User login
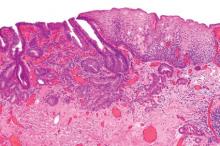
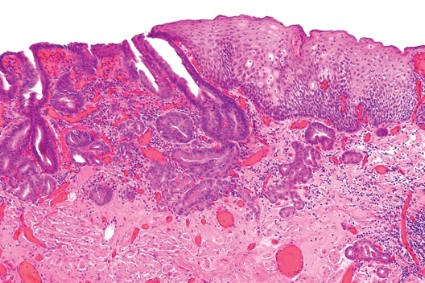
Interval CRC may be distinct pathology
As many as 6% of colorectal cancers are discovered between 6 and 60 months of colonoscopy – usually in the proximal colon, and usually in patients whose index colonoscopy revealed adenomas, according to Dr. N. Jewel Samadder and colleagues.
While the investigators speculated that "differences in the biology of interval versus detected cancers" might be to blame, "suboptimal management of precancerous lesions (such as incomplete polypectomy) likely also plays a role," they wrote. The report is in the April issue of Gastroenterology (doi:10.1053/j.gastro.2014.01.013).
Dr. Samadder of the University of Utah, Salt Lake City, and colleagues looked at data from 126,851 patients who underwent colonoscopies between 1995 and 2009. The two centers in this analysis serve more than 85% of the state’s population. Patients with a history of colorectal cancer before 1995 were excluded, the researchers wrote.
Overall, 2,659 patients were diagnosed with colorectal cancer during the study period, mostly at initial, index colonoscopy – the investigators referred to these as "detected" cancers.
However, 159 of these cases (6%) were diagnosed between 6 and 60 months of the index colonoscopy, and were therefore classified as "interval" cancers.
Interval cancer patients had a mean age of 67 years at index colonoscopy (range, 34-92 years), identical to that of detected cancer patients; both cohorts were split evenly between men and women.
The authors found that compared with detected cancers, the interval cancers were much more commonly discovered in the proximal colon – indeed, proximal cancers made up 55% of the cohort, with the remainder split evenly between the distal colon and the recto-rectosigmoid junction.
In comparison, only 39% of detected cancers were found in the proximal colon (P less than .001).
Patients with an adenoma or villous adenoma discovered at index colonoscopy were significantly more likely to develop interval cancer, compared with patients whose cancers were detected earlier (for adenoma, the odds ratio was 2.96, 95% confidence interval = 2.0-4.28; for villous adenoma, OR = 2.04, 95% CI = 1.34-3.11).
A family history also conferred an odds ratio of 2.27 for interval cancer, compared with detected cancers (95% CI, 1.24-4.16).
Finally, interval cancers were more likely to be associated with polypectomy or a biopsy at index colonoscopy (84.3%), compared with patients who did not undergo these procedures (51.8%).
According to the authors, "the association of proximal tumor location, earlier stage at diagnosis, and survival advantage compared with detected colorectal cancers suggests that tumor biology may play an important role in the pathogenesis of these lesions."
On the other hand, a 2010 study showing a much higher interval cancer rate suggests that inexpert polypectomy may be at least partially to blame, since most colonoscopies in that study were performed by nongastroenterologists (Am. J. Gastroenterol. 2010;105:2588-96).
The National Cancer Institute, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, the Huntsman Cancer Foundation, the Utah Department of Health, and the University of Utah funded the study. Dr Samadder and a coauthor disclosed consulting for Cook Medical, Covidien, and Myriad Genetics.
Colonoscopy has been associated with reduced risk for colorectal cancer incidence and mortality.
Yet up to 14% of all individuals with CRC have their cancer diagnosed within 5 years of a colonoscopy. These interval cancers after colonoscopy may be due to biologic factors (such as rapid CRC development after normal examination), quality factors (such as missed and/or incompletely removed lesions), or both (lesions that are easy to miss, hard to remove, and associated with rapid CRC development).
 |
| Dr. Samir Gupta |
Interestingly, other research has found that there are lesions that are difficult to detect and completely remove with colonoscopy, such as sessile serrated adenomas and flat adenomas, which tend to be located in the proximal colon (Gastroenterology. 2013;144:74-80; Gastrointest. Endosc. 2012;75:1218-25).
Notably, serrated adenomas and proximal small and/or flat adenomas are more likely to contain features believed associated with increased risk for cancer progression, such as microsatellite instability and high grade dysplasia, respectively (Cancer Res. 2013;73:2863-72; Clin. Gastroenterol. Hepatol. 2012;10:1395-401).
Thus, it is plausible that these lesions may be responsible for a substantial proportion of interval cancers. Overall, based on the work by Samadder et al. and others, we are reminded that we need to develop interventions to optimize colonoscopy outcomes, and can postulate that future interventions may need to specifically focus on optimizing high quality detection and removal of lesions likely to share biologic and clinical characteristics with interval cancers, such as sessile serrated adenomas and flat adenomas.
Dr. Samir Gupta, MSCS, is with the San Diego Veterans Affairs Healthcare System, and is in the division of gastroenterology, department of internal medicine, at Moores Cancer Center,University of California San Diego. Hereported no financial conflicts of interest.
Colonoscopy has been associated with reduced risk for colorectal cancer incidence and mortality.
Yet up to 14% of all individuals with CRC have their cancer diagnosed within 5 years of a colonoscopy. These interval cancers after colonoscopy may be due to biologic factors (such as rapid CRC development after normal examination), quality factors (such as missed and/or incompletely removed lesions), or both (lesions that are easy to miss, hard to remove, and associated with rapid CRC development).
 |
| Dr. Samir Gupta |
Interestingly, other research has found that there are lesions that are difficult to detect and completely remove with colonoscopy, such as sessile serrated adenomas and flat adenomas, which tend to be located in the proximal colon (Gastroenterology. 2013;144:74-80; Gastrointest. Endosc. 2012;75:1218-25).
Notably, serrated adenomas and proximal small and/or flat adenomas are more likely to contain features believed associated with increased risk for cancer progression, such as microsatellite instability and high grade dysplasia, respectively (Cancer Res. 2013;73:2863-72; Clin. Gastroenterol. Hepatol. 2012;10:1395-401).
Thus, it is plausible that these lesions may be responsible for a substantial proportion of interval cancers. Overall, based on the work by Samadder et al. and others, we are reminded that we need to develop interventions to optimize colonoscopy outcomes, and can postulate that future interventions may need to specifically focus on optimizing high quality detection and removal of lesions likely to share biologic and clinical characteristics with interval cancers, such as sessile serrated adenomas and flat adenomas.
Dr. Samir Gupta, MSCS, is with the San Diego Veterans Affairs Healthcare System, and is in the division of gastroenterology, department of internal medicine, at Moores Cancer Center,University of California San Diego. Hereported no financial conflicts of interest.
Colonoscopy has been associated with reduced risk for colorectal cancer incidence and mortality.
Yet up to 14% of all individuals with CRC have their cancer diagnosed within 5 years of a colonoscopy. These interval cancers after colonoscopy may be due to biologic factors (such as rapid CRC development after normal examination), quality factors (such as missed and/or incompletely removed lesions), or both (lesions that are easy to miss, hard to remove, and associated with rapid CRC development).
 |
| Dr. Samir Gupta |
Interestingly, other research has found that there are lesions that are difficult to detect and completely remove with colonoscopy, such as sessile serrated adenomas and flat adenomas, which tend to be located in the proximal colon (Gastroenterology. 2013;144:74-80; Gastrointest. Endosc. 2012;75:1218-25).
Notably, serrated adenomas and proximal small and/or flat adenomas are more likely to contain features believed associated with increased risk for cancer progression, such as microsatellite instability and high grade dysplasia, respectively (Cancer Res. 2013;73:2863-72; Clin. Gastroenterol. Hepatol. 2012;10:1395-401).
Thus, it is plausible that these lesions may be responsible for a substantial proportion of interval cancers. Overall, based on the work by Samadder et al. and others, we are reminded that we need to develop interventions to optimize colonoscopy outcomes, and can postulate that future interventions may need to specifically focus on optimizing high quality detection and removal of lesions likely to share biologic and clinical characteristics with interval cancers, such as sessile serrated adenomas and flat adenomas.
Dr. Samir Gupta, MSCS, is with the San Diego Veterans Affairs Healthcare System, and is in the division of gastroenterology, department of internal medicine, at Moores Cancer Center,University of California San Diego. Hereported no financial conflicts of interest.
As many as 6% of colorectal cancers are discovered between 6 and 60 months of colonoscopy – usually in the proximal colon, and usually in patients whose index colonoscopy revealed adenomas, according to Dr. N. Jewel Samadder and colleagues.
While the investigators speculated that "differences in the biology of interval versus detected cancers" might be to blame, "suboptimal management of precancerous lesions (such as incomplete polypectomy) likely also plays a role," they wrote. The report is in the April issue of Gastroenterology (doi:10.1053/j.gastro.2014.01.013).
Dr. Samadder of the University of Utah, Salt Lake City, and colleagues looked at data from 126,851 patients who underwent colonoscopies between 1995 and 2009. The two centers in this analysis serve more than 85% of the state’s population. Patients with a history of colorectal cancer before 1995 were excluded, the researchers wrote.
Overall, 2,659 patients were diagnosed with colorectal cancer during the study period, mostly at initial, index colonoscopy – the investigators referred to these as "detected" cancers.
However, 159 of these cases (6%) were diagnosed between 6 and 60 months of the index colonoscopy, and were therefore classified as "interval" cancers.
Interval cancer patients had a mean age of 67 years at index colonoscopy (range, 34-92 years), identical to that of detected cancer patients; both cohorts were split evenly between men and women.
The authors found that compared with detected cancers, the interval cancers were much more commonly discovered in the proximal colon – indeed, proximal cancers made up 55% of the cohort, with the remainder split evenly between the distal colon and the recto-rectosigmoid junction.
In comparison, only 39% of detected cancers were found in the proximal colon (P less than .001).
Patients with an adenoma or villous adenoma discovered at index colonoscopy were significantly more likely to develop interval cancer, compared with patients whose cancers were detected earlier (for adenoma, the odds ratio was 2.96, 95% confidence interval = 2.0-4.28; for villous adenoma, OR = 2.04, 95% CI = 1.34-3.11).
A family history also conferred an odds ratio of 2.27 for interval cancer, compared with detected cancers (95% CI, 1.24-4.16).
Finally, interval cancers were more likely to be associated with polypectomy or a biopsy at index colonoscopy (84.3%), compared with patients who did not undergo these procedures (51.8%).
According to the authors, "the association of proximal tumor location, earlier stage at diagnosis, and survival advantage compared with detected colorectal cancers suggests that tumor biology may play an important role in the pathogenesis of these lesions."
On the other hand, a 2010 study showing a much higher interval cancer rate suggests that inexpert polypectomy may be at least partially to blame, since most colonoscopies in that study were performed by nongastroenterologists (Am. J. Gastroenterol. 2010;105:2588-96).
The National Cancer Institute, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, the Huntsman Cancer Foundation, the Utah Department of Health, and the University of Utah funded the study. Dr Samadder and a coauthor disclosed consulting for Cook Medical, Covidien, and Myriad Genetics.
As many as 6% of colorectal cancers are discovered between 6 and 60 months of colonoscopy – usually in the proximal colon, and usually in patients whose index colonoscopy revealed adenomas, according to Dr. N. Jewel Samadder and colleagues.
While the investigators speculated that "differences in the biology of interval versus detected cancers" might be to blame, "suboptimal management of precancerous lesions (such as incomplete polypectomy) likely also plays a role," they wrote. The report is in the April issue of Gastroenterology (doi:10.1053/j.gastro.2014.01.013).
Dr. Samadder of the University of Utah, Salt Lake City, and colleagues looked at data from 126,851 patients who underwent colonoscopies between 1995 and 2009. The two centers in this analysis serve more than 85% of the state’s population. Patients with a history of colorectal cancer before 1995 were excluded, the researchers wrote.
