presence of an ICD‐9‐CM procedure code for injection or infusion of thrombolytic agent (code 99.10). Specific comorbid conditions associated with ischemic stroke were identified by searching for specific ICD‐9‐CM codes, including for heart failure, coronary artery disease, hypertension, diabetes mellitus, and atrial fibrillation. To provide a general assessment of the severity of illness of the patients, we calculated an adapted Charlson comorbidity score for each patient using available secondary discharge diagnosis codes.14 We also searched for codes corresponding to intracranial hemorrhage, a complication associated with tPA administration.
Statistical Analysis
We defined thrombolytic utilization rates as the number of patients hospitalized with a primary diagnosis of ischemic stroke who had a procedure code for thrombolysis divided by the total number of patients hospitalized with ischemic stroke. To calculate nationally representative prevalence rates, we used the sample weights provided by the NHDS to account for the complex sampling design of the survey. Differences in thrombolytic administration rates by patient and hospital characteristics were tested using chi‐squared tests for categorical variables and t‐tests for continuous variables. Variables underwent a backwards selection process with a significance level of 0.05 to develop the final multivariable model of predictors of thrombolytic administration. Length of stay and hospital discharge status were not included in the variable selection process, as the focus was on predictors of initial administration of thrombolytics. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
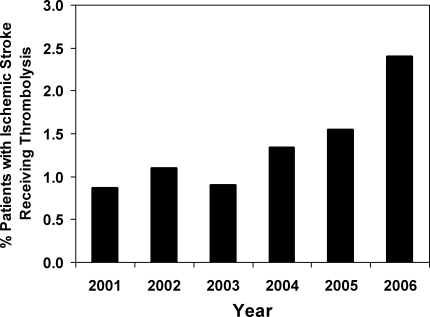
From years 2001 through 2006, we identified 22,842 patients with a primary diagnosis of ischemic stroke. Of these, 313 (1.37%, 95% confidence interval [CI], 1.22‐1.53%) had a procedure code for injection or infusion of thrombolysis. Using NHDS sample weights, these numbers corresponded to an estimated 2.55 million hospitalizations for ischemic stroke in the United States during the time period and to 35,082 patients receiving intravenous thrombolytics. Although the overall rate of thrombolysis administration was quite low overall, the administration rate increased over time, from 0.87% [95% CI, 0.61‐1.22%] of stroke patients in year 2001 to 2.40% [95% CI, 1.95‐2.93%] in year 2006 and with a particular increase especially noted after year 2003 (P <0.001 for trend, Figure 1).

On bivariate analysis, a lower proportion of African‐American patients received tPA compared to white patients (0.8% vs. 1.5%, P = 0.003), while a higher proportion of patients with atrial fibrillation received tPA (2.3% vs. 1.2%, P < 0.001). Older patients were less likely than younger patients to receive tPA (Table 1). The rate of intracranial hemorrhage was significantly higher in patients who received tPA (5.4% vs. 0.6%, P < 0.001) and the overall inpatient mortality in patients who received tPA was 9.0%. Mortality in patients receiving tPA continued to be higher than in patients who did not receive tPA even when patients with intracranial hemorrhage were excluded (8.1% vs. 5.3%, P < 0.001). Larger hospitals were more likely to administer tPA to patients with ischemic stroke, with a 1.79% administration rate in hospitals with 300 beds compared to 0.90% in hospitals with 100 to 199 beds and 0.52% in hospitals with 6 to 99 beds (P < 0.001).
