User login
Does turmeric relieve inflammatory conditions?
YES, but data aren’t plentiful. Limited evidence suggests that turmeric and its active compound, curcumin, are effective for rheumatoid arthritis and other inflammatory conditions (strength of recommendation [SOR]: C, primarily low-quality cohort studies with small patient numbers).
Curcumin has shown limited benefit for patients with psoriasis, inflammatory bowel disease (IBS), inflammatory eye diseases, familial adenomatous polyposis, and kidney transplantation (SOR: B, small, short randomized controlled trials [RCTs]).
No evidence indicates that curcumin helps patients with human immunodeficiency virus (HIV) (SOR: B, single RCT)
Evidence summary
Although extensive in vitro and animal studies have analyzed the effect of curcumin on inflammation and inflammatory mediators (including inhibition of lipoxygenase, cyclooxygenase-2, leukotrienes, thromboxane, prostaglandins, and tumor necrosis factor),1 few human studies have looked at patient-oriented outcomes.
Rheumatoid arthritis. One very small (N=18) double-blind crossover study showed a statistically significant improvement in morning stiffness, walking time, and joint swelling in rheumatoid arthritis patients taking curcumin.2
Psoriasis. A cohort study demonstrated that curcumin applied topically in a gel formulation to patients with psoriasis resulted in either resolution or reduction in psoriatic plaques after 8 weeks of treatment.3
IBS. Two studies have found curcumin to have a positive effect on patients with IBS. A cohort study (N=10) of patients with ulcerative colitis or Crohn’s disease demonstrated symptomatic improvement (more formed stools, less frequent bowel movements, and less abdominal pain and cramping) after consuming curcumin for 2 and 3 months, respectively.4 A randomized, double-blind, multicenter trial (N=89) showed that 6 months of daily curcumin improved the clinical activity index and maintained remission in patients with ulcerative colitis.5
Inflammatory eye diseases. A cohort study of 32 patients found that curcumin was as effective as corticosteroids for chronic anterior uveitis (as demonstrated by improved vision, decreased keratic precipitates, and a break of synechiae assessed by slit lamp examination).6 Another small cohort study (N=5) by the same authors showed that curcumin reduced or resolved inflammatory orbital pseudotumor (as evidenced by reduced ocular swelling, normal ocular movements, and absence of diplopia).7
Familial adenomatous polyposis. A small cohort study (N=5) demonstrated a decrease in size and number of adenomas in patients with familial adenomatous polyposis after a mean of 6 months of treatment with curcumin, although patients received quercetin concurrently during the treatment period.8
Kidney transplantation. A cohort study followed 43 dialysis-dependent cadaver kidney recipients who had taken curcumin for 1 month. Investigators observed reduced acute rejection and neurotoxicity over the course of 6 months.9
HIV. Curcumin didn’t reduce viral load or improve CD4 counts in 40 HIV patients in the single study identified in a Cochrane Review.10
Dosage and adverse effects.
Dosing varied across the studies reviewed in this Clinical Inquiry, but generally was 500 to 1000 mg, 1 to 3 times daily. Curcumin doses as high as 12,000 mg daily have been given in experimental settings without significant adverse events. Minor gastrointestinal side effects, including nausea and diarrhea, have been reported.11
Recommendations
The National Center for Complementary and Alternative Medicine of the National Institutes of Health states that little reliable evidence exists to support the use of turmeric for any health condition because few clinical trials have been conducted. Preliminary findings from animal and laboratory studies suggest that curcumin may have anti-inflammatory and anticancer properties, but these findings have not been confirmed in people.12
1. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). J Altern Complement Med. 2003;9:161-168.
2. Deodhar SD, Sethi R, Srimal RC. Preliminary studies on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res. 1980;71:632-634.
3. Heng MC, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological, and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
4. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191-2193.
5. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502-1506.
6. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
7. Lal B, Kapoor AK, Agrawal PK, et al. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14:443-447.
8. Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035-1038.
9. Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80:1556-1559.
10. Liu JP, Manheimer E, Yang M. Herbal medicines for treating HIV infection and AIDS. Cochrane Database Syst Rev. 2005;(3):CD003937.
11. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471-480.
12. National Center for Complementary and Alternative Medicine Herbs at a glance: turmeric. Updated August 12, 2010. Available at: http://nccam.nih.gov/health/turmeric/. Accessed October 11, 2010.
YES, but data aren’t plentiful. Limited evidence suggests that turmeric and its active compound, curcumin, are effective for rheumatoid arthritis and other inflammatory conditions (strength of recommendation [SOR]: C, primarily low-quality cohort studies with small patient numbers).
Curcumin has shown limited benefit for patients with psoriasis, inflammatory bowel disease (IBS), inflammatory eye diseases, familial adenomatous polyposis, and kidney transplantation (SOR: B, small, short randomized controlled trials [RCTs]).
No evidence indicates that curcumin helps patients with human immunodeficiency virus (HIV) (SOR: B, single RCT)
Evidence summary
Although extensive in vitro and animal studies have analyzed the effect of curcumin on inflammation and inflammatory mediators (including inhibition of lipoxygenase, cyclooxygenase-2, leukotrienes, thromboxane, prostaglandins, and tumor necrosis factor),1 few human studies have looked at patient-oriented outcomes.
Rheumatoid arthritis. One very small (N=18) double-blind crossover study showed a statistically significant improvement in morning stiffness, walking time, and joint swelling in rheumatoid arthritis patients taking curcumin.2
Psoriasis. A cohort study demonstrated that curcumin applied topically in a gel formulation to patients with psoriasis resulted in either resolution or reduction in psoriatic plaques after 8 weeks of treatment.3
IBS. Two studies have found curcumin to have a positive effect on patients with IBS. A cohort study (N=10) of patients with ulcerative colitis or Crohn’s disease demonstrated symptomatic improvement (more formed stools, less frequent bowel movements, and less abdominal pain and cramping) after consuming curcumin for 2 and 3 months, respectively.4 A randomized, double-blind, multicenter trial (N=89) showed that 6 months of daily curcumin improved the clinical activity index and maintained remission in patients with ulcerative colitis.5
Inflammatory eye diseases. A cohort study of 32 patients found that curcumin was as effective as corticosteroids for chronic anterior uveitis (as demonstrated by improved vision, decreased keratic precipitates, and a break of synechiae assessed by slit lamp examination).6 Another small cohort study (N=5) by the same authors showed that curcumin reduced or resolved inflammatory orbital pseudotumor (as evidenced by reduced ocular swelling, normal ocular movements, and absence of diplopia).7
Familial adenomatous polyposis. A small cohort study (N=5) demonstrated a decrease in size and number of adenomas in patients with familial adenomatous polyposis after a mean of 6 months of treatment with curcumin, although patients received quercetin concurrently during the treatment period.8
Kidney transplantation. A cohort study followed 43 dialysis-dependent cadaver kidney recipients who had taken curcumin for 1 month. Investigators observed reduced acute rejection and neurotoxicity over the course of 6 months.9
HIV. Curcumin didn’t reduce viral load or improve CD4 counts in 40 HIV patients in the single study identified in a Cochrane Review.10
Dosage and adverse effects.
Dosing varied across the studies reviewed in this Clinical Inquiry, but generally was 500 to 1000 mg, 1 to 3 times daily. Curcumin doses as high as 12,000 mg daily have been given in experimental settings without significant adverse events. Minor gastrointestinal side effects, including nausea and diarrhea, have been reported.11
Recommendations
The National Center for Complementary and Alternative Medicine of the National Institutes of Health states that little reliable evidence exists to support the use of turmeric for any health condition because few clinical trials have been conducted. Preliminary findings from animal and laboratory studies suggest that curcumin may have anti-inflammatory and anticancer properties, but these findings have not been confirmed in people.12
YES, but data aren’t plentiful. Limited evidence suggests that turmeric and its active compound, curcumin, are effective for rheumatoid arthritis and other inflammatory conditions (strength of recommendation [SOR]: C, primarily low-quality cohort studies with small patient numbers).
Curcumin has shown limited benefit for patients with psoriasis, inflammatory bowel disease (IBS), inflammatory eye diseases, familial adenomatous polyposis, and kidney transplantation (SOR: B, small, short randomized controlled trials [RCTs]).
No evidence indicates that curcumin helps patients with human immunodeficiency virus (HIV) (SOR: B, single RCT)
Evidence summary
Although extensive in vitro and animal studies have analyzed the effect of curcumin on inflammation and inflammatory mediators (including inhibition of lipoxygenase, cyclooxygenase-2, leukotrienes, thromboxane, prostaglandins, and tumor necrosis factor),1 few human studies have looked at patient-oriented outcomes.
Rheumatoid arthritis. One very small (N=18) double-blind crossover study showed a statistically significant improvement in morning stiffness, walking time, and joint swelling in rheumatoid arthritis patients taking curcumin.2
Psoriasis. A cohort study demonstrated that curcumin applied topically in a gel formulation to patients with psoriasis resulted in either resolution or reduction in psoriatic plaques after 8 weeks of treatment.3
IBS. Two studies have found curcumin to have a positive effect on patients with IBS. A cohort study (N=10) of patients with ulcerative colitis or Crohn’s disease demonstrated symptomatic improvement (more formed stools, less frequent bowel movements, and less abdominal pain and cramping) after consuming curcumin for 2 and 3 months, respectively.4 A randomized, double-blind, multicenter trial (N=89) showed that 6 months of daily curcumin improved the clinical activity index and maintained remission in patients with ulcerative colitis.5
Inflammatory eye diseases. A cohort study of 32 patients found that curcumin was as effective as corticosteroids for chronic anterior uveitis (as demonstrated by improved vision, decreased keratic precipitates, and a break of synechiae assessed by slit lamp examination).6 Another small cohort study (N=5) by the same authors showed that curcumin reduced or resolved inflammatory orbital pseudotumor (as evidenced by reduced ocular swelling, normal ocular movements, and absence of diplopia).7
Familial adenomatous polyposis. A small cohort study (N=5) demonstrated a decrease in size and number of adenomas in patients with familial adenomatous polyposis after a mean of 6 months of treatment with curcumin, although patients received quercetin concurrently during the treatment period.8
Kidney transplantation. A cohort study followed 43 dialysis-dependent cadaver kidney recipients who had taken curcumin for 1 month. Investigators observed reduced acute rejection and neurotoxicity over the course of 6 months.9
HIV. Curcumin didn’t reduce viral load or improve CD4 counts in 40 HIV patients in the single study identified in a Cochrane Review.10
Dosage and adverse effects.