Overall, 2,659 patients were diagnosed with colorectal cancer during the study period, mostly at initial, index colonoscopy – the investigators referred to these as "detected" cancers.
However, 159 of these cases (6%) were diagnosed between 6 and 60 months of the index colonoscopy, and were therefore classified as "interval" cancers.
Interval cancer patients had a mean age of 67 years at index colonoscopy (range, 34-92 years), identical to that of detected cancer patients; both cohorts were split evenly between men and women.
The authors found that compared with detected cancers, the interval cancers were much more commonly discovered in the proximal colon – indeed, proximal cancers made up 55% of the cohort, with the remainder split evenly between the distal colon and the recto-rectosigmoid junction.
In comparison, only 39% of detected cancers were found in the proximal colon (P less than .001).
Patients with an adenoma or villous adenoma discovered at index colonoscopy were significantly more likely to develop interval cancer, compared with patients whose cancers were detected earlier (for adenoma, the odds ratio was 2.96, 95% confidence interval = 2.0-4.28; for villous adenoma, OR = 2.04, 95% CI = 1.34-3.11).
A family history also conferred an odds ratio of 2.27 for interval cancer, compared with detected cancers (95% CI, 1.24-4.16).
Finally, interval cancers were more likely to be associated with polypectomy or a biopsy at index colonoscopy (84.3%), compared with patients who did not undergo these procedures (51.8%).
According to the authors, "the association of proximal tumor location, earlier stage at diagnosis, and survival advantage compared with detected colorectal cancers suggests that tumor biology may play an important role in the pathogenesis of these lesions."
On the other hand, a 2010 study showing a much higher interval cancer rate suggests that inexpert polypectomy may be at least partially to blame, since most colonoscopies in that study were performed by nongastroenterologists (Am. J. Gastroenterol. 2010;105:2588-96).
The National Cancer Institute, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, the Huntsman Cancer Foundation, the Utah Department of Health, and the University of Utah funded the study. Dr Samadder and a coauthor disclosed consulting for Cook Medical, Covidien, and Myriad Genetics.
FROM GASTROENTEROLOGY
Major finding: Up to 6% of colorectal cancers may not be detected until within 6-60 months of an index colonoscopy.
Data source: A population-based cohort study of 126,851 patients in Utah.
Disclosures: The National Cancer Institute, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, the Huntsman Cancer Foundation, the Utah Department of Health, and the University of Utah funded the study. Dr Samadder and a coauthor disclosed consulting for Cook Medical, Covidien, and Myriad Genetics.
Linaclotide effective even in severe IBS
Linaclotide is safe and effective, even in severe cases of irritable bowel syndrome with constipation, according to a post-hoc analysis of phase III trial data.
Indeed, more acute patients "responded ... just as well as, if not better than, the intent-to-treat population, which included patients with milder abdominal symptoms," wrote Dr. Satish S. C. Rao and colleagues in the April issue of Clinical Gastroenterology and Hepatology (doi:10.1053/j.gastro.2014.01.0130).
According to Dr. Rao of Georgia Regents University, Augusta, although the guanylate cyclase-C agonist is already Food and Drug Administration approved for irritable bowel syndrome–constipation (IBS-C) subtype in 2012, it has not been explicitly tested in patients with more severe disease.
In the current study, Dr. Rao and his fellow investigators looked specifically at several symptoms common to this population: pain, cramping, bloating, fullness, and discomfort.
Patients who ranked at least one of these as being greater than or equal to 7 at baseline were scored as severe, where 0 represents no symptoms and 10 represents the worst possible symptoms.
Overall, out of a total intent-to-treat population of 1,602 patients, 376 reported severe pain, 507 reported severe discomfort, 702 reported severe bloating, 708 reported severe fullness, and 359 reported severe cramping.
Given the overlap between the pain/cramping and bloating/fullness cohorts, the investigators organized the severe IBS group into four subgroups: patients reporting severe pain as their primary complaint (including cramping), severe bloating (including fullness), severe discomfort, and those patients who reported severe symptoms in all three realms.
Demographics were similar between each subgroup and in the overall study population, "although the subpopulations tended to be younger, more female, and more non-white than the intent-to-treat population," the investigators wrote.
Patients were randomized to receive placebo or linaclotide 290 mcg once daily; primary endpoints for this study were assessed at 12 weeks.
The investigators found that, among all severe IBS patients in all subgroups, the mean changes from baseline were significantly greater for linaclotide-treated patients compared with placebo patients for pain, discomfort, bloating, fullness, and cramping (P less than .0001 for all).
Looking at the whole severe IBS cohort, the authors found that 59%-61% of linaclotide patients reported adequate relief of IBS symptoms at week 12, compared with 28%-32% of placebo patients (P less than .0001).
Of those with severe IBS, 70%-77% of linaclotide-treated patients reported being "moderately, quite, or very satisfied" with treatment at week 12, but only 41%-43% of placebo-treated patients said the same (P less than .0001).
Finally, looking specifically at severe versus more mild IBS cases, the investigators found that, overall, the severe IBS cohort reported significantly greater improvements with the drug, compared with their less severe, drug-treated counterparts in the intent-to-treat population.
The most common side effect – diarrhea – occurred in almost one-fifth of linaclotide-treated patients with severe IBS, versus an occurrence rate of 1.6%-2.1% among placebo-treated patients.
In addition to the finding that linaclotide is effective in severe IBS-C, the investigators also wrote that their finding of severe bloating and fullness in many of these patients should give clinicians pause.
"Pain or discomfort is considered a clinical hallmark of IBS," they wrote, but "the presence of bloating and fullness in patients with IBS-C may warrant greater attention in clinical practice as well as in clinical trial design."
Several of the investigators are employees of Ironwood Pharmaceuticals, maker of linaclotide. Additionally, Dr. Rao and another investigator disclosed financial relationships with the pharmaceutical company, which also funded the study.
Irritable bowel syndrome with constipation is a heterogeneous disorder. Heterogeneity exists both with regard to the predominant symptom (constipation vs. pain vs. bloating) and the severity of those symptoms.
 |
|
The severity of IBS-C symptoms is unique to each patient and is influenced by multiple factors, including the duration, intensity, frequency, and unpredictability of symptoms; quality of life; fears and concerns about the disease state; coping skills; sex, ethnicity, and culture; and the presence of coexisting functional gastrointestinal disorders, psychological disorders, and medical disorders.
Understanding the level of severity in an IBS-C patient is important as it may influence both diagnostic tests and treatment options. However, despite the prevalence of IBS and its impact on the health care system, few research studies have attempted to categorize IBS-C patients by level of severity to determine their responsiveness to therapy.
In the study by Dr. Rao and his colleagues, a post-hoc analysis of two previously published phase III clinical trials of linaclotide in patients with IBS-C was performed to assess the prevalence of severe abdominal symptoms and the efficacy of linaclotide on these severe symptoms. Abdominal symptoms were evaluated prospectively in each trial using an 11-point numerical rating scale (0 = none; 10 = very severe).
In this post-hoc analysis, the effects of linaclotide were measured for patients with scores greater than 7.0 at baseline. Interestingly, 44% of patients (n = 1,602) reported severe bloating and abdominal fullness. Abdominal discomfort (32%), pain (23%), and cramping (22%) were less common. Not surprisingly, there was considerable overlap with these symptoms, and 21% reported that three symptoms were severe (bloating, pain, and discomfort). Linaclotide improved all five severe symptoms to a greater degree than did placebo, but, more importantly, improved symptoms to a greater degree in these severe patients compared with the study population as a whole.
This study sets the stage for future prospective therapeutic trials. Regardless of IBS subtype (constipation vs. diarrhea vs. mixed) we need to better understand the severity of symptoms of IBS patients enrolled in research studies.
It is likely that critical information is being lost by grouping all IBS patients together with regard to the efficacy of a particular therapeutic agent. This could explain the low therapeutic gain seen in some IBS drug trials. It is likely that differential effects will be observed based on symptom severity. Thus, a patient with moderate to severe IBS symptoms (and thus a reduced quality of life and greater impact on the health care system) may respond better to a particular medication than someone with very mild symptoms (usually with a better quality of life and less impact on the health care system).
One could envision a future practice strategy based on symptom severity with less risky/less expensive treatments (education, reassurance, diet, and exercise) recommended to those with mild symptoms and more aggressive, multimodal therapy (behavioral therapy, psychological therapy, and overlapping medications for visceral/somatic/psychological distress) recommended to those with more severe symptoms. This study provides a framework for future IBS studies to better categorize symptom severity.
Dr. Brian Lacy is section chief of gastroenterology and hepatology and associate professor of medicine at the Geisel School of Medicine, Dartmouth University, Lebanon, N.H. He has received research funding from the National Institutes of Health and is on the advisory boards of Takeda, Prometheus, and Ironwood.
Irritable bowel syndrome with constipation is a heterogeneous disorder. Heterogeneity exists both with regard to the predominant symptom (constipation vs. pain vs. bloating) and the severity of those symptoms.
 |
|
The severity of IBS-C symptoms is unique to each patient and is influenced by multiple factors, including the duration, intensity, frequency, and unpredictability of symptoms; quality of life; fears and concerns about the disease state; coping skills; sex, ethnicity, and culture; and the presence of coexisting functional gastrointestinal disorders, psychological disorders, and medical disorders.
Understanding the level of severity in an IBS-C patient is important as it may influence both diagnostic tests and treatment options. However, despite the prevalence of IBS and its impact on the health care system, few research studies have attempted to categorize IBS-C patients by level of severity to determine their responsiveness to therapy.
In the study by Dr. Rao and his colleagues, a post-hoc analysis of two previously published phase III clinical trials of linaclotide in patients with IBS-C was performed to assess the prevalence of severe abdominal symptoms and the efficacy of linaclotide on these severe symptoms. Abdominal symptoms were evaluated prospectively in each trial using an 11-point numerical rating scale (0 = none; 10 = very severe).
In this post-hoc analysis, the effects of linaclotide were measured for patients with scores greater than 7.0 at baseline. Interestingly, 44% of patients (n = 1,602) reported severe bloating and abdominal fullness. Abdominal discomfort (32%), pain (23%), and cramping (22%) were less common. Not surprisingly, there was considerable overlap with these symptoms, and 21% reported that three symptoms were severe (bloating, pain, and discomfort). Linaclotide improved all five severe symptoms to a greater degree than did placebo, but, more importantly, improved symptoms to a greater degree in these severe patients compared with the study population as a whole.
This study sets the stage for future prospective therapeutic trials. Regardless of IBS subtype (constipation vs. diarrhea vs. mixed) we need to better understand the severity of symptoms of IBS patients enrolled in research studies.
It is likely that critical information is being lost by grouping all IBS patients together with regard to the efficacy of a particular therapeutic agent. This could explain the low therapeutic gain seen in some IBS drug trials. It is likely that differential effects will be observed based on symptom severity. Thus, a patient with moderate to severe IBS symptoms (and thus a reduced quality of life and greater impact on the health care system) may respond better to a particular medication than someone with very mild symptoms (usually with a better quality of life and less impact on the health care system).
One could envision a future practice strategy based on symptom severity with less risky/less expensive treatments (education, reassurance, diet, and exercise) recommended to those with mild symptoms and more aggressive, multimodal therapy (behavioral therapy, psychological therapy, and overlapping medications for visceral/somatic/psychological distress) recommended to those with more severe symptoms. This study provides a framework for future IBS studies to better categorize symptom severity.
Dr. Brian Lacy is section chief of gastroenterology and hepatology and associate professor of medicine at the Geisel School of Medicine, Dartmouth University, Lebanon, N.H. He has received research funding from the National Institutes of Health and is on the advisory boards of Takeda, Prometheus, and Ironwood.