| Thrombolysis, n (%) | No Thrombolysis, n (%) | P Value | |
|---|---|---|---|
| |||
| Age | 0.001 | ||
| <60 | 76 (24.3) | 4478 (19.9) | |
| 60‐69 | 73 (23.3) | 3942 (17.5) | |
| 70‐79 | 82 (26.2) | 6265 (27.8) | |
| 80+ | 82 (26.2) | 7844 (34.8) | |
| Female | 155 (49.5) | 12625 (56.0) | 0.02 |
| Race | 0.003 | ||
| White | 173 (55.3) | 11542 (51.2) | |
| African American | 29 (9.3) | 3774 (16.8) | |
| Other | 16 (5.1) | 814 (3.6) | |
| Not stated | 95 (30.4) | 6399 (28.4) | |
| Region | 0.05 | ||
| Northeast | 78 (24.9) | 4570 (20.3) | |
| Midwest | 84 (26.8) | 6924 (30.7) | |
| South | 104 (33.2) | 8284 (36.8) | |
| West | 47 (15.0) | 2751 (12.2) | |
| Type of admission | <0.001 | ||
| Emergent | 247 (78.9) | 14233 (63.2) | |
| Urgent | 33 (10.5) | 3703 (16.4) | |
| Elective | 5 (1.6) | 1346 (6.0) | |
| Unknown | 28 (9.0) | 3247 (14.4) | |
| Length of stay, days [95% CI] | 7.2 [6.6‐7.8] | 6.0 [5.9‐6.1] | <0.001 |
| Hospital bedsize | <0.001 | ||
| 6‐99 | 16 (5.1) | 3065 (13.6) | |
| 100‐199 | 48 (15.3) | 5289 (23.5) | |
| 200‐299 | 86 (27.5) | 5212 (23.1) | |
| 300+ | 163 (52.1) | 8963 (39.8) | |
| Payment type | <0.001 | ||
| Medicare | 176 (56.2) | 15197 (67.5) | |
| Medicaid | 21 (6.7) | 1245 (5.5) | |
| Private | 45 (14.4) | 2483 (11.0) | |
| HMO/PPO | 39 (12.5) | 2224 (9.9) | |
| Other/unknown | 32 (10.2) | 1380 (6.1) | |
| Discharge status | <0.001 | ||
| Home | 98 (31.3) | 9507 (42.2) | |
| Short term care facility | 25 (8.0) | 1271 (5.6) | |
| Long term care facility | 62 (19.8) | 5400 (24.0) | |
| Alive, status unknown | 89 (28.4) | 4514 (20.0) | |
| Death | 28 (9.0) | 1218 (5.4) | |
| Unknown | 11 (3.5) | 619 (2.8) | |
| Comorbid conditions | |||
| Congestive heart failure | 48 (15.3) | 2769 (12.3) | 0.10 |
| Coronary artery disease | 49 (15.7) | 4082 (18.1) | 0.26 |
| Hypertension | 164 (52.4) | 12480 (55.4) | 0.29 |
| Diabetes mellitus | 42 (13.4) | 4965 (22.0) | <0.001 |
| Atrial fibrillation | 96 (30.7) | 4096 (18.2) | <0.001 |
| Intracranial hemorrhage | 17 (5.4) | 139 (0.6) | <0.001 |
| Charlson score14 (mean) | 2.48 [2.32‐2.64] | 2.38 [2.36‐2.40] | 0.23 |
After adjusting for patient and hospital characteristics, the absolute rate of thrombolysis administration increased by an average of 0.19% per year (95% CI, 0.12‐0.26%). Factors that were significantly associated with administration of thrombolytics included being hospitalized in a larger hospital, having a history of atrial fibrillation, and a higher Charlson comorbidity index (Table 2). Patients aged 80 years or older, African American patients, and those with diabetes mellitus were significantly less likely to receive thrombolysis.
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| |
| Year (per year, from 2001 to 2006) | 1.2 (1.1‐1.3) |
| Age, years | |
| <60 | Referent |
| 60‐69 | 1.0 (0.7‐1.4) |
| 70‐79 | 0.6 (0.5‐0.9) |
| 80+ | 0.4 (0.3‐0.6) |
| Race | |
| Not African American | Referent |
| African‐American | 0.4 (0.3‐0.7) |
| Unknown | 1.0 (0.8‐1.2) |
| Hospital bedsize | |
| 6‐99 | Referent |
| 100‐199 | 1.7 (1.0‐3.1) |
| 200‐299 | 3.2 (1.8‐5.4) |
| 300+ | 3.3 (2.0‐5.6) |
| Diabetes mellitus | 0.5 (0.3‐0.6) |
| Atrial fibrillation | 2.2 (1.7‐2.9) |
| Charlson comorbidity score14 (per point increase) | 1.1 (1.1‐1.2) |
Discussion
Despite strong recommendations from guidelines and regulatory agencies, national rates of intravenous thrombolysis for ischemic stroke continue to be quite low overall. However, tPA administration appears to have increased from previous years and particularly increased in years after the Joint Commission began to accredit institutions as Primary Stroke Centers.11 The oldest patients and African Americans were less likely to receive thrombolytics, while patients with atrial fibrillation were more likely to receive thrombolysis, potentially related to atrial fibrillation causing more severe strokes.15 A total of 5.4% of patients who received tPA were diagnosed with intracranial hemorrhage, and the inpatient mortality rate of patients with tPA was 9.0%.