Dosing varied across the studies reviewed in this Clinical Inquiry, but generally was 500 to 1000 mg, 1 to 3 times daily. Curcumin doses as high as 12,000 mg daily have been given in experimental settings without significant adverse events. Minor gastrointestinal side effects, including nausea and diarrhea, have been reported.11
Recommendations
The National Center for Complementary and Alternative Medicine of the National Institutes of Health states that little reliable evidence exists to support the use of turmeric for any health condition because few clinical trials have been conducted. Preliminary findings from animal and laboratory studies suggest that curcumin may have anti-inflammatory and anticancer properties, but these findings have not been confirmed in people.12
1. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). J Altern Complement Med. 2003;9:161-168.
2. Deodhar SD, Sethi R, Srimal RC. Preliminary studies on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res. 1980;71:632-634.
3. Heng MC, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological, and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
4. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191-2193.
5. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502-1506.
6. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
7. Lal B, Kapoor AK, Agrawal PK, et al. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14:443-447.
8. Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035-1038.
9. Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80:1556-1559.
10. Liu JP, Manheimer E, Yang M. Herbal medicines for treating HIV infection and AIDS. Cochrane Database Syst Rev. 2005;(3):CD003937.
11. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471-480.
12. National Center for Complementary and Alternative Medicine Herbs at a glance: turmeric. Updated August 12, 2010. Available at: http://nccam.nih.gov/health/turmeric/. Accessed October 11, 2010.
1. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). J Altern Complement Med. 2003;9:161-168.
2. Deodhar SD, Sethi R, Srimal RC. Preliminary studies on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res. 1980;71:632-634.
3. Heng MC, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological, and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
4. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191-2193.
5. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502-1506.
6. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
7. Lal B, Kapoor AK, Agrawal PK, et al. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14:443-447.
8. Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035-1038.
9. Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80:1556-1559.
10. Liu JP, Manheimer E, Yang M. Herbal medicines for treating HIV infection and AIDS. Cochrane Database Syst Rev. 2005;(3):CD003937.
11. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471-480.
12. National Center for Complementary and Alternative Medicine Herbs at a glance: turmeric. Updated August 12, 2010. Available at: http://nccam.nih.gov/health/turmeric/. Accessed October 11, 2010.
Evidence-based answers from the Family Physicians Inquiries Network
What screening tests should you use to evaluate a man with low testosterone?
Obtain a repeat morning testosterone level, as well as levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin to help understand the cause of low testosterone when there is a lack of adequate empiric evidence to guide evaluation, advise the experts. When low or normal FSH and LH levels accompany low testosterone, evaluation of the pituitary gland is recommended.
Chromosomal studies are indicated in prepubertal males with low testosterone and elevated FSH and LH levels to assess for Klinefelter syndrome. Perform a semen analysis if fertility is an issue. Bone densitometry is indicated in men with chronic hypogonadism to identify increased risk of hip fracture (strength of all recommendations: C, consensus guidelines and disease-oriented evidence).
Diagnosis is often straightforward, but treatment…not so much
Pamela A. Williams, MD
Uniformed Services University of the Health Sciences, Bethesda, Md
The causes of low testosterone are diverse and vary across the life span (TABLE).1,2
Although screening tests are integral to the evaluation, a successful diagnostic approach must begin with a detailed history and physical exam. Clinical clues coupled with judiciously selected tests typically lead to a straightforward diagnosis.
The decision whether or not to treat a patient diagnosed with partial androgen deficiency of aging is often less clear, especially when clinical symptoms are minimal or absent. The benefits of testosterone replacement therapy are significant, but so are the potential risks. Shared decision making with the patient is key to this dilemma.
Evidence summary
Our search retrieved no randomized controlled clinical trials evaluating the screening tests required to work-up a male with low testosterone. We therefore examined 2 consensus guidelines, 9 review articles, and disease-oriented evidence. The recommendations discussed here are based primarily on consensus guidelines and disease-oriented evidence.
Hypogonadism increases with age
Hypogonadism is a common endocrinologic disorder in men. Advancing age, increased life expectancy, and a rising prevalence of obesity and type 2 diabetes may increase the occurrence of hypogonadism.3 Many cases result from partial androgen deficiency in the aging male, because testosterone levels decline an estimated 1% to 2% per year in adult men.1,3 A focused, cost-effective work-up will become ever more critical because an estimated 19% of men will be 65 years or older by 2050.4
TABLE
Causes of hypogonadism
| CLASS | LOCATION | CAUSES |
|---|---|---|
| Primary (low testosterone, elevated FSH) | Testes | Congenital Biosynthesis and chromosomal disorders (rare) Klinefelter syndrome (most common, 1:500-1000 males) |
| Acquired Chemotherapeutic agents Autoimmune disorders Aging Drugs Toxins (eg, alcohol) Infection Trauma Radiation Idiopathic causes | ||
| Secondary (low testosterone, normal or low FSH) | Pituitary gland | Congenital Kallmann syndrome (1:10,000 male births) Idiopathic causes Abnormal structural hormone defects |
| Acquired Chronic disease Drugs (eg, chronic opioids) Infection (eg, HIV) Trauma Tumors Idiopathic causes | ||
| Age-related (low testosterone, normal or elevated FSH) | Testes/hypothalamus | |
| Aging (common; 1%-2% per year after 65 years of age, 30%-70% at 60-80 years of age) | ||
| FSH, follicle-stimulating hormone; HIV, human immunodeficiency virus. Sources: Darby E et al1 and Badar F et al.2 | ||
Serum testosterone: The first-choice test
Serum testosterone measurements are considered the initial test of choice be-cause they’re reliable, inexpensive, and widely available. Testosterone levels vary from hour to hour and diurnally, so a repeat morning measurement is recommended to confirm subnormal levels.3,5
In some cases—including patients with obesity, type 2 diabetes, or hypothyroidism—the total testosterone level can be misleading; tests for free testosterone and sex hormone-binding globulin levels should be ordered. These tests can also help evaluate men with low-normal total testosterone levels (200-400 ng/dL).6,7
Is the patient pre- or postpubertal?
Assessment of low testosterone should distinguish between pre- and postpubertal males. In prepubertal males, chromosomal analysis is indicated because hypothalamic-pituitary-gonadal axis defects are common—especially Klinefelter syndrome (1 in 500 males).6,8
Men with very low testosterone levels (<150 ng/dL) or signs and symptoms suggesting pituitary pathology warrant pituitary imaging and measurement of thyroxine, cortisol, and prolactin levels.6 Both pre- and postpubertal males with low testosterone should have FSH, LH, and prolactin levels tested to differentiate primary from secondary hypogonadism.6,9
Be alert for hemochromatosis and low bone density
Order biopsy or ultrasound examination of testicular masses and iron studies if hemochromatosis is suspected. Hemochromatosis is the most common single gene disorder of Caucasian Americans (1 in 250-300 are homozygous; 1 in 10 are heterozygous) and is associated with hypogonadotrophic hypogonadism.5,10 In a series of 3 studies, 30% (26 of 89) of men with hemochromatosis had hypogonadism.11 The prevalence of hemochromatosis in males with hypogonadism hasn’t been reported.
Because chronic hypogonadism leads to low bone density and increased risk of fracture, baseline bone densitometry may be prudent.12 A chart review study of nursing home residents found that 66% of men with hip fractures and 20% of men with vertebral fractures had low testosterone.13 Notably, 50% of men in their 80s have testosterone levels in the hypogonadal range (<300 ng/dL), compared with 12% of men <50 years.1,14
Recommendations
Scant guidance is available concerning what screening tests to order for a male with low testosterone. The United States Preventive Services Task Force and Canadian Task Force on Preventive Health Care make no recommendations; the Cochrane collaboration has no reviews on the topic. The American Association of Clinical Endocrinologists’ (AACE) guidelines are based on expert opinion.3
The AACE consensus guideline used peer review for validation and didn’t specify the method used to assess the quality and strength of the evidence used to write the statement. The AACE guideline recommends a history and physical exam, obtaining repeat morning testosterone levels, prolactin, FSH, LH, bone densitometry, and a semen analysis if fertility is an issue.
In acquired hypogonadism, pituitary imaging is recommended along with thyroid, adrenal, and growth hormone axis testing. Prepubertal males should undergo chromosome analysis, and men with a suspected mass should have a testicular ultrasound examination.
1. Darby E, Anawalt BD. Male hypogonadism: an update on diagnosis and treatment. Treat Endocrinol. 2005;4:293-309.
2. Badar F, Mirmira V, Hemady N. Hypogonadism in men: underdiagnosed and undertreated. Resid Staff Physician. 2006;52(6):6-12.
3. American Association of Clinical Endocrinologists American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Prac. 2002;8:440-456.
4. Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med. 1998;158:1309-1314.
5. Spratt DI, O’Dea LS, Schoenfeld D, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988;254(5 Part 1):E658-E666.
6. Seftel AD. Male hypogonadism. Part I: epidemiology of hypogonadism. Int J Impot Res. 2006;18:115-120.
7. Sadovsky R, Dhindsa S, Margo K. Testosterone deficiency: which patients should you screen and treat? J Fam Pract. 2007;56(5 suppl):S1-S24.
8. Bhasin S, Jameson DL. Disorders of the testes and male reproductive system. Ch 340. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008.
9. US Census Bureau. US Interim projections by age, sex, race, and Hispanic origin: 2000-2050. Created March 18, 2004. Available at: www.census.gov/ipc/www/usinterimproj/. Accessed March 14, 2006.
10. Schrier SL, Bacon BR. Clinical manifestations of hereditary hemochromatosis. Up to Date [online database]. Version 15.1. Waltham, Mass: UpToDate; 2008.
11. Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724-731.