Irritable bowel syndrome with constipation is a heterogeneous disorder. Heterogeneity exists both with regard to the predominant symptom (constipation vs. pain vs. bloating) and the severity of those symptoms.
 |
|
The severity of IBS-C symptoms is unique to each patient and is influenced by multiple factors, including the duration, intensity, frequency, and unpredictability of symptoms; quality of life; fears and concerns about the disease state; coping skills; sex, ethnicity, and culture; and the presence of coexisting functional gastrointestinal disorders, psychological disorders, and medical disorders.
Understanding the level of severity in an IBS-C patient is important as it may influence both diagnostic tests and treatment options. However, despite the prevalence of IBS and its impact on the health care system, few research studies have attempted to categorize IBS-C patients by level of severity to determine their responsiveness to therapy.
In the study by Dr. Rao and his colleagues, a post-hoc analysis of two previously published phase III clinical trials of linaclotide in patients with IBS-C was performed to assess the prevalence of severe abdominal symptoms and the efficacy of linaclotide on these severe symptoms. Abdominal symptoms were evaluated prospectively in each trial using an 11-point numerical rating scale (0 = none; 10 = very severe).
In this post-hoc analysis, the effects of linaclotide were measured for patients with scores greater than 7.0 at baseline. Interestingly, 44% of patients (n = 1,602) reported severe bloating and abdominal fullness. Abdominal discomfort (32%), pain (23%), and cramping (22%) were less common. Not surprisingly, there was considerable overlap with these symptoms, and 21% reported that three symptoms were severe (bloating, pain, and discomfort). Linaclotide improved all five severe symptoms to a greater degree than did placebo, but, more importantly, improved symptoms to a greater degree in these severe patients compared with the study population as a whole.
This study sets the stage for future prospective therapeutic trials. Regardless of IBS subtype (constipation vs. diarrhea vs. mixed) we need to better understand the severity of symptoms of IBS patients enrolled in research studies.
It is likely that critical information is being lost by grouping all IBS patients together with regard to the efficacy of a particular therapeutic agent. This could explain the low therapeutic gain seen in some IBS drug trials. It is likely that differential effects will be observed based on symptom severity. Thus, a patient with moderate to severe IBS symptoms (and thus a reduced quality of life and greater impact on the health care system) may respond better to a particular medication than someone with very mild symptoms (usually with a better quality of life and less impact on the health care system).
One could envision a future practice strategy based on symptom severity with less risky/less expensive treatments (education, reassurance, diet, and exercise) recommended to those with mild symptoms and more aggressive, multimodal therapy (behavioral therapy, psychological therapy, and overlapping medications for visceral/somatic/psychological distress) recommended to those with more severe symptoms. This study provides a framework for future IBS studies to better categorize symptom severity.
Dr. Brian Lacy is section chief of gastroenterology and hepatology and associate professor of medicine at the Geisel School of Medicine, Dartmouth University, Lebanon, N.H. He has received research funding from the National Institutes of Health and is on the advisory boards of Takeda, Prometheus, and Ironwood.
Linaclotide is safe and effective, even in severe cases of irritable bowel syndrome with constipation, according to a post-hoc analysis of phase III trial data.
Indeed, more acute patients "responded ... just as well as, if not better than, the intent-to-treat population, which included patients with milder abdominal symptoms," wrote Dr. Satish S. C. Rao and colleagues in the April issue of Clinical Gastroenterology and Hepatology (doi:10.1053/j.gastro.2014.01.0130).
According to Dr. Rao of Georgia Regents University, Augusta, although the guanylate cyclase-C agonist is already Food and Drug Administration approved for irritable bowel syndrome–constipation (IBS-C) subtype in 2012, it has not been explicitly tested in patients with more severe disease.
In the current study, Dr. Rao and his fellow investigators looked specifically at several symptoms common to this population: pain, cramping, bloating, fullness, and discomfort.
Patients who ranked at least one of these as being greater than or equal to 7 at baseline were scored as severe, where 0 represents no symptoms and 10 represents the worst possible symptoms.
Overall, out of a total intent-to-treat population of 1,602 patients, 376 reported severe pain, 507 reported severe discomfort, 702 reported severe bloating, 708 reported severe fullness, and 359 reported severe cramping.
Given the overlap between the pain/cramping and bloating/fullness cohorts, the investigators organized the severe IBS group into four subgroups: patients reporting severe pain as their primary complaint (including cramping), severe bloating (including fullness), severe discomfort, and those patients who reported severe symptoms in all three realms.
Demographics were similar between each subgroup and in the overall study population, "although the subpopulations tended to be younger, more female, and more non-white than the intent-to-treat population," the investigators wrote.
Patients were randomized to receive placebo or linaclotide 290 mcg once daily; primary endpoints for this study were assessed at 12 weeks.
The investigators found that, among all severe IBS patients in all subgroups, the mean changes from baseline were significantly greater for linaclotide-treated patients compared with placebo patients for pain, discomfort, bloating, fullness, and cramping (P less than .0001 for all).
Looking at the whole severe IBS cohort, the authors found that 59%-61% of linaclotide patients reported adequate relief of IBS symptoms at week 12, compared with 28%-32% of placebo patients (P less than .0001).
Of those with severe IBS, 70%-77% of linaclotide-treated patients reported being "moderately, quite, or very satisfied" with treatment at week 12, but only 41%-43% of placebo-treated patients said the same (P less than .0001).
Finally, looking specifically at severe versus more mild IBS cases, the investigators found that, overall, the severe IBS cohort reported significantly greater improvements with the drug, compared with their less severe, drug-treated counterparts in the intent-to-treat population.
The most common side effect – diarrhea – occurred in almost one-fifth of linaclotide-treated patients with severe IBS, versus an occurrence rate of 1.6%-2.1% among placebo-treated patients.
In addition to the finding that linaclotide is effective in severe IBS-C, the investigators also wrote that their finding of severe bloating and fullness in many of these patients should give clinicians pause.
"Pain or discomfort is considered a clinical hallmark of IBS," they wrote, but "the presence of bloating and fullness in patients with IBS-C may warrant greater attention in clinical practice as well as in clinical trial design."
Several of the investigators are employees of Ironwood Pharmaceuticals, maker of linaclotide. Additionally, Dr. Rao and another investigator disclosed financial relationships with the pharmaceutical company, which also funded the study.
Linaclotide is safe and effective, even in severe cases of irritable bowel syndrome with constipation, according to a post-hoc analysis of phase III trial data.
Indeed, more acute patients "responded ... just as well as, if not better than, the intent-to-treat population, which included patients with milder abdominal symptoms," wrote Dr. Satish S. C. Rao and colleagues in the April issue of Clinical Gastroenterology and Hepatology (doi:10.1053/j.gastro.2014.01.0130).
According to Dr. Rao of Georgia Regents University, Augusta, although the guanylate cyclase-C agonist is already Food and Drug Administration approved for irritable bowel syndrome–constipation (IBS-C) subtype in 2012, it has not been explicitly tested in patients with more severe disease.
In the current study, Dr. Rao and his fellow investigators looked specifically at several symptoms common to this population: pain, cramping, bloating, fullness, and discomfort.
Patients who ranked at least one of these as being greater than or equal to 7 at baseline were scored as severe, where 0 represents no symptoms and 10 represents the worst possible symptoms.
Overall, out of a total intent-to-treat population of 1,602 patients, 376 reported severe pain, 507 reported severe discomfort, 702 reported severe bloating, 708 reported severe fullness, and 359 reported severe cramping.
Given the overlap between the pain/cramping and bloating/fullness cohorts, the investigators organized the severe IBS group into four subgroups: patients reporting severe pain as their primary complaint (including cramping), severe bloating (including fullness), severe discomfort, and those patients who reported severe symptoms in all three realms.
Demographics were similar between each subgroup and in the overall study population, "although the subpopulations tended to be younger, more female, and more non-white than the intent-to-treat population," the investigators wrote.
Patients were randomized to receive placebo or linaclotide 290 mcg once daily; primary endpoints for this study were assessed at 12 weeks.
The investigators found that, among all severe IBS patients in all subgroups, the mean changes from baseline were significantly greater for linaclotide-treated patients compared with placebo patients for pain, discomfort, bloating, fullness, and cramping (P less than .0001 for all).
Looking at the whole severe IBS cohort, the authors found that 59%-61% of linaclotide patients reported adequate relief of IBS symptoms at week 12, compared with 28%-32% of placebo patients (P less than .0001).
Of those with severe IBS, 70%-77% of linaclotide-treated patients reported being "moderately, quite, or very satisfied" with treatment at week 12, but only 41%-43% of placebo-treated patients said the same (P less than .0001).
Finally, looking specifically at severe versus more mild IBS cases, the investigators found that, overall, the severe IBS cohort reported significantly greater improvements with the drug, compared with their less severe, drug-treated counterparts in the intent-to-treat population.
The most common side effect – diarrhea – occurred in almost one-fifth of linaclotide-treated patients with severe IBS, versus an occurrence rate of 1.6%-2.1% among placebo-treated patients.
In addition to the finding that linaclotide is effective in severe IBS-C, the investigators also wrote that their finding of severe bloating and fullness in many of these patients should give clinicians pause.
"Pain or discomfort is considered a clinical hallmark of IBS," they wrote, but "the presence of bloating and fullness in patients with IBS-C may warrant greater attention in clinical practice as well as in clinical trial design."
Several of the investigators are employees of Ironwood Pharmaceuticals, maker of linaclotide. Additionally, Dr. Rao and another investigator disclosed financial relationships with the pharmaceutical company, which also funded the study.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Major finding: Up to 61% of linaclotide-treated patients with severe irritable bowel symptoms had adequate relief by 12 weeks, compared with 32% of placebo patients (P less than .0001).
Data source: A post hoc analysis of 1,602 patients in a phase III study.
Disclosures: Several investigators are employees of Ironwood Pharmaceuticals, maker of linaclotide. Additionally, Dr. Rao and another investigator disclosed financial relationships with the pharmaceutical company, which also funded the study.
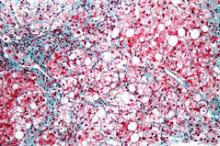
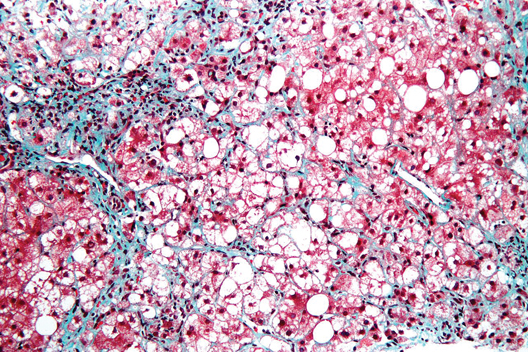
Liver histology crucial at Wilson disease diagnosis
Wilson disease patients without cirrhosis at diagnosis generally have good long-term prognoses, but the presence of cirrhosis on diagnosis of this rare condition necessitates transplant in up to 13% of cases and is the strongest predictor of mortality, according to findings from a retrospective analysis.
The report appears in the April issue of Clinical Gastroenterology and Hepatology (doi:10.1016/j.cgh.2013.09.025).
The finding "[reinforces] the histologic assessment of liver fibrosis to be performed in all patients suspected to have Wilson disease, regardless of whether they present with neurologic or hepatic symptoms," wrote Dr. Sandra Beinhardt of the Medical University of Vienna. She and her colleagues looked at 229 patients (48% of whom were male) who were diagnosed with Wilson disease in Austria between 1961 and 2013.
Courtesy: American Gastroenterological Association
"In patients with evaluable symptoms at diagnosis as well as reliable information on the time of the onset of symptoms" (90% of the total cohort), diagnosis of Wilson disease was established by 3 years after symptom onset in 75% of patients, the researchers noted.