The exact optimal rate of thrombolysis administration for the patients in our study is unknown, as the NHDS database lacked detailed information on factors that would preclude tPA administration such as late timing of presentation and mild stroke symptoms.3 Studies conducted in stroke registries and regional settings have found that only approximately 15% to 32% of patients presenting with ischemic stroke arrive within 3 hours of symptom onset, and of these, only about 40% to 50% are eligible for tPA clinically.9, 10, 1619 However, even among presumed eligible patients, tPA administration rates only range between 25% and 43%,17, 19, 20 and the ideal rate is likely to be higher than the very low rates we observed in our study. Newer evidence that extending the time window where tPA may be given safely may increase the number of eligible patients.21
Patients who received thrombolysis had higher mortality rates than patients who did not. Although we were unable to determine a causal association, prior observational studies of tPA administration for acute stroke have found that patients with more severe neurologic deficits were more likely to receive thrombolysis.17, 18 The 9.0% inpatient case‐fatality rate observed in our study compares favorably to the 13.4% mortality rate after tPA reported in a post‐approval meta‐analysis of safety outcomes22 and the rate of intracranial hemorrhage in our analysis was similar to those observed in other settings.9, 2225 We were unable to determine whether intracranial hemorrhages in our study were as a result of tPA administration or whether patients who received tPA were more likely to have intracranial hemorrhages detected, such as may be due to increased frequency of head imaging.
Larger hospitals were more likely to administer tPA. This may reflect regionalization of stroke care, particularly in those designated as stroke centers of excellence. As well, there is some evidence that there is a learning curve with thrombolysis administration, where guideline‐recommended practice and use of tPA increases with additional experience with the drug.9, 26 Promoting systems that allow for rapid triage and diagnosis of acute stroke should be encouraged and hospital leaders should develop strategies that allow for early recognition of potential tPA candidates.
There are several limitations to our analysis. The NHDS does not collect detailed data on clinical or presenting features of stroke, and so we lacked information on stroke severity and eligibility for administration of thrombolysis. Our study may have underestimated the overall rates of thrombolysis, as it was dependent on diagnostic codes. A previous study of 34 patients who received tPA found that although the 99.10 code was 100% specific, the code identified only 17 patients who actually received tPA (sensitivity of 50%).20 Another study comparing Medicare administrative claims data to actual pharmacy billing charges for tPA found that administrative data underestimated the rate of tPA administration by approximately 25% to 30%.12 If a diagnostic code sensitivity of 50% was assumed, rates of tPA administration in our study may have been as high as 4.8% (95% CI, 4.1‐5.5%) by year 2006.
Conclusion
In conclusion, the use of intravenous thrombolysis in patients admitted with acute ischemic stroke in the United States has risen over time, but overall use remains very low. Further efforts to improve appropriate administration rates should be encouraged, particularly as the acceptable time‐window for using tPA widens.
Acknowledgements
The authors thank Mr. Loren Yglecias for his assistance with manuscript text and references.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rT‐PA stroke trials.Lancet.2004;363:768–774.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): An observational study.Lancet.2007;369:275–282.
- ,,, et al.Recommendations for the establishment of primary stroke centers.JAMA.2000;283:3102–3109.
- ,.Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative.Lancet Neurol.2003;2:698–701.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Circulation.2007;115:e478–534.
- The Joint Commission Primary Stroke Center Certification. Available at: http://www.jointcommission.org/CertificationPrograms/PrimaryStrokeCenters. Accessed February 2010.