12. Kaufman JM, Johnell O, Abadie E, et al. Background for studies on the treatment of male osteoporosis: state of the art. Ann Rheum Dis. 2000;59:765-772.
13. Abbasi AA, Rudman D, Wilson CR, et al. Observations on nursing home residents with a history of hip fracture. Am J Med Sci. 1995;310:229-234.
14. Pietrangelo A. Hereditary hemochromatosis—a new look at an old disease. N Engl J Med. 2004;350:2383-2397.
Obtain a repeat morning testosterone level, as well as levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin to help understand the cause of low testosterone when there is a lack of adequate empiric evidence to guide evaluation, advise the experts. When low or normal FSH and LH levels accompany low testosterone, evaluation of the pituitary gland is recommended.
Chromosomal studies are indicated in prepubertal males with low testosterone and elevated FSH and LH levels to assess for Klinefelter syndrome. Perform a semen analysis if fertility is an issue. Bone densitometry is indicated in men with chronic hypogonadism to identify increased risk of hip fracture (strength of all recommendations: C, consensus guidelines and disease-oriented evidence).
Diagnosis is often straightforward, but treatment…not so much
Pamela A. Williams, MD
Uniformed Services University of the Health Sciences, Bethesda, Md
The causes of low testosterone are diverse and vary across the life span (TABLE).1,2
Although screening tests are integral to the evaluation, a successful diagnostic approach must begin with a detailed history and physical exam. Clinical clues coupled with judiciously selected tests typically lead to a straightforward diagnosis.
The decision whether or not to treat a patient diagnosed with partial androgen deficiency of aging is often less clear, especially when clinical symptoms are minimal or absent. The benefits of testosterone replacement therapy are significant, but so are the potential risks. Shared decision making with the patient is key to this dilemma.
Evidence summary
Our search retrieved no randomized controlled clinical trials evaluating the screening tests required to work-up a male with low testosterone. We therefore examined 2 consensus guidelines, 9 review articles, and disease-oriented evidence. The recommendations discussed here are based primarily on consensus guidelines and disease-oriented evidence.
Hypogonadism increases with age
Hypogonadism is a common endocrinologic disorder in men. Advancing age, increased life expectancy, and a rising prevalence of obesity and type 2 diabetes may increase the occurrence of hypogonadism.3 Many cases result from partial androgen deficiency in the aging male, because testosterone levels decline an estimated 1% to 2% per year in adult men.1,3 A focused, cost-effective work-up will become ever more critical because an estimated 19% of men will be 65 years or older by 2050.4
TABLE
Causes of hypogonadism
| CLASS | LOCATION | CAUSES |
|---|---|---|
| Primary (low testosterone, elevated FSH) | Testes | Congenital Biosynthesis and chromosomal disorders (rare) Klinefelter syndrome (most common, 1:500-1000 males) |
| Acquired Chemotherapeutic agents Autoimmune disorders Aging Drugs Toxins (eg, alcohol) Infection Trauma Radiation Idiopathic causes | ||
| Secondary (low testosterone, normal or low FSH) | Pituitary gland | Congenital Kallmann syndrome (1:10,000 male births) Idiopathic causes Abnormal structural hormone defects |
| Acquired Chronic disease Drugs (eg, chronic opioids) Infection (eg, HIV) Trauma Tumors Idiopathic causes | ||
| Age-related (low testosterone, normal or elevated FSH) | Testes/hypothalamus | |
| Aging (common; 1%-2% per year after 65 years of age, 30%-70% at 60-80 years of age) | ||
| FSH, follicle-stimulating hormone; HIV, human immunodeficiency virus. Sources: Darby E et al1 and Badar F et al.2 | ||
Serum testosterone: The first-choice test
Serum testosterone measurements are considered the initial test of choice be-cause they’re reliable, inexpensive, and widely available. Testosterone levels vary from hour to hour and diurnally, so a repeat morning measurement is recommended to confirm subnormal levels.3,5
In some cases—including patients with obesity, type 2 diabetes, or hypothyroidism—the total testosterone level can be misleading; tests for free testosterone and sex hormone-binding globulin levels should be ordered. These tests can also help evaluate men with low-normal total testosterone levels (200-400 ng/dL).6,7
Is the patient pre- or postpubertal?
Assessment of low testosterone should distinguish between pre- and postpubertal males. In prepubertal males, chromosomal analysis is indicated because hypothalamic-pituitary-gonadal axis defects are common—especially Klinefelter syndrome (1 in 500 males).6,8
Men with very low testosterone levels (<150 ng/dL) or signs and symptoms suggesting pituitary pathology warrant pituitary imaging and measurement of thyroxine, cortisol, and prolactin levels.6 Both pre- and postpubertal males with low testosterone should have FSH, LH, and prolactin levels tested to differentiate primary from secondary hypogonadism.6,9
Be alert for hemochromatosis and low bone density
Order biopsy or ultrasound examination of testicular masses and iron studies if hemochromatosis is suspected. Hemochromatosis is the most common single gene disorder of Caucasian Americans (1 in 250-300 are homozygous; 1 in 10 are heterozygous) and is associated with hypogonadotrophic hypogonadism.5,10 In a series of 3 studies, 30% (26 of 89) of men with hemochromatosis had hypogonadism.11 The prevalence of hemochromatosis in males with hypogonadism hasn’t been reported.
Because chronic hypogonadism leads to low bone density and increased risk of fracture, baseline bone densitometry may be prudent.12 A chart review study of nursing home residents found that 66% of men with hip fractures and 20% of men with vertebral fractures had low testosterone.13 Notably, 50% of men in their 80s have testosterone levels in the hypogonadal range (<300 ng/dL), compared with 12% of men <50 years.1,14
Recommendations
Scant guidance is available concerning what screening tests to order for a male with low testosterone. The United States Preventive Services Task Force and Canadian Task Force on Preventive Health Care make no recommendations; the Cochrane collaboration has no reviews on the topic. The American Association of Clinical Endocrinologists’ (AACE) guidelines are based on expert opinion.3
The AACE consensus guideline used peer review for validation and didn’t specify the method used to assess the quality and strength of the evidence used to write the statement. The AACE guideline recommends a history and physical exam, obtaining repeat morning testosterone levels, prolactin, FSH, LH, bone densitometry, and a semen analysis if fertility is an issue.
In acquired hypogonadism, pituitary imaging is recommended along with thyroid, adrenal, and growth hormone axis testing. Prepubertal males should undergo chromosome analysis, and men with a suspected mass should have a testicular ultrasound examination.
Obtain a repeat morning testosterone level, as well as levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin to help understand the cause of low testosterone when there is a lack of adequate empiric evidence to guide evaluation, advise the experts. When low or normal FSH and LH levels accompany low testosterone, evaluation of the pituitary gland is recommended.
Chromosomal studies are indicated in prepubertal males with low testosterone and elevated FSH and LH levels to assess for Klinefelter syndrome. Perform a semen analysis if fertility is an issue. Bone densitometry is indicated in men with chronic hypogonadism to identify increased risk of hip fracture (strength of all recommendations: C, consensus guidelines and disease-oriented evidence).
Diagnosis is often straightforward, but treatment…not so much
Pamela A. Williams, MD
Uniformed Services University of the Health Sciences, Bethesda, Md
The causes of low testosterone are diverse and vary across the life span (TABLE).1,2
Although screening tests are integral to the evaluation, a successful diagnostic approach must begin with a detailed history and physical exam. Clinical clues coupled with judiciously selected tests typically lead to a straightforward diagnosis.
The decision whether or not to treat a patient diagnosed with partial androgen deficiency of aging is often less clear, especially when clinical symptoms are minimal or absent. The benefits of testosterone replacement therapy are significant, but so are the potential risks. Shared decision making with the patient is key to this dilemma.
Evidence summary
Our search retrieved no randomized controlled clinical trials evaluating the screening tests required to work-up a male with low testosterone. We therefore examined 2 consensus guidelines, 9 review articles, and disease-oriented evidence. The recommendations discussed here are based primarily on consensus guidelines and disease-oriented evidence.
Hypogonadism increases with age
Hypogonadism is a common endocrinologic disorder in men. Advancing age, increased life expectancy, and a rising prevalence of obesity and type 2 diabetes may increase the occurrence of hypogonadism.3 Many cases result from partial androgen deficiency in the aging male, because testosterone levels decline an estimated 1% to 2% per year in adult men.1,3 A focused, cost-effective work-up will become ever more critical because an estimated 19% of men will be 65 years or older by 2050.4
TABLE
Causes of hypogonadism
| CLASS | LOCATION | CAUSES |
|---|---|---|
| Primary (low testosterone, elevated FSH) | Testes | Congenital Biosynthesis and chromosomal disorders (rare) Klinefelter syndrome (most common, 1:500-1000 males) |
| Acquired Chemotherapeutic agents Autoimmune disorders Aging Drugs Toxins (eg, alcohol) Infection Trauma Radiation Idiopathic causes | ||
| Secondary (low testosterone, normal or low FSH) | Pituitary gland | Congenital Kallmann syndrome (1:10,000 male births) Idiopathic causes Abnormal structural hormone defects |
| Acquired Chronic disease Drugs (eg, chronic opioids) Infection (eg, HIV) Trauma Tumors Idiopathic causes | ||
| Age-related (low testosterone, normal or elevated FSH) | Testes/hypothalamus | |
| Aging (common; 1%-2% per year after 65 years of age, 30%-70% at 60-80 years of age) | ||
| FSH, follicle-stimulating hormone; HIV, human immunodeficiency virus. Sources: Darby E et al1 and Badar F et al.2 | ||
Serum testosterone: The first-choice test
Serum testosterone measurements are considered the initial test of choice be-cause they’re reliable, inexpensive, and widely available. Testosterone levels vary from hour to hour and diurnally, so a repeat morning measurement is recommended to confirm subnormal levels.3,5
In some cases—including patients with obesity, type 2 diabetes, or hypothyroidism—the total testosterone level can be misleading; tests for free testosterone and sex hormone-binding globulin levels should be ordered. These tests can also help evaluate men with low-normal total testosterone levels (200-400 ng/dL).6,7
Is the patient pre- or postpubertal?