Overall, 140 patients presented with predominantly hepatic symptoms; 61 with neurologic symptoms; and 23 were diagnosed by screening before any symptoms were noted.
In the remaining 5 patients, the presenting symptoms could not be determined.
Looking at long-term prognosis, the authors found that patients presenting with neurologic symptoms had a significantly longer mean follow-up period than did patients with hepatic symptoms at diagnosis (21.1 years versus 11.8 years, respectively; P less than .001).
"Nevertheless, 34% of patients predominantly presenting with neurologic symptoms at diagnosis already had developed cirrhosis," they wrote.
Overall, 30 patients received liver transplants between 1986 and 2013, because of fulminant hepatic failure in 11 patients, neurologic worsening in 1 patient, and decompensated liver cirrhosis in the remaining 18.
As for mortality, among the 17 patients who died during the study period, the researchers reported that most of the deaths could be attributed to Wilson disease. Seven patients died of liver failure, four died of complications resulting from the liver transplant.
One patient died of suicide, reportedly following severe depression stemming from the Wilson disease diagnosis.
That translated to an overall 20-year cumulative survival rate of 0.920 by Kaplan-Meier analysis. However, that figure dipped to 0.84 for patients with cirrhosis on diagnosis (compared with 0.97 for all others, P = .008).
Indeed, "By univariate logistic regression analysis, liver cirrhosis at diagnosis was the best predictor for the need of liver transplant (odds ratio, 0.07; 95% confidence interval, 0.016-0.307; P less than .001) as well as for death (OR, 6.8; 95% CI, 1.5-31.03; P = .013)," wrote the authors.
The cohort they studied represents nearly all cases of Wilson disease that could have been expected to occur in Austria during the study period, given an incidence of 30 cases per million people in that nation of 8 million inhabitants, they wrote.
And while the retrospective nature of their study limits analysis, because of the rarity of Wilson disease, "prospective studies with reasonably large sample sizes are almost impossible to perform," they noted.
Nevertheless, "the long-term prognosis in patients with Wilson disease surviving more than 10 years after diagnosis and treatment initiation is excellent," especially if the diagnosis is early, wrote Dr. Beinhardt and her colleagues.
The authors stated that they had no conflicts of interest.
Wilson disease patients without cirrhosis at diagnosis generally have good long-term prognoses, but the presence of cirrhosis on diagnosis of this rare condition necessitates transplant in up to 13% of cases and is the strongest predictor of mortality, according to findings from a retrospective analysis.
The report appears in the April issue of Clinical Gastroenterology and Hepatology (doi:10.1016/j.cgh.2013.09.025).
The finding "[reinforces] the histologic assessment of liver fibrosis to be performed in all patients suspected to have Wilson disease, regardless of whether they present with neurologic or hepatic symptoms," wrote Dr. Sandra Beinhardt of the Medical University of Vienna. She and her colleagues looked at 229 patients (48% of whom were male) who were diagnosed with Wilson disease in Austria between 1961 and 2013.
Courtesy: American Gastroenterological Association
"In patients with evaluable symptoms at diagnosis as well as reliable information on the time of the onset of symptoms" (90% of the total cohort), diagnosis of Wilson disease was established by 3 years after symptom onset in 75% of patients, the researchers noted.
Overall, 140 patients presented with predominantly hepatic symptoms; 61 with neurologic symptoms; and 23 were diagnosed by screening before any symptoms were noted.
In the remaining 5 patients, the presenting symptoms could not be determined.
Looking at long-term prognosis, the authors found that patients presenting with neurologic symptoms had a significantly longer mean follow-up period than did patients with hepatic symptoms at diagnosis (21.1 years versus 11.8 years, respectively; P less than .001).
"Nevertheless, 34% of patients predominantly presenting with neurologic symptoms at diagnosis already had developed cirrhosis," they wrote.
Overall, 30 patients received liver transplants between 1986 and 2013, because of fulminant hepatic failure in 11 patients, neurologic worsening in 1 patient, and decompensated liver cirrhosis in the remaining 18.
As for mortality, among the 17 patients who died during the study period, the researchers reported that most of the deaths could be attributed to Wilson disease. Seven patients died of liver failure, four died of complications resulting from the liver transplant.
One patient died of suicide, reportedly following severe depression stemming from the Wilson disease diagnosis.
That translated to an overall 20-year cumulative survival rate of 0.920 by Kaplan-Meier analysis. However, that figure dipped to 0.84 for patients with cirrhosis on diagnosis (compared with 0.97 for all others, P = .008).
Indeed, "By univariate logistic regression analysis, liver cirrhosis at diagnosis was the best predictor for the need of liver transplant (odds ratio, 0.07; 95% confidence interval, 0.016-0.307; P less than .001) as well as for death (OR, 6.8; 95% CI, 1.5-31.03; P = .013)," wrote the authors.
The cohort they studied represents nearly all cases of Wilson disease that could have been expected to occur in Austria during the study period, given an incidence of 30 cases per million people in that nation of 8 million inhabitants, they wrote.
And while the retrospective nature of their study limits analysis, because of the rarity of Wilson disease, "prospective studies with reasonably large sample sizes are almost impossible to perform," they noted.
Nevertheless, "the long-term prognosis in patients with Wilson disease surviving more than 10 years after diagnosis and treatment initiation is excellent," especially if the diagnosis is early, wrote Dr. Beinhardt and her colleagues.
The authors stated that they had no conflicts of interest.
Wilson disease patients without cirrhosis at diagnosis generally have good long-term prognoses, but the presence of cirrhosis on diagnosis of this rare condition necessitates transplant in up to 13% of cases and is the strongest predictor of mortality, according to findings from a retrospective analysis.
The report appears in the April issue of Clinical Gastroenterology and Hepatology (doi:10.1016/j.cgh.2013.09.025).
The finding "[reinforces] the histologic assessment of liver fibrosis to be performed in all patients suspected to have Wilson disease, regardless of whether they present with neurologic or hepatic symptoms," wrote Dr. Sandra Beinhardt of the Medical University of Vienna. She and her colleagues looked at 229 patients (48% of whom were male) who were diagnosed with Wilson disease in Austria between 1961 and 2013.
Courtesy: American Gastroenterological Association
"In patients with evaluable symptoms at diagnosis as well as reliable information on the time of the onset of symptoms" (90% of the total cohort), diagnosis of Wilson disease was established by 3 years after symptom onset in 75% of patients, the researchers noted.
Overall, 140 patients presented with predominantly hepatic symptoms; 61 with neurologic symptoms; and 23 were diagnosed by screening before any symptoms were noted.
In the remaining 5 patients, the presenting symptoms could not be determined.
Looking at long-term prognosis, the authors found that patients presenting with neurologic symptoms had a significantly longer mean follow-up period than did patients with hepatic symptoms at diagnosis (21.1 years versus 11.8 years, respectively; P less than .001).
"Nevertheless, 34% of patients predominantly presenting with neurologic symptoms at diagnosis already had developed cirrhosis," they wrote.
Overall, 30 patients received liver transplants between 1986 and 2013, because of fulminant hepatic failure in 11 patients, neurologic worsening in 1 patient, and decompensated liver cirrhosis in the remaining 18.
As for mortality, among the 17 patients who died during the study period, the researchers reported that most of the deaths could be attributed to Wilson disease. Seven patients died of liver failure, four died of complications resulting from the liver transplant.
One patient died of suicide, reportedly following severe depression stemming from the Wilson disease diagnosis.
That translated to an overall 20-year cumulative survival rate of 0.920 by Kaplan-Meier analysis. However, that figure dipped to 0.84 for patients with cirrhosis on diagnosis (compared with 0.97 for all others, P = .008).
Indeed, "By univariate logistic regression analysis, liver cirrhosis at diagnosis was the best predictor for the need of liver transplant (odds ratio, 0.07; 95% confidence interval, 0.016-0.307; P less than .001) as well as for death (OR, 6.8; 95% CI, 1.5-31.03; P = .013)," wrote the authors.
The cohort they studied represents nearly all cases of Wilson disease that could have been expected to occur in Austria during the study period, given an incidence of 30 cases per million people in that nation of 8 million inhabitants, they wrote.
And while the retrospective nature of their study limits analysis, because of the rarity of Wilson disease, "prospective studies with reasonably large sample sizes are almost impossible to perform," they noted.
Nevertheless, "the long-term prognosis in patients with Wilson disease surviving more than 10 years after diagnosis and treatment initiation is excellent," especially if the diagnosis is early, wrote Dr. Beinhardt and her colleagues.
The authors stated that they had no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Major finding: In Wilson disease patients, liver cirrhosis at diagnosis was the best predictor for liver transplant as well as for death (OR, 6.8).
Data source: A retrospective analysis of a well-characterized Austrian cohort of 229 patients with Wilson disease.
Disclosures: The authors stated that they had no conflicts of interest.
Chronic pancreatitis life expectancy dramatically decreased
Patients with chronic pancreatitis have a life expectancy that is roughly 8 years shorter than that of the general population.
Indeed, the "beyond a doubt" finding of a mortality rate up to five times higher in this cohort illustrates "the great impact the presence of this disease has on the accompanying complications," wrote Dr. Ulrich Christian Bang. The report appears in the April issue of Gastroenterology (doi:10.1053/j.gastro.2013.12.033).
Dr. Bang, of Copenhagen University Hospital Hvidovre, looked at 11,972 patients (33.5% were women) with a primary diagnosis of chronic pancreatitis between 1995 and 2010, and 119,720 age- and sex-matched controls. Median age was 54 years.
Alcoholic pancreatitis was present in just over half (52.7%) of chronic pancreatitis cases.
The primary endpoint was mortality, but the authors also assessed all inpatient and outpatient diagnoses accumulated during the study period.
Overall, the age at death was significantly lower for pancreatitis patients compared with controls (63.7 years versus 72.1 years for controls; P less than .0001).
Indeed, per 1,000 patient-years, mortality rates were 77.4 among cases (95% confidence interval, 75.4-79.5) and 16.9 among controls (95% CI, 16.7-17.2) translating to an adjusted hazard ratio of 5.0 (95% CI, 4.8-5.2).
Although mortality rates predictably increased with age for both cases and controls, "the adjusted relative risks of death were significantly higher for the younger chronic pancreatitis cases than among older patients (P less than .0001)," wrote Dr. Bang and colleagues.
Fatal diseases of the alimentary tract were the most common cause of death in cases (10.6%), followed closely by cancer (10.2%) and circulatory system diseases (5.5%).
In comparison, 0.4% of controls had mortality associated with gastrointestinal disease (adjusted HR for cases, 26.1); 3.3% developed a fatal malignancy (HR for cases, 1.4), and 3.2% had mortality caused by diseases of the circulatory system (HR for cases, 1.9).
Looking generally at all comorbidities, the researchers found that the proportion of patients with any morbidity excluding chronic pancreatitis was significantly higher among cases (78%) compared with controls (38%) (P less than .0001). That included the presence of cerebrovascular disease (adjusted HR for cases, 1.3; 94% CI, 1.2-1.4), chronic pulmonary disease (adjusted HR for cases, 1.9; 95% CI, 1.8-2.1), and chronic kidney disease (adjusted HR for cases, 1.7; 95% CI, 1.5-1.9) as well as diabetes and ulcer disease.
Only the incidence of myocardial infarction was not elevated among cases, compared with controls; in fact, after adjustment for socioeconomic status, chronic pulmonary disease, and diabetes, cases had a trend toward a slightly lower risk, at 0.9 (95% CI, 0.8-1.0).
According to the authors, one of the weaknesses of their study was that they were unable to control for lifestyle factors such as smoking and drinking, which may especially contribute to the higher mortality among younger patients with chronic pancreatitis.
"Older age groups may be able to compensate for more advanced chronic pancreatitis accompanied by fat malabsorption and secondary diabetes by adopting a healthier lifestyle including tobacco and alcohol cessation as well as better compliance with routine follow-up evaluation and medication," they added.
