Article
Implant Survivorship and Complication Rates After Total Knee Arthroplasty With a Third-Generation Cemented System: 15-Year Follow-Up
- Author:
- Muthana Sartawi, MD
- David Zurakowski, PhD
- Aaron Rosenberg, MD
Publish date: March 28, 2018
Article
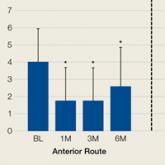
Comparison of Anterior and Posterior Corticosteroid Injections for Pain Relief and Functional Improvement in Shoulder Impingement Syndrome
- Author:
- Arun Ramappa, MD
- Kempland C. Walley, BSc
- Lindsay M. Herder, BA
- Sravisht Iyer, MD
- David Zurakowski, PhD
- Amber Hall, MPH
- Joseph P. DeAngelis, MD
Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain....