Assessment of low testosterone should distinguish between pre- and postpubertal males. In prepubertal males, chromosomal analysis is indicated because hypothalamic-pituitary-gonadal axis defects are common—especially Klinefelter syndrome (1 in 500 males).6,8
Men with very low testosterone levels (<150 ng/dL) or signs and symptoms suggesting pituitary pathology warrant pituitary imaging and measurement of thyroxine, cortisol, and prolactin levels.6 Both pre- and postpubertal males with low testosterone should have FSH, LH, and prolactin levels tested to differentiate primary from secondary hypogonadism.6,9
Be alert for hemochromatosis and low bone density
Order biopsy or ultrasound examination of testicular masses and iron studies if hemochromatosis is suspected. Hemochromatosis is the most common single gene disorder of Caucasian Americans (1 in 250-300 are homozygous; 1 in 10 are heterozygous) and is associated with hypogonadotrophic hypogonadism.5,10 In a series of 3 studies, 30% (26 of 89) of men with hemochromatosis had hypogonadism.11 The prevalence of hemochromatosis in males with hypogonadism hasn’t been reported.
Because chronic hypogonadism leads to low bone density and increased risk of fracture, baseline bone densitometry may be prudent.12 A chart review study of nursing home residents found that 66% of men with hip fractures and 20% of men with vertebral fractures had low testosterone.13 Notably, 50% of men in their 80s have testosterone levels in the hypogonadal range (<300 ng/dL), compared with 12% of men <50 years.1,14
Recommendations
Scant guidance is available concerning what screening tests to order for a male with low testosterone. The United States Preventive Services Task Force and Canadian Task Force on Preventive Health Care make no recommendations; the Cochrane collaboration has no reviews on the topic. The American Association of Clinical Endocrinologists’ (AACE) guidelines are based on expert opinion.3
The AACE consensus guideline used peer review for validation and didn’t specify the method used to assess the quality and strength of the evidence used to write the statement. The AACE guideline recommends a history and physical exam, obtaining repeat morning testosterone levels, prolactin, FSH, LH, bone densitometry, and a semen analysis if fertility is an issue.
In acquired hypogonadism, pituitary imaging is recommended along with thyroid, adrenal, and growth hormone axis testing. Prepubertal males should undergo chromosome analysis, and men with a suspected mass should have a testicular ultrasound examination.
1. Darby E, Anawalt BD. Male hypogonadism: an update on diagnosis and treatment. Treat Endocrinol. 2005;4:293-309.
2. Badar F, Mirmira V, Hemady N. Hypogonadism in men: underdiagnosed and undertreated. Resid Staff Physician. 2006;52(6):6-12.
3. American Association of Clinical Endocrinologists American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Prac. 2002;8:440-456.
4. Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med. 1998;158:1309-1314.
5. Spratt DI, O’Dea LS, Schoenfeld D, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988;254(5 Part 1):E658-E666.
6. Seftel AD. Male hypogonadism. Part I: epidemiology of hypogonadism. Int J Impot Res. 2006;18:115-120.
7. Sadovsky R, Dhindsa S, Margo K. Testosterone deficiency: which patients should you screen and treat? J Fam Pract. 2007;56(5 suppl):S1-S24.
8. Bhasin S, Jameson DL. Disorders of the testes and male reproductive system. Ch 340. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008.
9. US Census Bureau. US Interim projections by age, sex, race, and Hispanic origin: 2000-2050. Created March 18, 2004. Available at: www.census.gov/ipc/www/usinterimproj/. Accessed March 14, 2006.
10. Schrier SL, Bacon BR. Clinical manifestations of hereditary hemochromatosis. Up to Date [online database]. Version 15.1. Waltham, Mass: UpToDate; 2008.
11. Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724-731.
12. Kaufman JM, Johnell O, Abadie E, et al. Background for studies on the treatment of male osteoporosis: state of the art. Ann Rheum Dis. 2000;59:765-772.
13. Abbasi AA, Rudman D, Wilson CR, et al. Observations on nursing home residents with a history of hip fracture. Am J Med Sci. 1995;310:229-234.
14. Pietrangelo A. Hereditary hemochromatosis—a new look at an old disease. N Engl J Med. 2004;350:2383-2397.
1. Darby E, Anawalt BD. Male hypogonadism: an update on diagnosis and treatment. Treat Endocrinol. 2005;4:293-309.
2. Badar F, Mirmira V, Hemady N. Hypogonadism in men: underdiagnosed and undertreated. Resid Staff Physician. 2006;52(6):6-12.
3. American Association of Clinical Endocrinologists American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Prac. 2002;8:440-456.
4. Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med. 1998;158:1309-1314.
5. Spratt DI, O’Dea LS, Schoenfeld D, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988;254(5 Part 1):E658-E666.
6. Seftel AD. Male hypogonadism. Part I: epidemiology of hypogonadism. Int J Impot Res. 2006;18:115-120.
7. Sadovsky R, Dhindsa S, Margo K. Testosterone deficiency: which patients should you screen and treat? J Fam Pract. 2007;56(5 suppl):S1-S24.
8. Bhasin S, Jameson DL. Disorders of the testes and male reproductive system. Ch 340. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008.
9. US Census Bureau. US Interim projections by age, sex, race, and Hispanic origin: 2000-2050. Created March 18, 2004. Available at: www.census.gov/ipc/www/usinterimproj/. Accessed March 14, 2006.
10. Schrier SL, Bacon BR. Clinical manifestations of hereditary hemochromatosis. Up to Date [online database]. Version 15.1. Waltham, Mass: UpToDate; 2008.
11. Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724-731.
12. Kaufman JM, Johnell O, Abadie E, et al. Background for studies on the treatment of male osteoporosis: state of the art. Ann Rheum Dis. 2000;59:765-772.
13. Abbasi AA, Rudman D, Wilson CR, et al. Observations on nursing home residents with a history of hip fracture. Am J Med Sci. 1995;310:229-234.
14. Pietrangelo A. Hereditary hemochromatosis—a new look at an old disease. N Engl J Med. 2004;350:2383-2397.
Evidence-based answers from the Family Physicians Inquiries Network
When should you treat scabies empirically?
Empirically treat patients when they have pruritus and lesions typical of scabies in at least 2 places—even if there is no known household contact diagnosed with scabies, and even if the diagnosis cannot be confirmed by light microscopy (strength of recommendation [SOR]: B, based on a single large cohort study). Also give empiric treatment to all sexual and household contacts of anyone diagnosed with scabies (SOR: C, based on expert opinion).
In institutional settings such as hospitals, nursing homes, or residential facilities, treat the entire at-risk population empirically to prevent epidemics (SOR: C, based on expert opinion). In hospital settings, give empiric treatment to health care workers with skin exposure to patients with scabies (SOR: B, based on case-control study).
Treating empirically saves money (and unnecessary itching)
Barbara Walker, DO
New Hanover regional Medical Center residency in Family Medicine, University of North Carolina, Wilmington
During my medical training and years in the military, I have seen patients who suffered prolonged itching because they had no microscopic confirmation of scabies, but who cleared quickly with treatment after a skin biopsy identified scabies. This has given me a “short fuse” for treating empirically in my own clinics.
Though I always encourage the residents to do a scraping—since the microscopic confirmation is one of those “oh, wow!” findings when it is positive (FIGURE)—it is reassuring to know that evidence exists for opting to treat without confirmation. It also saves the patient the cost of the skin scraping and microscopy—important for the increasing numbers of cash-paying, uninsured patients.
Permethrin is relatively safe (rated category B in pregnancy), usually affordable, and well-tolerated; the hardest part of the empiric treatment may be the emotional impact on the patient who is told his skin has a “parasitic infestation.” (I’m itching at the thought!)
Evidence summary
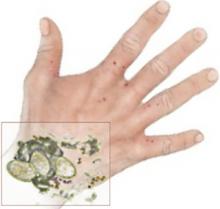
Clinical diagnosis of scabies begins with pruritus, typical lesions in a distribution consistent with scabies—finger webs, wrists, axillae, elbows, buttocks, genitalia of men, breasts of women—and possible exposure. Clinical diagnosis can be confirmed by skin scrapings from characteristic lesions, such as burrows. When these scrapings are examined under light microscopy, they can show mites, eggs, or feces from the mites (FIGURE). However, this technique depends greatly on operator experience and skill, and a lack of light microscopy findings does not rule out scabies.1
The only study we found that investigated the sensitivity of clinical features in diagnosing scabies was done in sub-Saharan Africa.2 In this study, the presence of diffuse itching, plus lesions in at least 2 locations typical with scabies or a household member with itch, had 100% sensitivity and 96.9% specificity for scabies infection. This study used the evaluation of a dermatologist as a gold standard. The authors propose that treatment based on clinical findings with or without microscopic confirmation is appropriate; however, it is not clear how these data translate to a primary care population with a lower prevalence of scabies.
FIGURE
The “Oh, wow!” test
I always encourage residents to do a scraping, since the microscopic confirmation is one of those “oh, wow!” findings when it is positive.
It is reassuring to know that evidence exists for opting to treat without confirmation. It also saves the patient the cost of the skin scraping and microscopy—important for the increasing numbers of cash-paying, uninsured patients.
—Barbara Walker, Do
Long stretches without symptoms play role in treatment
To date, no controlled trials address whether empiric treatment of asymptomatic contacts or family members of those with scabies decreases its spread. However, it is known that an initial infestation with scabies will not lead to pruritus for up to 4 to 6 weeks.1 Asymptomatic contacts can be infected with scabies, and can transmit this infection to others before symptoms even occur.
Given the long period of asymptomatic infestation, prevention of epidemics in institutions such as hospitals, nursing homes, and residential facilities is of particular importance. One case-control study, performed at a large tertiary-care teaching hospital, demonstrated that health care workers on a service having a patient with undiagnosed scabies were 5.3 times more likely to develop a pruritic rash than those in other units.3
Health care workers with more skin-to-skin contact with the patients (nurses, nursing students, and physical therapists) were 4.5 times more likely to develop scabies compared with those in less physical contact (physicians, medical students, and occupational therapists). Among the symptomatic health care workers, 17% of their household contacts developed scabies, too.