Copenhagen University Hospital Hvidovre funded the study. The authors stated that they had no conflicts of interest.
Patients with chronic pancreatitis have a life expectancy that is roughly 8 years shorter than that of the general population.
Indeed, the "beyond a doubt" finding of a mortality rate up to five times higher in this cohort illustrates "the great impact the presence of this disease has on the accompanying complications," wrote Dr. Ulrich Christian Bang. The report appears in the April issue of Gastroenterology (doi:10.1053/j.gastro.2013.12.033).
Dr. Bang, of Copenhagen University Hospital Hvidovre, looked at 11,972 patients (33.5% were women) with a primary diagnosis of chronic pancreatitis between 1995 and 2010, and 119,720 age- and sex-matched controls. Median age was 54 years.
Alcoholic pancreatitis was present in just over half (52.7%) of chronic pancreatitis cases.
The primary endpoint was mortality, but the authors also assessed all inpatient and outpatient diagnoses accumulated during the study period.
Overall, the age at death was significantly lower for pancreatitis patients compared with controls (63.7 years versus 72.1 years for controls; P less than .0001).
Indeed, per 1,000 patient-years, mortality rates were 77.4 among cases (95% confidence interval, 75.4-79.5) and 16.9 among controls (95% CI, 16.7-17.2) translating to an adjusted hazard ratio of 5.0 (95% CI, 4.8-5.2).
Although mortality rates predictably increased with age for both cases and controls, "the adjusted relative risks of death were significantly higher for the younger chronic pancreatitis cases than among older patients (P less than .0001)," wrote Dr. Bang and colleagues.
Fatal diseases of the alimentary tract were the most common cause of death in cases (10.6%), followed closely by cancer (10.2%) and circulatory system diseases (5.5%).
In comparison, 0.4% of controls had mortality associated with gastrointestinal disease (adjusted HR for cases, 26.1); 3.3% developed a fatal malignancy (HR for cases, 1.4), and 3.2% had mortality caused by diseases of the circulatory system (HR for cases, 1.9).
Looking generally at all comorbidities, the researchers found that the proportion of patients with any morbidity excluding chronic pancreatitis was significantly higher among cases (78%) compared with controls (38%) (P less than .0001). That included the presence of cerebrovascular disease (adjusted HR for cases, 1.3; 94% CI, 1.2-1.4), chronic pulmonary disease (adjusted HR for cases, 1.9; 95% CI, 1.8-2.1), and chronic kidney disease (adjusted HR for cases, 1.7; 95% CI, 1.5-1.9) as well as diabetes and ulcer disease.
Only the incidence of myocardial infarction was not elevated among cases, compared with controls; in fact, after adjustment for socioeconomic status, chronic pulmonary disease, and diabetes, cases had a trend toward a slightly lower risk, at 0.9 (95% CI, 0.8-1.0).
According to the authors, one of the weaknesses of their study was that they were unable to control for lifestyle factors such as smoking and drinking, which may especially contribute to the higher mortality among younger patients with chronic pancreatitis.
"Older age groups may be able to compensate for more advanced chronic pancreatitis accompanied by fat malabsorption and secondary diabetes by adopting a healthier lifestyle including tobacco and alcohol cessation as well as better compliance with routine follow-up evaluation and medication," they added.
Copenhagen University Hospital Hvidovre funded the study. The authors stated that they had no conflicts of interest.
Patients with chronic pancreatitis have a life expectancy that is roughly 8 years shorter than that of the general population.
Indeed, the "beyond a doubt" finding of a mortality rate up to five times higher in this cohort illustrates "the great impact the presence of this disease has on the accompanying complications," wrote Dr. Ulrich Christian Bang. The report appears in the April issue of Gastroenterology (doi:10.1053/j.gastro.2013.12.033).
Dr. Bang, of Copenhagen University Hospital Hvidovre, looked at 11,972 patients (33.5% were women) with a primary diagnosis of chronic pancreatitis between 1995 and 2010, and 119,720 age- and sex-matched controls. Median age was 54 years.
Alcoholic pancreatitis was present in just over half (52.7%) of chronic pancreatitis cases.
The primary endpoint was mortality, but the authors also assessed all inpatient and outpatient diagnoses accumulated during the study period.
Overall, the age at death was significantly lower for pancreatitis patients compared with controls (63.7 years versus 72.1 years for controls; P less than .0001).
Indeed, per 1,000 patient-years, mortality rates were 77.4 among cases (95% confidence interval, 75.4-79.5) and 16.9 among controls (95% CI, 16.7-17.2) translating to an adjusted hazard ratio of 5.0 (95% CI, 4.8-5.2).
Although mortality rates predictably increased with age for both cases and controls, "the adjusted relative risks of death were significantly higher for the younger chronic pancreatitis cases than among older patients (P less than .0001)," wrote Dr. Bang and colleagues.
Fatal diseases of the alimentary tract were the most common cause of death in cases (10.6%), followed closely by cancer (10.2%) and circulatory system diseases (5.5%).
In comparison, 0.4% of controls had mortality associated with gastrointestinal disease (adjusted HR for cases, 26.1); 3.3% developed a fatal malignancy (HR for cases, 1.4), and 3.2% had mortality caused by diseases of the circulatory system (HR for cases, 1.9).
Looking generally at all comorbidities, the researchers found that the proportion of patients with any morbidity excluding chronic pancreatitis was significantly higher among cases (78%) compared with controls (38%) (P less than .0001). That included the presence of cerebrovascular disease (adjusted HR for cases, 1.3; 94% CI, 1.2-1.4), chronic pulmonary disease (adjusted HR for cases, 1.9; 95% CI, 1.8-2.1), and chronic kidney disease (adjusted HR for cases, 1.7; 95% CI, 1.5-1.9) as well as diabetes and ulcer disease.
Only the incidence of myocardial infarction was not elevated among cases, compared with controls; in fact, after adjustment for socioeconomic status, chronic pulmonary disease, and diabetes, cases had a trend toward a slightly lower risk, at 0.9 (95% CI, 0.8-1.0).
According to the authors, one of the weaknesses of their study was that they were unable to control for lifestyle factors such as smoking and drinking, which may especially contribute to the higher mortality among younger patients with chronic pancreatitis.
"Older age groups may be able to compensate for more advanced chronic pancreatitis accompanied by fat malabsorption and secondary diabetes by adopting a healthier lifestyle including tobacco and alcohol cessation as well as better compliance with routine follow-up evaluation and medication," they added.
Copenhagen University Hospital Hvidovre funded the study. The authors stated that they had no conflicts of interest.
FROM GASTROENTEROLOGY
Major finding: The age at death was significantly lower for patients with chronic pancreatitis compared with controls (63.7 years versus 72.1 years, respectively; P less than .0001).
Data source: A matched, retrospective cohort study of 11,972 patients and 119,720 controls in the Danish National Patient Register.
Disclosures: Copenhagen University Hospital Hvidovre funded the study. The authors stated that they had no conflicts of interest.
In situ melanoma high risk for subsequent diagnosis
In situ melanoma patients have a significant, substantially elevated risk for subsequent invasive or in situ melanoma – nearly as high as the risk for patients presenting with more invasive disease.
The finding means that "education and continued surveillance are paramount not only for persons with an invasive melanoma but also for persons with an in situ melanoma," wrote Danny R. Youlden in JAMA Dermatology, published online March 19 (doi:10.1001/jamadermatol.2013.9852).
Mr. Youlden, a biostatistician with Cancer Council Queensland, in Brisbane, Australia, and his colleagues looked at 39,668 cases of first primary invasive melanoma, and 22,845 patients with first primary in situ melanoma, diagnosed between 1982 and 2005.
Patients were followed for a median of more than 9 years, during which time there were 5,358 subsequent primary invasive melanomas; 3,520 (66%) of these occurred in patients with previous invasive melanomas.
Compared with the general Australia population, that amounted to a standardized incidence ratio for primary invasive melanoma of 5.42 for patients with previous invasive melanoma diagnoses (95% CI, 5.23-5.61) and 4.59 for patients with previous in situ melanoma (95% CI, 4.37-4.82).
Moreover, the authors found that the body site of the second melanoma was typically the same as for the first invasive or in situ diagnosis, especially on the head and lower extremities; in particular, females with a first primary invasive melanoma on the head had a standardized incidence ratio of 13.32 for a second primary invasive melanoma of the head, compared with the general population (95% CI, 10.28-16.98).
The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
In situ melanoma patients have a significant, substantially elevated risk for subsequent invasive or in situ melanoma – nearly as high as the risk for patients presenting with more invasive disease.
The finding means that "education and continued surveillance are paramount not only for persons with an invasive melanoma but also for persons with an in situ melanoma," wrote Danny R. Youlden in JAMA Dermatology, published online March 19 (doi:10.1001/jamadermatol.2013.9852).
Mr. Youlden, a biostatistician with Cancer Council Queensland, in Brisbane, Australia, and his colleagues looked at 39,668 cases of first primary invasive melanoma, and 22,845 patients with first primary in situ melanoma, diagnosed between 1982 and 2005.
Patients were followed for a median of more than 9 years, during which time there were 5,358 subsequent primary invasive melanomas; 3,520 (66%) of these occurred in patients with previous invasive melanomas.
Compared with the general Australia population, that amounted to a standardized incidence ratio for primary invasive melanoma of 5.42 for patients with previous invasive melanoma diagnoses (95% CI, 5.23-5.61) and 4.59 for patients with previous in situ melanoma (95% CI, 4.37-4.82).
Moreover, the authors found that the body site of the second melanoma was typically the same as for the first invasive or in situ diagnosis, especially on the head and lower extremities; in particular, females with a first primary invasive melanoma on the head had a standardized incidence ratio of 13.32 for a second primary invasive melanoma of the head, compared with the general population (95% CI, 10.28-16.98).
The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
In situ melanoma patients have a significant, substantially elevated risk for subsequent invasive or in situ melanoma – nearly as high as the risk for patients presenting with more invasive disease.
The finding means that "education and continued surveillance are paramount not only for persons with an invasive melanoma but also for persons with an in situ melanoma," wrote Danny R. Youlden in JAMA Dermatology, published online March 19 (doi:10.1001/jamadermatol.2013.9852).
Mr. Youlden, a biostatistician with Cancer Council Queensland, in Brisbane, Australia, and his colleagues looked at 39,668 cases of first primary invasive melanoma, and 22,845 patients with first primary in situ melanoma, diagnosed between 1982 and 2005.
Patients were followed for a median of more than 9 years, during which time there were 5,358 subsequent primary invasive melanomas; 3,520 (66%) of these occurred in patients with previous invasive melanomas.
Compared with the general Australia population, that amounted to a standardized incidence ratio for primary invasive melanoma of 5.42 for patients with previous invasive melanoma diagnoses (95% CI, 5.23-5.61) and 4.59 for patients with previous in situ melanoma (95% CI, 4.37-4.82).
Moreover, the authors found that the body site of the second melanoma was typically the same as for the first invasive or in situ diagnosis, especially on the head and lower extremities; in particular, females with a first primary invasive melanoma on the head had a standardized incidence ratio of 13.32 for a second primary invasive melanoma of the head, compared with the general population (95% CI, 10.28-16.98).
The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
FROM JAMA DERMATOLOGY
Major finding: In situ melanoma patients have a nearly fivefold greater risk of subsequent primary invasive melanoma, compared with the general population.
Data source: A retrospective cohort study of the Queensland Cancer Registry.
Disclosures: The authors disclosed no conflicts of interest; several reported grants from the National Health and Medical Research Council.
No benefit from androgen deprivation therapy in localized prostate cancer
For most men with localized prostate cancer who defer surgery or radiation, primary androgen deprivation therapy offers no mortality benefit, Dr. Arnold L. Potosky reported.