- ,,, et al.Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience.JAMA.2000;283:1151–1158.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- ,,, et al.Thrombolysis for ischemic stroke in the united states: Data from National Hospital Discharge Survey 1999–2001.Neurosurgery.2005;57:647–654; discussion647–654.
- ,,,,.National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate.Stroke.2008;39:924–928.
- US Department of Health and Human Services Public Health Service and National Center for Health Statistics.National Hospital Discharge Durvey 1991–2006. Multi‐year public‐use data file documentation.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.Stroke severity in atrial fibrillation. The Framingham study.Stroke.1996;27:1760–1764.
- ,,,,.Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility.Neurology.2001;57:1739–1740.
- ,,,,,.Utilization of intravenous tissue plasminogen activator for acute ischemic stroke.Arch Neurol.2004;61:346–350.
- ,,, et al.Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study.Stroke.2004;35:27–29.
- ,,, et al.Tpa use for stroke in the registry of the Canadian stroke network.Can J Neurol Sci.2005;32:433–439.
- ,,, et al.Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity.Stroke.2001;32:1061–1068.
- ,,, et al.Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3‐ to 4.5‐hour window: Joint outcome table analysis of the ECASS 3 trial.Stroke.2009;40:2433–2437.
- .Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta‐analysis of safety data.Stroke.2003;34:2847–2850.
- ,,, et al.Early intravenous thrombolysis for acute ischemic stroke in a community‐based approach.Stroke.1998;29:1544–1549.
- ,,, et al.Factors associated with in‐hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the Nationwide Inpatient Sample 1999 to 2002.Stroke.2006;37:440–446.
- ,,, et al.Intravenous t‐PA for acute ischemic stroke: therapeutic yield of a stroke code system.Neurology.1998;50:501–503.
- ,,, et al.Intravenous tissue‐type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000.Arch Neurol.2001;58:2009–2013.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rT‐PA stroke trials.Lancet.2004;363:768–774.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): An observational study.Lancet.2007;369:275–282.
- ,,, et al.Recommendations for the establishment of primary stroke centers.JAMA.2000;283:3102–3109.
- ,.Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative.Lancet Neurol.2003;2:698–701.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Circulation.2007;115:e478–534.
- The Joint Commission Primary Stroke Center Certification. Available at: http://www.jointcommission.org/CertificationPrograms/PrimaryStrokeCenters. Accessed February 2010.
- ,,, et al.Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience.JAMA.2000;283:1151–1158.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- ,,, et al.Thrombolysis for ischemic stroke in the united states: Data from National Hospital Discharge Survey 1999–2001.Neurosurgery.2005;57:647–654; discussion647–654.
- ,,,,.National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate.Stroke.2008;39:924–928.
- US Department of Health and Human Services Public Health Service and National Center for Health Statistics.National Hospital Discharge Durvey 1991–2006. Multi‐year public‐use data file documentation.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.Stroke severity in atrial fibrillation. The Framingham study.Stroke.1996;27:1760–1764.
- ,,,,.Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility.Neurology.2001;57:1739–1740.
- ,,,,,.Utilization of intravenous tissue plasminogen activator for acute ischemic stroke.Arch Neurol.2004;61:346–350.
- ,,, et al.Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study.Stroke.2004;35:27–29.
- ,,, et al.Tpa use for stroke in the registry of the Canadian stroke network.Can J Neurol Sci.2005;32:433–439.
- ,,, et al.Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity.Stroke.2001;32:1061–1068.
- ,,, et al.Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3‐ to 4.5‐hour window: Joint outcome table analysis of the ECASS 3 trial.Stroke.2009;40:2433–2437.
- .Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta‐analysis of safety data.Stroke.2003;34:2847–2850.
- ,,, et al.Early intravenous thrombolysis for acute ischemic stroke in a community‐based approach.Stroke.1998;29:1544–1549.
- ,,, et al.Factors associated with in‐hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the Nationwide Inpatient Sample 1999 to 2002.Stroke.2006;37:440–446.
- ,,, et al.Intravenous t‐PA for acute ischemic stroke: therapeutic yield of a stroke code system.Neurology.1998;50:501–503.
- ,,, et al.Intravenous tissue‐type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000.Arch Neurol.2001;58:2009–2013.
Copyright © 2010 Society of Hospital Medicine