Permethrin vs lindane? Which is better?
A 2000 Cochrane review, updated in 2002, concluded that permethrin was superior to lindane for topical treatment of scabies.4,5 Combining 4 trials with 718 patients, permethrin 5% appeared better than lindane 1% (odds ratio=0.66; 95% confidence interval, 0.46–0.95). However, there was significant heterogeneity between the studies, and the largest trial (n=467) found no difference.
Oral ivermectin, though costly, is an effective alternative for those who do not tolerate topical treatment. See the TABLE for a summary of treatment recommendations.
TABLE
Recommended treatment for scabies infection
| DIAGNOSIS | RECOMMENDED THERAPY | SOR |
|---|---|---|
| High-risk individual with exposure | Permethrin 5% topical solution (single overnight application) | A |
| Typical scabies infection | Permethrin 5% topical solution (single overnight application) | A |
| Crusted (Norwegian) scabies | oral ivermectin 200 mcg/kg single dose repeated in 14 days | B |
| Scabies in patient with HIV | Oral ivermectin 200 mcg/kg single dose repeated in 14 days | B |
| Data taken from 2000 Cochrane Systematic Review.4 and 2002 update5 | ||
| SOR, strength of recommendation. | ||
Recommendations from others
Guidelines released by the Centers for Disease Control and Prevention in 2002 regarding the treatment of sexually transmitted diseases state that both sexual and close personal or household contacts of patients diagnosed with scabies within the preceding month should be examined and treated.6
Another guideline, developed by the British Association of Sexual Health and HIV, recommends empiric treatment of sexual, household, and institutional contacts of those with scabies. This guideline recommends treating those who were in contact with the scabies patient within 2 months of his diagnosis; this time frame, though, is arbitrary.7 No evidence grading was given for these recommendations, which are based on expert opinion.
1. Orion E, Marcos B, Davidovici B, Wolf R. Itch and scratch: scabies and pediculosis. Clin Dermatol 2006;24:168-175.
2. Mahe A, Faye O, N’Diaye HT, et al. Definition of an algorithm for the management of common skin diseases at primary health care level in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2005;99:39-47.
3. Obasanjo OO, Wu P, Conlon M, et al. An outbreak of scabies in a teaching hospital: lessons learned. Infect Control Hosp Epidemiol 2001;22:13-18.
4. Walker GJA, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev 2000;(3):CD000320.
5. Walker G, Johnstone P. Scabies. Clin Evid 2002;8:1745-1752.
6. Ectoparasitic infections. Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002;51:6709.
7. Scott G. 2002 National Guideline on the management of scabies. Clinical Effectiveness Group. Developed by British Association of Sexual Health and HIV–Medical Specialty Society 2002.
Empirically treat patients when they have pruritus and lesions typical of scabies in at least 2 places—even if there is no known household contact diagnosed with scabies, and even if the diagnosis cannot be confirmed by light microscopy (strength of recommendation [SOR]: B, based on a single large cohort study). Also give empiric treatment to all sexual and household contacts of anyone diagnosed with scabies (SOR: C, based on expert opinion).
In institutional settings such as hospitals, nursing homes, or residential facilities, treat the entire at-risk population empirically to prevent epidemics (SOR: C, based on expert opinion). In hospital settings, give empiric treatment to health care workers with skin exposure to patients with scabies (SOR: B, based on case-control study).
Treating empirically saves money (and unnecessary itching)
Barbara Walker, DO
New Hanover regional Medical Center residency in Family Medicine, University of North Carolina, Wilmington
During my medical training and years in the military, I have seen patients who suffered prolonged itching because they had no microscopic confirmation of scabies, but who cleared quickly with treatment after a skin biopsy identified scabies. This has given me a “short fuse” for treating empirically in my own clinics.
Though I always encourage the residents to do a scraping—since the microscopic confirmation is one of those “oh, wow!” findings when it is positive (FIGURE)—it is reassuring to know that evidence exists for opting to treat without confirmation. It also saves the patient the cost of the skin scraping and microscopy—important for the increasing numbers of cash-paying, uninsured patients.
Permethrin is relatively safe (rated category B in pregnancy), usually affordable, and well-tolerated; the hardest part of the empiric treatment may be the emotional impact on the patient who is told his skin has a “parasitic infestation.” (I’m itching at the thought!)
Evidence summary
Clinical diagnosis of scabies begins with pruritus, typical lesions in a distribution consistent with scabies—finger webs, wrists, axillae, elbows, buttocks, genitalia of men, breasts of women—and possible exposure. Clinical diagnosis can be confirmed by skin scrapings from characteristic lesions, such as burrows. When these scrapings are examined under light microscopy, they can show mites, eggs, or feces from the mites (FIGURE). However, this technique depends greatly on operator experience and skill, and a lack of light microscopy findings does not rule out scabies.1
The only study we found that investigated the sensitivity of clinical features in diagnosing scabies was done in sub-Saharan Africa.2 In this study, the presence of diffuse itching, plus lesions in at least 2 locations typical with scabies or a household member with itch, had 100% sensitivity and 96.9% specificity for scabies infection. This study used the evaluation of a dermatologist as a gold standard. The authors propose that treatment based on clinical findings with or without microscopic confirmation is appropriate; however, it is not clear how these data translate to a primary care population with a lower prevalence of scabies.
FIGURE
The “Oh, wow!” test
I always encourage residents to do a scraping, since the microscopic confirmation is one of those “oh, wow!” findings when it is positive.
It is reassuring to know that evidence exists for opting to treat without confirmation. It also saves the patient the cost of the skin scraping and microscopy—important for the increasing numbers of cash-paying, uninsured patients.
—Barbara Walker, Do
Long stretches without symptoms play role in treatment
To date, no controlled trials address whether empiric treatment of asymptomatic contacts or family members of those with scabies decreases its spread. However, it is known that an initial infestation with scabies will not lead to pruritus for up to 4 to 6 weeks.1 Asymptomatic contacts can be infected with scabies, and can transmit this infection to others before symptoms even occur.
Given the long period of asymptomatic infestation, prevention of epidemics in institutions such as hospitals, nursing homes, and residential facilities is of particular importance. One case-control study, performed at a large tertiary-care teaching hospital, demonstrated that health care workers on a service having a patient with undiagnosed scabies were 5.3 times more likely to develop a pruritic rash than those in other units.3
Health care workers with more skin-to-skin contact with the patients (nurses, nursing students, and physical therapists) were 4.5 times more likely to develop scabies compared with those in less physical contact (physicians, medical students, and occupational therapists). Among the symptomatic health care workers, 17% of their household contacts developed scabies, too.
Permethrin vs lindane? Which is better?
A 2000 Cochrane review, updated in 2002, concluded that permethrin was superior to lindane for topical treatment of scabies.4,5 Combining 4 trials with 718 patients, permethrin 5% appeared better than lindane 1% (odds ratio=0.66; 95% confidence interval, 0.46–0.95). However, there was significant heterogeneity between the studies, and the largest trial (n=467) found no difference.
Oral ivermectin, though costly, is an effective alternative for those who do not tolerate topical treatment. See the TABLE for a summary of treatment recommendations.
TABLE
Recommended treatment for scabies infection
| DIAGNOSIS | RECOMMENDED THERAPY | SOR |
|---|---|---|
| High-risk individual with exposure | Permethrin 5% topical solution (single overnight application) | A |
| Typical scabies infection | Permethrin 5% topical solution (single overnight application) | A |
| Crusted (Norwegian) scabies | oral ivermectin 200 mcg/kg single dose repeated in 14 days | B |
| Scabies in patient with HIV | Oral ivermectin 200 mcg/kg single dose repeated in 14 days | B |
| Data taken from 2000 Cochrane Systematic Review.4 and 2002 update5 | ||
| SOR, strength of recommendation. | ||
Recommendations from others
Guidelines released by the Centers for Disease Control and Prevention in 2002 regarding the treatment of sexually transmitted diseases state that both sexual and close personal or household contacts of patients diagnosed with scabies within the preceding month should be examined and treated.6
Another guideline, developed by the British Association of Sexual Health and HIV, recommends empiric treatment of sexual, household, and institutional contacts of those with scabies. This guideline recommends treating those who were in contact with the scabies patient within 2 months of his diagnosis; this time frame, though, is arbitrary.7 No evidence grading was given for these recommendations, which are based on expert opinion.
Empirically treat patients when they have pruritus and lesions typical of scabies in at least 2 places—even if there is no known household contact diagnosed with scabies, and even if the diagnosis cannot be confirmed by light microscopy (strength of recommendation [SOR]: B, based on a single large cohort study). Also give empiric treatment to all sexual and household contacts of anyone diagnosed with scabies (SOR: C, based on expert opinion).
In institutional settings such as hospitals, nursing homes, or residential facilities, treat the entire at-risk population empirically to prevent epidemics (SOR: C, based on expert opinion). In hospital settings, give empiric treatment to health care workers with skin exposure to patients with scabies (SOR: B, based on case-control study).
Treating empirically saves money (and unnecessary itching)
Barbara Walker, DO
New Hanover regional Medical Center residency in Family Medicine, University of North Carolina, Wilmington
During my medical training and years in the military, I have seen patients who suffered prolonged itching because they had no microscopic confirmation of scabies, but who cleared quickly with treatment after a skin biopsy identified scabies. This has given me a “short fuse” for treating empirically in my own clinics.
Though I always encourage the residents to do a scraping—since the microscopic confirmation is one of those “oh, wow!” findings when it is positive (FIGURE)—it is reassuring to know that evidence exists for opting to treat without confirmation. It also saves the patient the cost of the skin scraping and microscopy—important for the increasing numbers of cash-paying, uninsured patients.
Permethrin is relatively safe (rated category B in pregnancy), usually affordable, and well-tolerated; the hardest part of the empiric treatment may be the emotional impact on the patient who is told his skin has a “parasitic infestation.” (I’m itching at the thought!)