Dr. Potosky of Georgetown University Medical Center, Washington, D.C., and colleagues looked at 15,170 men with newly diagnosed, clinically localized prostate cancer who did not receive curative therapy, including radiation, radical prostatectomy, or chemotherapy (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.2043]).
Overall, 23% of the cohort had primary androgen deprivation therapy (PADT) initiated within the first year of diagnosis. After adjustment for factors including age, baseline prostate-specific antigen, Gleason score, and T stage, the authors found that there was no difference between PADT-treated men and their PADT-naive counterparts in all-cause mortality (hazard ratio, 1.04; 95% confidence interval, 0.97-1.11) or prostate-cancer specific mortality (HR, 1.03; 95% CI, 0.89-1.19).
Indeed, despite its widespread use among patients who do not undergo curative-intent treatments, "the risk of serious adverse events and the high costs associated with its use mitigate any clinical or policy rationale for PADT use in these men," the authors wrote.
They did report a small but significant decreased risk of all-cause mortality (HR, 0.88; 95% CI, 0.78-0.97) in men with high-risk cancer (pretreatment PSA greater than 20; Gleason score, 8-10; clinical stage, T2c-T3a), but "the observed benefit was relatively small and should not be taken as definitive, given the limitations of our data," they noted.
An increased risk of death was reported in men with low-risk cancer (HR, 1.41; 95% CI, 0.99-1.82) and no difference in risk for men with intermediate-risk cancer (HR, 1.12; 95% CI, 0.92-1.32). There were no differences in risk of prostate cancer mortality by risk group category.
The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
For most men with localized prostate cancer who defer surgery or radiation, primary androgen deprivation therapy offers no mortality benefit, Dr. Arnold L. Potosky reported.
Dr. Potosky of Georgetown University Medical Center, Washington, D.C., and colleagues looked at 15,170 men with newly diagnosed, clinically localized prostate cancer who did not receive curative therapy, including radiation, radical prostatectomy, or chemotherapy (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.2043]).
Overall, 23% of the cohort had primary androgen deprivation therapy (PADT) initiated within the first year of diagnosis. After adjustment for factors including age, baseline prostate-specific antigen, Gleason score, and T stage, the authors found that there was no difference between PADT-treated men and their PADT-naive counterparts in all-cause mortality (hazard ratio, 1.04; 95% confidence interval, 0.97-1.11) or prostate-cancer specific mortality (HR, 1.03; 95% CI, 0.89-1.19).
Indeed, despite its widespread use among patients who do not undergo curative-intent treatments, "the risk of serious adverse events and the high costs associated with its use mitigate any clinical or policy rationale for PADT use in these men," the authors wrote.
They did report a small but significant decreased risk of all-cause mortality (HR, 0.88; 95% CI, 0.78-0.97) in men with high-risk cancer (pretreatment PSA greater than 20; Gleason score, 8-10; clinical stage, T2c-T3a), but "the observed benefit was relatively small and should not be taken as definitive, given the limitations of our data," they noted.
An increased risk of death was reported in men with low-risk cancer (HR, 1.41; 95% CI, 0.99-1.82) and no difference in risk for men with intermediate-risk cancer (HR, 1.12; 95% CI, 0.92-1.32). There were no differences in risk of prostate cancer mortality by risk group category.
The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
For most men with localized prostate cancer who defer surgery or radiation, primary androgen deprivation therapy offers no mortality benefit, Dr. Arnold L. Potosky reported.
Dr. Potosky of Georgetown University Medical Center, Washington, D.C., and colleagues looked at 15,170 men with newly diagnosed, clinically localized prostate cancer who did not receive curative therapy, including radiation, radical prostatectomy, or chemotherapy (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.2043]).
Overall, 23% of the cohort had primary androgen deprivation therapy (PADT) initiated within the first year of diagnosis. After adjustment for factors including age, baseline prostate-specific antigen, Gleason score, and T stage, the authors found that there was no difference between PADT-treated men and their PADT-naive counterparts in all-cause mortality (hazard ratio, 1.04; 95% confidence interval, 0.97-1.11) or prostate-cancer specific mortality (HR, 1.03; 95% CI, 0.89-1.19).
Indeed, despite its widespread use among patients who do not undergo curative-intent treatments, "the risk of serious adverse events and the high costs associated with its use mitigate any clinical or policy rationale for PADT use in these men," the authors wrote.
They did report a small but significant decreased risk of all-cause mortality (HR, 0.88; 95% CI, 0.78-0.97) in men with high-risk cancer (pretreatment PSA greater than 20; Gleason score, 8-10; clinical stage, T2c-T3a), but "the observed benefit was relatively small and should not be taken as definitive, given the limitations of our data," they noted.
An increased risk of death was reported in men with low-risk cancer (HR, 1.41; 95% CI, 0.99-1.82) and no difference in risk for men with intermediate-risk cancer (HR, 1.12; 95% CI, 0.92-1.32). There were no differences in risk of prostate cancer mortality by risk group category.
The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Men with localized prostate cancer who deferred curative treatment but opted for primary androgen deprivation therapy saw no significant decrease in mortality (hazard ratio, 0.97; 95% confidence interval, 0.97-1.11).
Data source: A retrospective cohort study using comprehensive utilization and cancer registry data from three integrated health plans (n = 15,170).
Disclosures: The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
Endoscopic resection for adenocarcinoma
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care. That's according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gasro.2013.11.006).
Dr. Pech of the University of Regensburg (Germany) and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, referred to a single center from October 1996 to September 2010.
All patients had mucosal Barrett's carcinoma; lesions judged resectable were subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett's esophagus, and the remainder had long-segment Barrett's. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae. En bloc resection was performed in 508 patients; piecemeal resection in the rest.
Complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection. That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1). He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, they determined that long-segment Barrett's as well as poorly differentiated mucosal adenocarcinoma had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort. Additionally, over the long course of the study, best practices for Barrett's esophagus and high-grade dysplasia have evolved considerably.
The authors had no disclosures.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care. That's according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gasro.2013.11.006).
Dr. Pech of the University of Regensburg (Germany) and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, referred to a single center from October 1996 to September 2010.
All patients had mucosal Barrett's carcinoma; lesions judged resectable were subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett's esophagus, and the remainder had long-segment Barrett's. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae. En bloc resection was performed in 508 patients; piecemeal resection in the rest.
Complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection. That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1). He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, they determined that long-segment Barrett's as well as poorly differentiated mucosal adenocarcinoma had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort. Additionally, over the long course of the study, best practices for Barrett's esophagus and high-grade dysplasia have evolved considerably.
The authors had no disclosures.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care. That's according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gasro.2013.11.006).
Dr. Pech of the University of Regensburg (Germany) and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, referred to a single center from October 1996 to September 2010.
All patients had mucosal Barrett's carcinoma; lesions judged resectable were subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett's esophagus, and the remainder had long-segment Barrett's. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae. En bloc resection was performed in 508 patients; piecemeal resection in the rest.
Complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection. That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1). He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, they determined that long-segment Barrett's as well as poorly differentiated mucosal adenocarcinoma had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort. Additionally, over the long course of the study, best practices for Barrett's esophagus and high-grade dysplasia have evolved considerably.
The authors had no disclosures.
Major finding: Endoscopic resection of esophageal adenocarcinoma resulted in a long-term complete remission rate of 93.8%.
Data source: Data from 1,000 consecutive patients with mucosal adenocarcinoma of the esophagus.
Disclosures: The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
Endoscopic mucosal resection new gold standard for esophageal adenocarcinoma
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care.
That’s according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gastro.2013.11.006).
Dr. Pech, of the University of Regensburg, Germany, and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, who were referred to a single center between October 1996 and September 2010.
All patients had mucosal Barrett’s carcinoma; lesions judged resectable were first subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett’s esophagus, and the remainder had long-segment Barrett’s. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae.
En bloc resection was performed in 508 patients and piecemeal resection in the rest.
The authors found that complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of the 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection.
That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1).
He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, the authors determined that long-segment Barrett’s as well as poorly differentiated mucosal adenocarcinoma (as opposed to well-differentiated lesions) had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort, "because it is possible that only patients with early Barrett’s carcinoma that was endoscopically well treatable may have been referred."
Additionally, over the long course of the study, best practices for Barrett’s esophagus and high grade-dysplasia have evolved considerably, "moving away from multimodal therapy for early Barrett’s carcinoma using a combination of [endoscopic resection], photodynamic therapy, [argon plasma coagulation], and laser toward a strict and purely resectional form of treatment."
Nevertheless, "the data presented here on the largest series published to date on endoscopic therapy for mucosal adenocarcinomas in 1,000 patients confirm the safety of endoscopic resection," the authors wrote.
"Endoscopic therapy for mucosal Barrett’s carcinoma should therefore become the international gold standard for treatment," they added.
The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
In this month’s issue of Gastroenterology, Oliver Pech, Christian Ell, and colleagues continue their pioneering work on endoscopic treatment of Barrett’s neoplasia with a study of the long-term efficacy and safety of endoscopic resection for T1a esophageal adenocarcinoma. Based on prior work by this German group and several others around the world, endoscopic resection of nodular high-grade dysplasia followed by ablation or resection of all residual Barrett’s has become the standard of care for high-grade dysplasia. There are fewer data available on endoscopic treatment of T1a lesions, which have a small but nonzero incidence of lymph node spread that needs to be considered in treatment algorithms.
While many therapeutic endoscopy programs have embraced endoscopic treatment of T1a esophageal adenocarcinoma with favorable histology, some skepticism remains, and the current study will go a long way toward justifying endoscopic treatment of these tumors. The scope and magnitude of this study are striking – 1,000 consecutive patients with T1a tumors treated by endoscopic resection were followed for an average of nearly 5 years. The results are outstanding, with a long-term remission rate of 94% and only two deaths from Barrett’s cancer. Major complications were rare and were successfully treated endoscopically. Very few patients ultimately required surgery – for lymphatic infiltration, inability to resect the lesion endoscopically due to scarring, poor wound healing, incorrect assessment of the tumor stage during initial treatment, or additional cancer developing during the study. Even those patients ultimately did well.
Given the well-documented mortality risk of esophagectomy even in expert centers (2%-5%), as well as the high morbidity of surgery, it is clear from this study that endoscopic treatment of T1a esophageal adenocarcinoma needs to be the standard of care.
Dr. Shai Friedland is an associate professor of medicine at Stanford (Calif.) University. He is a consultant for C2 Medical.
In this month’s issue of Gastroenterology, Oliver Pech, Christian Ell, and colleagues continue their pioneering work on endoscopic treatment of Barrett’s neoplasia with a study of the long-term efficacy and safety of endoscopic resection for T1a esophageal adenocarcinoma. Based on prior work by this German group and several others around the world, endoscopic resection of nodular high-grade dysplasia followed by ablation or resection of all residual Barrett’s has become the standard of care for high-grade dysplasia. There are fewer data available on endoscopic treatment of T1a lesions, which have a small but nonzero incidence of lymph node spread that needs to be considered in treatment algorithms.
While many therapeutic endoscopy programs have embraced endoscopic treatment of T1a esophageal adenocarcinoma with favorable histology, some skepticism remains, and the current study will go a long way toward justifying endoscopic treatment of these tumors. The scope and magnitude of this study are striking – 1,000 consecutive patients with T1a tumors treated by endoscopic resection were followed for an average of nearly 5 years. The results are outstanding, with a long-term remission rate of 94% and only two deaths from Barrett’s cancer. Major complications were rare and were successfully treated endoscopically. Very few patients ultimately required surgery – for lymphatic infiltration, inability to resect the lesion endoscopically due to scarring, poor wound healing, incorrect assessment of the tumor stage during initial treatment, or additional cancer developing during the study. Even those patients ultimately did well.