Evidence summary
Clinical diagnosis of scabies begins with pruritus, typical lesions in a distribution consistent with scabies—finger webs, wrists, axillae, elbows, buttocks, genitalia of men, breasts of women—and possible exposure. Clinical diagnosis can be confirmed by skin scrapings from characteristic lesions, such as burrows. When these scrapings are examined under light microscopy, they can show mites, eggs, or feces from the mites (FIGURE). However, this technique depends greatly on operator experience and skill, and a lack of light microscopy findings does not rule out scabies.1
The only study we found that investigated the sensitivity of clinical features in diagnosing scabies was done in sub-Saharan Africa.2 In this study, the presence of diffuse itching, plus lesions in at least 2 locations typical with scabies or a household member with itch, had 100% sensitivity and 96.9% specificity for scabies infection. This study used the evaluation of a dermatologist as a gold standard. The authors propose that treatment based on clinical findings with or without microscopic confirmation is appropriate; however, it is not clear how these data translate to a primary care population with a lower prevalence of scabies.
FIGURE
The “Oh, wow!” test
I always encourage residents to do a scraping, since the microscopic confirmation is one of those “oh, wow!” findings when it is positive.
It is reassuring to know that evidence exists for opting to treat without confirmation. It also saves the patient the cost of the skin scraping and microscopy—important for the increasing numbers of cash-paying, uninsured patients.
—Barbara Walker, Do
Long stretches without symptoms play role in treatment
To date, no controlled trials address whether empiric treatment of asymptomatic contacts or family members of those with scabies decreases its spread. However, it is known that an initial infestation with scabies will not lead to pruritus for up to 4 to 6 weeks.1 Asymptomatic contacts can be infected with scabies, and can transmit this infection to others before symptoms even occur.
Given the long period of asymptomatic infestation, prevention of epidemics in institutions such as hospitals, nursing homes, and residential facilities is of particular importance. One case-control study, performed at a large tertiary-care teaching hospital, demonstrated that health care workers on a service having a patient with undiagnosed scabies were 5.3 times more likely to develop a pruritic rash than those in other units.3
Health care workers with more skin-to-skin contact with the patients (nurses, nursing students, and physical therapists) were 4.5 times more likely to develop scabies compared with those in less physical contact (physicians, medical students, and occupational therapists). Among the symptomatic health care workers, 17% of their household contacts developed scabies, too.
Permethrin vs lindane? Which is better?
A 2000 Cochrane review, updated in 2002, concluded that permethrin was superior to lindane for topical treatment of scabies.4,5 Combining 4 trials with 718 patients, permethrin 5% appeared better than lindane 1% (odds ratio=0.66; 95% confidence interval, 0.46–0.95). However, there was significant heterogeneity between the studies, and the largest trial (n=467) found no difference.
Oral ivermectin, though costly, is an effective alternative for those who do not tolerate topical treatment. See the TABLE for a summary of treatment recommendations.
TABLE
Recommended treatment for scabies infection
| DIAGNOSIS | RECOMMENDED THERAPY | SOR |
|---|---|---|
| High-risk individual with exposure | Permethrin 5% topical solution (single overnight application) | A |
| Typical scabies infection | Permethrin 5% topical solution (single overnight application) | A |
| Crusted (Norwegian) scabies | oral ivermectin 200 mcg/kg single dose repeated in 14 days | B |
| Scabies in patient with HIV | Oral ivermectin 200 mcg/kg single dose repeated in 14 days | B |
| Data taken from 2000 Cochrane Systematic Review.4 and 2002 update5 | ||
| SOR, strength of recommendation. | ||
Recommendations from others
Guidelines released by the Centers for Disease Control and Prevention in 2002 regarding the treatment of sexually transmitted diseases state that both sexual and close personal or household contacts of patients diagnosed with scabies within the preceding month should be examined and treated.6
Another guideline, developed by the British Association of Sexual Health and HIV, recommends empiric treatment of sexual, household, and institutional contacts of those with scabies. This guideline recommends treating those who were in contact with the scabies patient within 2 months of his diagnosis; this time frame, though, is arbitrary.7 No evidence grading was given for these recommendations, which are based on expert opinion.
1. Orion E, Marcos B, Davidovici B, Wolf R. Itch and scratch: scabies and pediculosis. Clin Dermatol 2006;24:168-175.
2. Mahe A, Faye O, N’Diaye HT, et al. Definition of an algorithm for the management of common skin diseases at primary health care level in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2005;99:39-47.
3. Obasanjo OO, Wu P, Conlon M, et al. An outbreak of scabies in a teaching hospital: lessons learned. Infect Control Hosp Epidemiol 2001;22:13-18.
4. Walker GJA, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev 2000;(3):CD000320.
5. Walker G, Johnstone P. Scabies. Clin Evid 2002;8:1745-1752.
6. Ectoparasitic infections. Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002;51:6709.
7. Scott G. 2002 National Guideline on the management of scabies. Clinical Effectiveness Group. Developed by British Association of Sexual Health and HIV–Medical Specialty Society 2002.
1. Orion E, Marcos B, Davidovici B, Wolf R. Itch and scratch: scabies and pediculosis. Clin Dermatol 2006;24:168-175.
2. Mahe A, Faye O, N’Diaye HT, et al. Definition of an algorithm for the management of common skin diseases at primary health care level in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2005;99:39-47.
3. Obasanjo OO, Wu P, Conlon M, et al. An outbreak of scabies in a teaching hospital: lessons learned. Infect Control Hosp Epidemiol 2001;22:13-18.
4. Walker GJA, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev 2000;(3):CD000320.
5. Walker G, Johnstone P. Scabies. Clin Evid 2002;8:1745-1752.
6. Ectoparasitic infections. Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002;51:6709.
7. Scott G. 2002 National Guideline on the management of scabies. Clinical Effectiveness Group. Developed by British Association of Sexual Health and HIV–Medical Specialty Society 2002.
Evidence-based answers from the Family Physicians Inquiries Network
Are any alternative therapies effective in treating asthma?
Yes, some are. Acupuncture relieves subjective symptoms of asthma and reduces medication use in mild to moderate asthma (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials [RCTs] of variable quality). Herbal medications, such as Ginkgo biloba, appear to improve lung function, while herbs such as Tylophora indica and Tsumura saiboku-to may decrease asthma symptoms (SOR: B, based on systematic review of RCTs with poor methodology). No evidence, however, supports the use of room air ionizers, manual therapy, homeopathy, or mind-body therapy for treatment of asthma (SOR: A, based on systematic reviews and meta-analyses of RCTs and individual RCTs).
Though this research is interesting, we should adhere to current guidelines
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Guidelines for the diagnosis and management of asthma are widely disseminated by the National Asthma Education and Prevention Program through its Expert Panel Reports (updated in 2002).1 Nevertheless, nearly 500,000 hospitalizations, 2 million emergency department visits, and 5000 deaths were reported annually in the US among those who have asthma.2 Furthermore, a significant difference in asthma prevalence, health care use, and mortality was found among different ethnic groups.1
Poor patient understanding of asthma control, nonadherence to medication regimens, cultural beliefs, and disparity of access to the health care system, together with physicians’ lack of close monitoring and inadequate compliance with national asthma guidelines, contribute to suboptimal control of chronic asthma. Family physicians must guide and empower their patients with the knowledge and responsibility of how to manage their asthma. For now, we should adhere to current national guidelines of management of asthma and avoid routine recommendation of any complimentary alternative treatments.
Evidence summary
Although complementary and alternative medicine (CAM) therapies are widely used, the overall body of research into CAM for asthma is still small and of limited quality. Interpreting the research is hampered by lack of standardized therapeutic approaches, lack of accepted methods for appropriate trials, and the fact that many CAM treatments are used as part of a multi-pronged, individualized approach to treatment in actual practice. Our search found 4 good-quality systematic reviews of RCTs, 1 good-quality systematic review of randomized trials, and 1 small additional pilot RCT of various CAM treatments for asthma.
Acupuncture and herbals provide some benefit
While a Cochrane review of 11 RCTs with variable trial quality and a total of 324 participants found that acupuncture had no significant effect on pulmonary function or global assessment of well-being, the review noted that some studies reported significant positive changes in daily symptoms, reductions in medication use, and improved quality of life. This suggests that some patients with mild to moderate asthma may benefit from acupuncture.3 In 1 RCT, improvement in general well-being was reported by 79% of 38 patients receiving acupuncture compared with 47% of 18 patients in the control group.4
When it comes to herbal remedies, a good-quality systematic review5 of 17 trials, with overall poor methodological quality and a total of 1445 participants, reported significant improvements in clinically relevant measures with 6 different herbal medicines.
- Ginkgo biloba liquor increased forced expiratory volume in 1 second (FEV1) by 10% at 4 weeks and by a more clinically relevant 15% at 8 weeks (significantly greater than placebo, P<.05).
- Invigorating Kidney for Preventing Asthma (IKPA) tablets increased FEV1 by 30% at 3 months compared with 17% in controls (P<.05).
- Wenyang Tonglulo Mixture (WTM) improved FEV1 by 30% at 8 weeks compared with a 16% increase in the control group using oral salbutamol and inhaled beclomethasone (P<.05).
- Dried ivy extract, thought to work as both a secretolytic and bronchospasmolytic, reduced airway resistance in children by 23.6% compared with placebo (P=.036).
- Tylophora indica (a rare herb also known as Indian ipecac) provided significant improvement in nocturnal dyspnea when compared with controls (P<.01) in a study that relied on patients’ symptom diaries.
- Tsumura saiboku-to (TJ-96) provided patients in one RCT with significant, but unspecified, asthma symptom relief when compared with those in a control group (P<.01).5
Other therapies didn’t quite make the grade
Homeopathy. A Cochrane review of 6 RCTs of mixed quality, with a total of 556 patients, concluded the evidence is insufficient to evaluate the possible role of homeopathy for the treatment of asthma, due to heterogeneity of interventions, patient populations, and outcome assessments. Each study evaluated a different homeopathic remedy, making any overall assessment difficult.
The review notes there have been only limited attempts to study a complete “package of care,” which includes the in-depth, one-on-one consultation, treatment, and follow-up that characterizes most homeopathic treatment in practice.6
Room air ionizers. A Cochrane review of 6 good-quality trials with a total of 106 participants reported no significant effect of room air ionizers on pulmonary function measures, symptoms, or medication use.7
Manual therapy. A Cochrane review8 of 3 moderate- to poor-quality RCTs with 156 participants reported no significant effect of chiropractic spinal manipulation (2 trials) or massage therapy (1 trial) on lung function, asthma symptoms, or medication use.
Mind-body therapy. A pilot RCT9 with 33 adults found a nonsignificant reduction in medication use among the subjects practicing mental imagery, but no overall effect on lung function or quality-of-life measures.
Recommendations from others
The New Zealand Guideline Group (NZGG)10 gives a Grade B recommendation for Buteyko Breathing Techniques as an intervention that may be helpful in reducing acute exacerbation medication use and improving patient quality of life. However, the NZGG did not find other benefits to this intervention and noted that it might be costly for the patient to obtain training in these techniques. The NZGG further recommends as a good practice point that healthcare professionals be open to the use of CAM therapies and that such therapies be tried by patients who are interested in them, with monitoring and self-assessment to assist patients in determining which therapies are of value.
1. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. Available at: www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed on March 30, 2007.
2. Mannino DM, Home DW, Akinbami LJ, Morrman JE, Guynn C, Redd SC. Surveillance of Asthma—1980–1999. MMWR Surveill Summ 2002;51:1-13.
3. McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000008.-
4. Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complementary Med 2000;6:519-525.
5. Huntley A, Ernst E. Herbal medicines for asthma: a systemic review. Thorax 2000;55:925-929.
6. McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000353.-
7. Blackhall K, Appleton S, Cates FJ. Ionisers for chronic asthma. Cochrane Database Syst Rev 2003;(3):CD002986.-
8. Hondras MA, Jones LK, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev 2005;(2):CD001002.-
9. Epstein GN, Halper JP, Barrett EA, et al. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Alternative Therapies 2004;10:66-71.
10. New Zealand Guidelines Group (NZGG) The diagnosis and treatment of adult asthma. Best Practice Evidence-Based Guideline. Wellington, NZ: NZGG; 2007. Available at: www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf. Accessed on March 30, 2007.
Yes, some are. Acupuncture relieves subjective symptoms of asthma and reduces medication use in mild to moderate asthma (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials [RCTs] of variable quality). Herbal medications, such as Ginkgo biloba, appear to improve lung function, while herbs such as Tylophora indica and Tsumura saiboku-to may decrease asthma symptoms (SOR: B, based on systematic review of RCTs with poor methodology). No evidence, however, supports the use of room air ionizers, manual therapy, homeopathy, or mind-body therapy for treatment of asthma (SOR: A, based on systematic reviews and meta-analyses of RCTs and individual RCTs).
Though this research is interesting, we should adhere to current guidelines
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Guidelines for the diagnosis and management of asthma are widely disseminated by the National Asthma Education and Prevention Program through its Expert Panel Reports (updated in 2002).1 Nevertheless, nearly 500,000 hospitalizations, 2 million emergency department visits, and 5000 deaths were reported annually in the US among those who have asthma.2 Furthermore, a significant difference in asthma prevalence, health care use, and mortality was found among different ethnic groups.1
Poor patient understanding of asthma control, nonadherence to medication regimens, cultural beliefs, and disparity of access to the health care system, together with physicians’ lack of close monitoring and inadequate compliance with national asthma guidelines, contribute to suboptimal control of chronic asthma. Family physicians must guide and empower their patients with the knowledge and responsibility of how to manage their asthma. For now, we should adhere to current national guidelines of management of asthma and avoid routine recommendation of any complimentary alternative treatments.
Evidence summary
Although complementary and alternative medicine (CAM) therapies are widely used, the overall body of research into CAM for asthma is still small and of limited quality. Interpreting the research is hampered by lack of standardized therapeutic approaches, lack of accepted methods for appropriate trials, and the fact that many CAM treatments are used as part of a multi-pronged, individualized approach to treatment in actual practice. Our search found 4 good-quality systematic reviews of RCTs, 1 good-quality systematic review of randomized trials, and 1 small additional pilot RCT of various CAM treatments for asthma.
Acupuncture and herbals provide some benefit
While a Cochrane review of 11 RCTs with variable trial quality and a total of 324 participants found that acupuncture had no significant effect on pulmonary function or global assessment of well-being, the review noted that some studies reported significant positive changes in daily symptoms, reductions in medication use, and improved quality of life. This suggests that some patients with mild to moderate asthma may benefit from acupuncture.3 In 1 RCT, improvement in general well-being was reported by 79% of 38 patients receiving acupuncture compared with 47% of 18 patients in the control group.4
When it comes to herbal remedies, a good-quality systematic review5 of 17 trials, with overall poor methodological quality and a total of 1445 participants, reported significant improvements in clinically relevant measures with 6 different herbal medicines.
- Ginkgo biloba liquor increased forced expiratory volume in 1 second (FEV1) by 10% at 4 weeks and by a more clinically relevant 15% at 8 weeks (significantly greater than placebo, P<.05).
- Invigorating Kidney for Preventing Asthma (IKPA) tablets increased FEV1 by 30% at 3 months compared with 17% in controls (P<.05).
- Wenyang Tonglulo Mixture (WTM) improved FEV1 by 30% at 8 weeks compared with a 16% increase in the control group using oral salbutamol and inhaled beclomethasone (P<.05).
- Dried ivy extract, thought to work as both a secretolytic and bronchospasmolytic, reduced airway resistance in children by 23.6% compared with placebo (P=.036).
- Tylophora indica (a rare herb also known as Indian ipecac) provided significant improvement in nocturnal dyspnea when compared with controls (P<.01) in a study that relied on patients’ symptom diaries.
- Tsumura saiboku-to (TJ-96) provided patients in one RCT with significant, but unspecified, asthma symptom relief when compared with those in a control group (P<.01).5
Other therapies didn’t quite make the grade
Homeopathy. A Cochrane review of 6 RCTs of mixed quality, with a total of 556 patients, concluded the evidence is insufficient to evaluate the possible role of homeopathy for the treatment of asthma, due to heterogeneity of interventions, patient populations, and outcome assessments. Each study evaluated a different homeopathic remedy, making any overall assessment difficult.
The review notes there have been only limited attempts to study a complete “package of care,” which includes the in-depth, one-on-one consultation, treatment, and follow-up that characterizes most homeopathic treatment in practice.6
Room air ionizers. A Cochrane review of 6 good-quality trials with a total of 106 participants reported no significant effect of room air ionizers on pulmonary function measures, symptoms, or medication use.7
Manual therapy. A Cochrane review8 of 3 moderate- to poor-quality RCTs with 156 participants reported no significant effect of chiropractic spinal manipulation (2 trials) or massage therapy (1 trial) on lung function, asthma symptoms, or medication use.
Mind-body therapy. A pilot RCT9 with 33 adults found a nonsignificant reduction in medication use among the subjects practicing mental imagery, but no overall effect on lung function or quality-of-life measures.
Recommendations from others
The New Zealand Guideline Group (NZGG)10 gives a Grade B recommendation for Buteyko Breathing Techniques as an intervention that may be helpful in reducing acute exacerbation medication use and improving patient quality of life. However, the NZGG did not find other benefits to this intervention and noted that it might be costly for the patient to obtain training in these techniques. The NZGG further recommends as a good practice point that healthcare professionals be open to the use of CAM therapies and that such therapies be tried by patients who are interested in them, with monitoring and self-assessment to assist patients in determining which therapies are of value.
Yes, some are. Acupuncture relieves subjective symptoms of asthma and reduces medication use in mild to moderate asthma (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials [RCTs] of variable quality). Herbal medications, such as Ginkgo biloba, appear to improve lung function, while herbs such as Tylophora indica and Tsumura saiboku-to may decrease asthma symptoms (SOR: B, based on systematic review of RCTs with poor methodology). No evidence, however, supports the use of room air ionizers, manual therapy, homeopathy, or mind-body therapy for treatment of asthma (SOR: A, based on systematic reviews and meta-analyses of RCTs and individual RCTs).
Though this research is interesting, we should adhere to current guidelines
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Guidelines for the diagnosis and management of asthma are widely disseminated by the National Asthma Education and Prevention Program through its Expert Panel Reports (updated in 2002).1 Nevertheless, nearly 500,000 hospitalizations, 2 million emergency department visits, and 5000 deaths were reported annually in the US among those who have asthma.2 Furthermore, a significant difference in asthma prevalence, health care use, and mortality was found among different ethnic groups.1
Poor patient understanding of asthma control, nonadherence to medication regimens, cultural beliefs, and disparity of access to the health care system, together with physicians’ lack of close monitoring and inadequate compliance with national asthma guidelines, contribute to suboptimal control of chronic asthma. Family physicians must guide and empower their patients with the knowledge and responsibility of how to manage their asthma. For now, we should adhere to current national guidelines of management of asthma and avoid routine recommendation of any complimentary alternative treatments.
Evidence summary
Although complementary and alternative medicine (CAM) therapies are widely used, the overall body of research into CAM for asthma is still small and of limited quality. Interpreting the research is hampered by lack of standardized therapeutic approaches, lack of accepted methods for appropriate trials, and the fact that many CAM treatments are used as part of a multi-pronged, individualized approach to treatment in actual practice. Our search found 4 good-quality systematic reviews of RCTs, 1 good-quality systematic review of randomized trials, and 1 small additional pilot RCT of various CAM treatments for asthma.
Acupuncture and herbals provide some benefit
While a Cochrane review of 11 RCTs with variable trial quality and a total of 324 participants found that acupuncture had no significant effect on pulmonary function or global assessment of well-being, the review noted that some studies reported significant positive changes in daily symptoms, reductions in medication use, and improved quality of life. This suggests that some patients with mild to moderate asthma may benefit from acupuncture.3 In 1 RCT, improvement in general well-being was reported by 79% of 38 patients receiving acupuncture compared with 47% of 18 patients in the control group.4
When it comes to herbal remedies, a good-quality systematic review5 of 17 trials, with overall poor methodological quality and a total of 1445 participants, reported significant improvements in clinically relevant measures with 6 different herbal medicines.