Given the well-documented mortality risk of esophagectomy even in expert centers (2%-5%), as well as the high morbidity of surgery, it is clear from this study that endoscopic treatment of T1a esophageal adenocarcinoma needs to be the standard of care.
Dr. Shai Friedland is an associate professor of medicine at Stanford (Calif.) University. He is a consultant for C2 Medical.
In this month’s issue of Gastroenterology, Oliver Pech, Christian Ell, and colleagues continue their pioneering work on endoscopic treatment of Barrett’s neoplasia with a study of the long-term efficacy and safety of endoscopic resection for T1a esophageal adenocarcinoma. Based on prior work by this German group and several others around the world, endoscopic resection of nodular high-grade dysplasia followed by ablation or resection of all residual Barrett’s has become the standard of care for high-grade dysplasia. There are fewer data available on endoscopic treatment of T1a lesions, which have a small but nonzero incidence of lymph node spread that needs to be considered in treatment algorithms.
While many therapeutic endoscopy programs have embraced endoscopic treatment of T1a esophageal adenocarcinoma with favorable histology, some skepticism remains, and the current study will go a long way toward justifying endoscopic treatment of these tumors. The scope and magnitude of this study are striking – 1,000 consecutive patients with T1a tumors treated by endoscopic resection were followed for an average of nearly 5 years. The results are outstanding, with a long-term remission rate of 94% and only two deaths from Barrett’s cancer. Major complications were rare and were successfully treated endoscopically. Very few patients ultimately required surgery – for lymphatic infiltration, inability to resect the lesion endoscopically due to scarring, poor wound healing, incorrect assessment of the tumor stage during initial treatment, or additional cancer developing during the study. Even those patients ultimately did well.
Given the well-documented mortality risk of esophagectomy even in expert centers (2%-5%), as well as the high morbidity of surgery, it is clear from this study that endoscopic treatment of T1a esophageal adenocarcinoma needs to be the standard of care.
Dr. Shai Friedland is an associate professor of medicine at Stanford (Calif.) University. He is a consultant for C2 Medical.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care.
That’s according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gastro.2013.11.006).
Dr. Pech, of the University of Regensburg, Germany, and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, who were referred to a single center between October 1996 and September 2010.
All patients had mucosal Barrett’s carcinoma; lesions judged resectable were first subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett’s esophagus, and the remainder had long-segment Barrett’s. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae.
En bloc resection was performed in 508 patients and piecemeal resection in the rest.
The authors found that complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of the 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection.
That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1).
He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, the authors determined that long-segment Barrett’s as well as poorly differentiated mucosal adenocarcinoma (as opposed to well-differentiated lesions) had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort, "because it is possible that only patients with early Barrett’s carcinoma that was endoscopically well treatable may have been referred."
Additionally, over the long course of the study, best practices for Barrett’s esophagus and high grade-dysplasia have evolved considerably, "moving away from multimodal therapy for early Barrett’s carcinoma using a combination of [endoscopic resection], photodynamic therapy, [argon plasma coagulation], and laser toward a strict and purely resectional form of treatment."
Nevertheless, "the data presented here on the largest series published to date on endoscopic therapy for mucosal adenocarcinomas in 1,000 patients confirm the safety of endoscopic resection," the authors wrote.
"Endoscopic therapy for mucosal Barrett’s carcinoma should therefore become the international gold standard for treatment," they added.
The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care.
That’s according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gastro.2013.11.006).
Dr. Pech, of the University of Regensburg, Germany, and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, who were referred to a single center between October 1996 and September 2010.
All patients had mucosal Barrett’s carcinoma; lesions judged resectable were first subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett’s esophagus, and the remainder had long-segment Barrett’s. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae.
En bloc resection was performed in 508 patients and piecemeal resection in the rest.
The authors found that complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of the 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection.
That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1).
He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, the authors determined that long-segment Barrett’s as well as poorly differentiated mucosal adenocarcinoma (as opposed to well-differentiated lesions) had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort, "because it is possible that only patients with early Barrett’s carcinoma that was endoscopically well treatable may have been referred."
Additionally, over the long course of the study, best practices for Barrett’s esophagus and high grade-dysplasia have evolved considerably, "moving away from multimodal therapy for early Barrett’s carcinoma using a combination of [endoscopic resection], photodynamic therapy, [argon plasma coagulation], and laser toward a strict and purely resectional form of treatment."
Nevertheless, "the data presented here on the largest series published to date on endoscopic therapy for mucosal adenocarcinomas in 1,000 patients confirm the safety of endoscopic resection," the authors wrote.
"Endoscopic therapy for mucosal Barrett’s carcinoma should therefore become the international gold standard for treatment," they added.
The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
FROM GASTROENTEROLOGY
Major finding: Endoscopic resection of esophageal adenocarcinoma resulted in a long-term complete remission rate of 93.8%.
Data source: Data from 1,000 consecutive patients with mucosal adenocarcinoma of the esophagus.
Disclosures: The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
NASH liver transplant mortality differs from non-NASH
Cardiovascular complications and sepsis are two major concerns after liver transplant for nonalcoholic steatohepatitis, more so than after liver transplant for other indications.
The finding comes from what the authors called the first systematic review and meta-analysis to investigate the cumulative clinical experience of liver transplant for NASH, wrote Dr. Xiaofei Wang and colleagues in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2013.09.023).
In their analysis, Dr. Wang, of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China, and colleagues performed a search of PubMed, Embase, the Cochrane Library, and the Web of Science for studies published through Sept. 1, 2012, that looked at liver transplant for nonalcoholic fatty liver disease (NAFLD) or NASH. Reviews were excluded, as were studies in which NASH patients could not be separated out from non-NASH liver transplant recipients.
Overall, nine studies were analyzed, all of which measured outcomes after liver transplant for NASH. Other indications for transplant included primary biliary cirrhosis/primary sclerosing cholangitis, alcoholic liver disease, hepatitis C, hepatitis B, and cryptogenic cirrhosis.
The researchers found that, on the whole, survival rates at post-transplant years 1, 3, and 5 did not significantly differ between NASH patients and their non-NASH counterparts (1-year odds ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05; 3-year OR, 0.97; 95% CI, 0.67-1.40; P = .86; 5-year OR, 1.09; 95% CI, 0.77-1.56; P = .63).
Nevertheless, NASH patients registered more deaths due to "cardiovascular events" compared with non-NASH liver transplant recipients, the authors wrote (OR, 1.65; 95% CI, 1.01-2.70; P = .05).
The authors also found that NASH patients were significantly more likely to die of sepsis post transplant than were non-NASH patients (OR, 1.71; 95% CI, 1.17-2.50; P = .006).
On the other hand, NASH patients had fewer deaths caused by graft failure than did patients undergoing liver transplant for other indications (OR, 0.21; 95% CI, 0.05-0.89; P = .03).
In an attempt to explain their findings, the authors pointed out that while cardiovascular events are the top cause of non–graft-related mortality in all liver transplants, NASH patients might be especially susceptible, given that their diagnosis is "frequently associated" with cardiovascular risk factors such as obesity, diabetes, and hypertension. Indeed, these same characteristics might also predispose these patients to postoperative infection and sepsis, they added.
And regarding the finding that graft-related mortality is actually lower among NASH patients, Dr. Wang and colleagues postulated that this may be due to the "lower likelihood of disease recurrence, and this may mean lower rates of graft failure compared with other liver diseases, such as hepatitis C virus and hepatitis B virus infection."
The authors conceded that there were several limitations to their analysis, not the least being heterogeneity between included studies. Moreover, two large population-based studies using national databases were ultimately excluded due to patient duplication and poor accuracy with regard to the cause of death.
In any case, the current analysis shows that "more attention and careful consideration are required in selecting patients with NASH for liver transplant, along with aggressive management of cardiovascular complications and sepsis after transplantation."
The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
Cardiovascular complications and sepsis are two major concerns after liver transplant for nonalcoholic steatohepatitis, more so than after liver transplant for other indications.
The finding comes from what the authors called the first systematic review and meta-analysis to investigate the cumulative clinical experience of liver transplant for NASH, wrote Dr. Xiaofei Wang and colleagues in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2013.09.023).
In their analysis, Dr. Wang, of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China, and colleagues performed a search of PubMed, Embase, the Cochrane Library, and the Web of Science for studies published through Sept. 1, 2012, that looked at liver transplant for nonalcoholic fatty liver disease (NAFLD) or NASH. Reviews were excluded, as were studies in which NASH patients could not be separated out from non-NASH liver transplant recipients.
Overall, nine studies were analyzed, all of which measured outcomes after liver transplant for NASH. Other indications for transplant included primary biliary cirrhosis/primary sclerosing cholangitis, alcoholic liver disease, hepatitis C, hepatitis B, and cryptogenic cirrhosis.
The researchers found that, on the whole, survival rates at post-transplant years 1, 3, and 5 did not significantly differ between NASH patients and their non-NASH counterparts (1-year odds ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05; 3-year OR, 0.97; 95% CI, 0.67-1.40; P = .86; 5-year OR, 1.09; 95% CI, 0.77-1.56; P = .63).
Nevertheless, NASH patients registered more deaths due to "cardiovascular events" compared with non-NASH liver transplant recipients, the authors wrote (OR, 1.65; 95% CI, 1.01-2.70; P = .05).
The authors also found that NASH patients were significantly more likely to die of sepsis post transplant than were non-NASH patients (OR, 1.71; 95% CI, 1.17-2.50; P = .006).
On the other hand, NASH patients had fewer deaths caused by graft failure than did patients undergoing liver transplant for other indications (OR, 0.21; 95% CI, 0.05-0.89; P = .03).
In an attempt to explain their findings, the authors pointed out that while cardiovascular events are the top cause of non–graft-related mortality in all liver transplants, NASH patients might be especially susceptible, given that their diagnosis is "frequently associated" with cardiovascular risk factors such as obesity, diabetes, and hypertension. Indeed, these same characteristics might also predispose these patients to postoperative infection and sepsis, they added.
And regarding the finding that graft-related mortality is actually lower among NASH patients, Dr. Wang and colleagues postulated that this may be due to the "lower likelihood of disease recurrence, and this may mean lower rates of graft failure compared with other liver diseases, such as hepatitis C virus and hepatitis B virus infection."
The authors conceded that there were several limitations to their analysis, not the least being heterogeneity between included studies. Moreover, two large population-based studies using national databases were ultimately excluded due to patient duplication and poor accuracy with regard to the cause of death.
In any case, the current analysis shows that "more attention and careful consideration are required in selecting patients with NASH for liver transplant, along with aggressive management of cardiovascular complications and sepsis after transplantation."
The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
Cardiovascular complications and sepsis are two major concerns after liver transplant for nonalcoholic steatohepatitis, more so than after liver transplant for other indications.
The finding comes from what the authors called the first systematic review and meta-analysis to investigate the cumulative clinical experience of liver transplant for NASH, wrote Dr. Xiaofei Wang and colleagues in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2013.09.023).
In their analysis, Dr. Wang, of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China, and colleagues performed a search of PubMed, Embase, the Cochrane Library, and the Web of Science for studies published through Sept. 1, 2012, that looked at liver transplant for nonalcoholic fatty liver disease (NAFLD) or NASH. Reviews were excluded, as were studies in which NASH patients could not be separated out from non-NASH liver transplant recipients.
Overall, nine studies were analyzed, all of which measured outcomes after liver transplant for NASH. Other indications for transplant included primary biliary cirrhosis/primary sclerosing cholangitis, alcoholic liver disease, hepatitis C, hepatitis B, and cryptogenic cirrhosis.
The researchers found that, on the whole, survival rates at post-transplant years 1, 3, and 5 did not significantly differ between NASH patients and their non-NASH counterparts (1-year odds ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05; 3-year OR, 0.97; 95% CI, 0.67-1.40; P = .86; 5-year OR, 1.09; 95% CI, 0.77-1.56; P = .63).
Nevertheless, NASH patients registered more deaths due to "cardiovascular events" compared with non-NASH liver transplant recipients, the authors wrote (OR, 1.65; 95% CI, 1.01-2.70; P = .05).
The authors also found that NASH patients were significantly more likely to die of sepsis post transplant than were non-NASH patients (OR, 1.71; 95% CI, 1.17-2.50; P = .006).
On the other hand, NASH patients had fewer deaths caused by graft failure than did patients undergoing liver transplant for other indications (OR, 0.21; 95% CI, 0.05-0.89; P = .03).
In an attempt to explain their findings, the authors pointed out that while cardiovascular events are the top cause of non–graft-related mortality in all liver transplants, NASH patients might be especially susceptible, given that their diagnosis is "frequently associated" with cardiovascular risk factors such as obesity, diabetes, and hypertension. Indeed, these same characteristics might also predispose these patients to postoperative infection and sepsis, they added.
And regarding the finding that graft-related mortality is actually lower among NASH patients, Dr. Wang and colleagues postulated that this may be due to the "lower likelihood of disease recurrence, and this may mean lower rates of graft failure compared with other liver diseases, such as hepatitis C virus and hepatitis B virus infection."
The authors conceded that there were several limitations to their analysis, not the least being heterogeneity between included studies. Moreover, two large population-based studies using national databases were ultimately excluded due to patient duplication and poor accuracy with regard to the cause of death.
In any case, the current analysis shows that "more attention and careful consideration are required in selecting patients with NASH for liver transplant, along with aggressive management of cardiovascular complications and sepsis after transplantation."
The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Major finding: Patients undergoing liver transplant for nonalcoholic steatohepatitis are at a greater risk for death due to sepsis and cardiovascular complications.
Data source: A meta-analysis of nine studies published through September 2012.
Disclosures: The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
Colonoscopy risk reductions go beyond screening
The risk of colorectal cancer is greatly reduced for up to 10 years following even nonscreening colonoscopies, such as those performed in the setting of a positive fecal occult blood test or abdominal symptoms, a study showed.
Cancer risk was also lowered in the right colon, regardless of the indication for the colonoscopy, reported Dr. Hermann Brenner and his colleagues in the March issue of Gastroenterology (doi:10.1053/j.gastro.2013.09.001).
The finding is mostly good news for patients and practitioners. However, the investigators pointed out that as the use of diagnostic colonoscopy becomes more widespread in the United States and beyond, future researchers risk "contamination by taking diagnostic colonoscopies into account in the design and analysis of results from screening endoscopy randomized controlled trials."
Dr. Brenner of the University of Heidelberg (Germany) looked at 2,516 patients with a first diagnosis of colorectal cancer and 2,284 healthy controls culled from the DACHS study, a population-based case-control study conducted in the Rhine-Neckar region of Germany since 2003.
Patients were excluded if they were younger than 50 years old (in which case screening colonoscopy is not generally recommended) and if they had any history of inflammatory bowel disease, which requires frequent colonoscopies.
The median age was 70 years; 59% of all participants were men.
Overall, reported the authors, slightly more than a third (38.3%) of controls had a previous colonoscopy, most commonly for screening (12.0%) followed by abdominal symptoms (9.1%).
The other possible indications were screening, a positive fecal occult blood test result, surveillance, rectal bleeding, or "other."
Looking at both cases and controls, the investigators said that although only a small fraction of colonoscopies conducted until 2002 were performed for screening, screening was the most common indication for colonoscopies conducted from 2003 on.
The authors found that even though there was a stronger risk reduction in colorectal cancer among patients who underwent screening colonoscopy between 1 and 10 years prior (and this reduction was profound, with an odds ratio of 0.09 and a 95% confidence interval of 0.07-0.13), there was still a significant reduction after colonoscopies performed for other indications, including a positive fecal occult blood test result (OR, 0.33; 95% CI, 0.19-0.57), surveillance after a preceding colonoscopy (OR, 0.33; 95% CI, 0.24-0.45), rectal bleeding (OR, 0.28; 95% CI, 0.20-0.40), and abdominal symptoms (OR, 0.15; 95% CI, 0.10-0.21).
Unspecified other indications were associated with an odds ratio of 0.21 for cancer (95% CI, 0.14-0.30).
"All risk reductions were substantially less pronounced (and partly not statistically significant) for cancer in the right colon compared with cancer in the left colon and rectum," wrote the authors.
Nevertheless, colonoscopy was also associated with a reduced risk of cancer in the right colon regardless of the indication, although the effect was more pronounced specifically after a screening exam (OR for screening colonoscopy, 0.22; 95% CI, 0.14-0.33).
"To our knowledge, only two case-control studies from Canada and the United States have previously provided separate estimates of colorectal cancer risk after diagnostic and screening colonoscopy," wrote the authors.
"However, with ORs of 0.69 and 0.81, risk reduction was much less pronounced in the Canadian study than in the U.S. study and our study."
The authors disclosed no conflicts of interest related to this study; they stated that funding was provided by the German Research Council as well as the German Federal Ministry of Education and Research.
The risk of colorectal cancer is greatly reduced for up to 10 years following even nonscreening colonoscopies, such as those performed in the setting of a positive fecal occult blood test or abdominal symptoms, a study showed.
Cancer risk was also lowered in the right colon, regardless of the indication for the colonoscopy, reported Dr. Hermann Brenner and his colleagues in the March issue of Gastroenterology (doi:10.1053/j.gastro.2013.09.001).
The finding is mostly good news for patients and practitioners. However, the investigators pointed out that as the use of diagnostic colonoscopy becomes more widespread in the United States and beyond, future researchers risk "contamination by taking diagnostic colonoscopies into account in the design and analysis of results from screening endoscopy randomized controlled trials."
Dr. Brenner of the University of Heidelberg (Germany) looked at 2,516 patients with a first diagnosis of colorectal cancer and 2,284 healthy controls culled from the DACHS study, a population-based case-control study conducted in the Rhine-Neckar region of Germany since 2003.
Patients were excluded if they were younger than 50 years old (in which case screening colonoscopy is not generally recommended) and if they had any history of inflammatory bowel disease, which requires frequent colonoscopies.
The median age was 70 years; 59% of all participants were men.
Overall, reported the authors, slightly more than a third (38.3%) of controls had a previous colonoscopy, most commonly for screening (12.0%) followed by abdominal symptoms (9.1%).
The other possible indications were screening, a positive fecal occult blood test result, surveillance, rectal bleeding, or "other."
Looking at both cases and controls, the investigators said that although only a small fraction of colonoscopies conducted until 2002 were performed for screening, screening was the most common indication for colonoscopies conducted from 2003 on.
The authors found that even though there was a stronger risk reduction in colorectal cancer among patients who underwent screening colonoscopy between 1 and 10 years prior (and this reduction was profound, with an odds ratio of 0.09 and a 95% confidence interval of 0.07-0.13), there was still a significant reduction after colonoscopies performed for other indications, including a positive fecal occult blood test result (OR, 0.33; 95% CI, 0.19-0.57), surveillance after a preceding colonoscopy (OR, 0.33; 95% CI, 0.24-0.45), rectal bleeding (OR, 0.28; 95% CI, 0.20-0.40), and abdominal symptoms (OR, 0.15; 95% CI, 0.10-0.21).
Unspecified other indications were associated with an odds ratio of 0.21 for cancer (95% CI, 0.14-0.30).
"All risk reductions were substantially less pronounced (and partly not statistically significant) for cancer in the right colon compared with cancer in the left colon and rectum," wrote the authors.
Nevertheless, colonoscopy was also associated with a reduced risk of cancer in the right colon regardless of the indication, although the effect was more pronounced specifically after a screening exam (OR for screening colonoscopy, 0.22; 95% CI, 0.14-0.33).
"To our knowledge, only two case-control studies from Canada and the United States have previously provided separate estimates of colorectal cancer risk after diagnostic and screening colonoscopy," wrote the authors.
"However, with ORs of 0.69 and 0.81, risk reduction was much less pronounced in the Canadian study than in the U.S. study and our study."
The authors disclosed no conflicts of interest related to this study; they stated that funding was provided by the German Research Council as well as the German Federal Ministry of Education and Research.
The risk of colorectal cancer is greatly reduced for up to 10 years following even nonscreening colonoscopies, such as those performed in the setting of a positive fecal occult blood test or abdominal symptoms, a study showed.
Cancer risk was also lowered in the right colon, regardless of the indication for the colonoscopy, reported Dr. Hermann Brenner and his colleagues in the March issue of Gastroenterology (doi:10.1053/j.gastro.2013.09.001).
The finding is mostly good news for patients and practitioners. However, the investigators pointed out that as the use of diagnostic colonoscopy becomes more widespread in the United States and beyond, future researchers risk "contamination by taking diagnostic colonoscopies into account in the design and analysis of results from screening endoscopy randomized controlled trials."
Dr. Brenner of the University of Heidelberg (Germany) looked at 2,516 patients with a first diagnosis of colorectal cancer and 2,284 healthy controls culled from the DACHS study, a population-based case-control study conducted in the Rhine-Neckar region of Germany since 2003.
Patients were excluded if they were younger than 50 years old (in which case screening colonoscopy is not generally recommended) and if they had any history of inflammatory bowel disease, which requires frequent colonoscopies.
The median age was 70 years; 59% of all participants were men.
Overall, reported the authors, slightly more than a third (38.3%) of controls had a previous colonoscopy, most commonly for screening (12.0%) followed by abdominal symptoms (9.1%).
The other possible indications were screening, a positive fecal occult blood test result, surveillance, rectal bleeding, or "other."
Looking at both cases and controls, the investigators said that although only a small fraction of colonoscopies conducted until 2002 were performed for screening, screening was the most common indication for colonoscopies conducted from 2003 on.
The authors found that even though there was a stronger risk reduction in colorectal cancer among patients who underwent screening colonoscopy between 1 and 10 years prior (and this reduction was profound, with an odds ratio of 0.09 and a 95% confidence interval of 0.07-0.13), there was still a significant reduction after colonoscopies performed for other indications, including a positive fecal occult blood test result (OR, 0.33; 95% CI, 0.19-0.57), surveillance after a preceding colonoscopy (OR, 0.33; 95% CI, 0.24-0.45), rectal bleeding (OR, 0.28; 95% CI, 0.20-0.40), and abdominal symptoms (OR, 0.15; 95% CI, 0.10-0.21).
Unspecified other indications were associated with an odds ratio of 0.21 for cancer (95% CI, 0.14-0.30).
"All risk reductions were substantially less pronounced (and partly not statistically significant) for cancer in the right colon compared with cancer in the left colon and rectum," wrote the authors.
Nevertheless, colonoscopy was also associated with a reduced risk of cancer in the right colon regardless of the indication, although the effect was more pronounced specifically after a screening exam (OR for screening colonoscopy, 0.22; 95% CI, 0.14-0.33).
"To our knowledge, only two case-control studies from Canada and the United States have previously provided separate estimates of colorectal cancer risk after diagnostic and screening colonoscopy," wrote the authors.
"However, with ORs of 0.69 and 0.81, risk reduction was much less pronounced in the Canadian study than in the U.S. study and our study."
The authors disclosed no conflicts of interest related to this study; they stated that funding was provided by the German Research Council as well as the German Federal Ministry of Education and Research.
FROM GASTROENTEROLOGY
Major finding: Colonoscopies performed for reasons other than screening were strongly and significantly associated with a reduction in colorectal cancer, even after 10 years.
Data source: The DACHS study, a population-based case-control study conducted in the Rhine-Neckar region of Germany since 2003.
Disclosures: The authors disclosed no conflicts of interest related to this study; they stated that funding was provided by the German Research Council as well as the German Federal Ministry of Education and Research.