- Ginkgo biloba liquor increased forced expiratory volume in 1 second (FEV1) by 10% at 4 weeks and by a more clinically relevant 15% at 8 weeks (significantly greater than placebo, P<.05).
- Invigorating Kidney for Preventing Asthma (IKPA) tablets increased FEV1 by 30% at 3 months compared with 17% in controls (P<.05).
- Wenyang Tonglulo Mixture (WTM) improved FEV1 by 30% at 8 weeks compared with a 16% increase in the control group using oral salbutamol and inhaled beclomethasone (P<.05).
- Dried ivy extract, thought to work as both a secretolytic and bronchospasmolytic, reduced airway resistance in children by 23.6% compared with placebo (P=.036).
- Tylophora indica (a rare herb also known as Indian ipecac) provided significant improvement in nocturnal dyspnea when compared with controls (P<.01) in a study that relied on patients’ symptom diaries.
- Tsumura saiboku-to (TJ-96) provided patients in one RCT with significant, but unspecified, asthma symptom relief when compared with those in a control group (P<.01).5
Other therapies didn’t quite make the grade
Homeopathy. A Cochrane review of 6 RCTs of mixed quality, with a total of 556 patients, concluded the evidence is insufficient to evaluate the possible role of homeopathy for the treatment of asthma, due to heterogeneity of interventions, patient populations, and outcome assessments. Each study evaluated a different homeopathic remedy, making any overall assessment difficult.
The review notes there have been only limited attempts to study a complete “package of care,” which includes the in-depth, one-on-one consultation, treatment, and follow-up that characterizes most homeopathic treatment in practice.6
Room air ionizers. A Cochrane review of 6 good-quality trials with a total of 106 participants reported no significant effect of room air ionizers on pulmonary function measures, symptoms, or medication use.7
Manual therapy. A Cochrane review8 of 3 moderate- to poor-quality RCTs with 156 participants reported no significant effect of chiropractic spinal manipulation (2 trials) or massage therapy (1 trial) on lung function, asthma symptoms, or medication use.
Mind-body therapy. A pilot RCT9 with 33 adults found a nonsignificant reduction in medication use among the subjects practicing mental imagery, but no overall effect on lung function or quality-of-life measures.
Recommendations from others
The New Zealand Guideline Group (NZGG)10 gives a Grade B recommendation for Buteyko Breathing Techniques as an intervention that may be helpful in reducing acute exacerbation medication use and improving patient quality of life. However, the NZGG did not find other benefits to this intervention and noted that it might be costly for the patient to obtain training in these techniques. The NZGG further recommends as a good practice point that healthcare professionals be open to the use of CAM therapies and that such therapies be tried by patients who are interested in them, with monitoring and self-assessment to assist patients in determining which therapies are of value.
1. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. Available at: www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed on March 30, 2007.
2. Mannino DM, Home DW, Akinbami LJ, Morrman JE, Guynn C, Redd SC. Surveillance of Asthma—1980–1999. MMWR Surveill Summ 2002;51:1-13.
3. McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000008.-
4. Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complementary Med 2000;6:519-525.
5. Huntley A, Ernst E. Herbal medicines for asthma: a systemic review. Thorax 2000;55:925-929.
6. McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000353.-
7. Blackhall K, Appleton S, Cates FJ. Ionisers for chronic asthma. Cochrane Database Syst Rev 2003;(3):CD002986.-
8. Hondras MA, Jones LK, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev 2005;(2):CD001002.-
9. Epstein GN, Halper JP, Barrett EA, et al. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Alternative Therapies 2004;10:66-71.
10. New Zealand Guidelines Group (NZGG) The diagnosis and treatment of adult asthma. Best Practice Evidence-Based Guideline. Wellington, NZ: NZGG; 2007. Available at: www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf. Accessed on March 30, 2007.
1. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. Available at: www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed on March 30, 2007.
2. Mannino DM, Home DW, Akinbami LJ, Morrman JE, Guynn C, Redd SC. Surveillance of Asthma—1980–1999. MMWR Surveill Summ 2002;51:1-13.
3. McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000008.-
4. Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complementary Med 2000;6:519-525.
5. Huntley A, Ernst E. Herbal medicines for asthma: a systemic review. Thorax 2000;55:925-929.
6. McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000353.-
7. Blackhall K, Appleton S, Cates FJ. Ionisers for chronic asthma. Cochrane Database Syst Rev 2003;(3):CD002986.-
8. Hondras MA, Jones LK, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev 2005;(2):CD001002.-
9. Epstein GN, Halper JP, Barrett EA, et al. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Alternative Therapies 2004;10:66-71.
10. New Zealand Guidelines Group (NZGG) The diagnosis and treatment of adult asthma. Best Practice Evidence-Based Guideline. Wellington, NZ: NZGG; 2007. Available at: www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf. Accessed on March 30, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best test for HSV-2 after exposure?
Enzyme-linked immunosorbent assay (ELISA) tests based on herpes simplex virus 2’s (HSV-2) glycoprotein G have demonstrated high sensitivity and specificity in determining seropositivity for HSV-2 antibodies (strength of recommendation [SOR]: C, based on cross-sectional studies).
ELISA tests not based on glycoprotein G are also highly sensitive, but they are less specific for HSV-2 and are prone to false-positive results because of cross-reactivity with HSV-1 antibodies (SOR: C, based on cross-sectional studies).
Random anogenital cultures are not sensitive for diagnosing HSV-2 infection (TABLE) (SOR: B, based on extrapolation from a well-designed prospective cohort study). No studies have found patient-oriented benefits to testing asymptomatic patients for HSV-2 infection.
Consider offering these tests to patients at high risk of coinfection with HSV
Manjula Julka, MD
University of Texas Southwestern, Dallas
An estimated 1.6 million new cases of genital herpes are diagnosed annually. The viral shedding among asymptomatic patients poses a great challenge in controlling its transmission. Older methods of detecting HSV infection by non–glycoprotein G-based ELISA tests are nonspecific and do not differentiate between HSV-1 and HSV-2. The newer serologic tests that detect antibodies to HSV glycoproteins G1 and G2 are available for rapid detection and typing of genital herpes. Sensitivity and specificity of these tests are also higher than older tests.
Although US Preventive Services Task Force (USPSTF) guidelines do not recommend routine screening of all patients for HSV, it’s important that you consider offering these tests to patients at high risk of coinfection with HSV, such as those who are HIV-positive. Good-quality evidence demonstrates that systemic antiviral therapy along with condom use effectively reduces the viral shedding and therefore reduces the risk of genital HSV transmission.
TABLE
Summary of HSV test characteristics
| TEST | SN (%) | SP (%) | LR+ | COST (EST.) | TIME TO RESULT |
|---|---|---|---|---|---|
| Genital culture3 | 5* | 100 | NA† | $90 | 24 hours |
| Western blot1,6 | 100 | 100 | >99‡ | $104 | 2 weeks |
| Glycoprotein G ELISA1,6 | 90–100 | 90–100 | 19‡ | $4–$20 | 1–2 weeks |
| Non–glycoprotein G ELISA1,6 | 95–100 | 60–85 | 3.5‡ | Not available | Not available |
| * Calculation using development of symptoms as gold standard. | |||||
| † Specificity=100%, so likelihood ratio=infinity and positive predictive value=1. | |||||
| ‡ LR calculated based on median sensitivity and specificity from sources cited in table. | |||||
| SN, sensitivity; SP, specificity; LR+, positive likelihood ratio; ELISA, enzyme-linked immunosorbent assay. | |||||
Evidence summary
Our literature search failed to find any randomized controlled trials comparing diagnostic tests for HSV-2 infection among asymptomatic populations. Data from cross-sectional studies, however, are available.
Glycoprotein G ELISA has better specificity
Using the Western blot technique as the gold standard, a total of 158 serum samples from patients with either HSV-1 or HSV-2 infection—without mention of symptomatology—were used to compare the performance of several commercially available ELISA assays.1 The glycoprotein G and non–glycoprotein G ELISA tests were both found to have sensitivities >90%, but the non–glycoprotein G ELISA tests had specificities under 90%.
In 47% to 82% of the samples tested with non–glycoprotein G ELISA, there was cross-reactivity between HSV-1 and HSV-2 antibodies.1 The College of American Pathologists found that 46% to 84% of laboratories using non–glycoprotein G ELISA tests incorrectly identified an HSV-1 sample as being HSV-2. All laboratories reporting use of glycoprotein G ELISA tests correctly identified the sample as containing only HSV-1 antibodies.2 Neither study included controls, delineated symptom status, or measured patient-oriented outcomes.
Genital culture has poor sensitivity
A prospective cohort study compared the viral shedding by Western blot among 52 asymptomatic seropositive patients with 90 seropositive and symptomatic patients.3 Daily genital swabs were done for 3 months for each patient. The asymptomatic individuals had HSV-2 positive cultures on 3% of culture days.3 Genital culture appears to have a very poor sensitivity (5%) for diagnosis of HSV-2 infection among asymptomatic individuals.
We found no studies that measured patient-oriented harms or benefits arising from testing asymptomatic individuals for HSV-2 infection.
Recommendations from others
The USPSTF recommends against routine serological screening for HSV in asymptomatic adolescents and adults (D recommendation, fair or good evidence that the service is ineffective or that the harms outweigh the benefits).4 The California Sexually Transmitted Diseases Controllers Association recommends that serologic testing is likely to benefit HIV-infected patients, those whose sexual partners have genital herpes, and those at high risk of STDs motivated to reduce their sexual risk behavior.5
1. Martins TB, Woolstenhulme RD, Jaskowski TD, et al. Comparison of four enzyme immunoassays with a Western Blot assay for the determination of type-specific antibodies to herpes simplex virus. Am J Clin Pathol 2001;115:272-277.
2. Morrow RA, Brown ZA. Common use of inaccurate antibody assays to identify infection status with herpes simplex virus type 2. Am J Obstet Gynecol 2004;193:361-362.
3. Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 2000;342:844-850.
4. US Preventive Services Task Force (USPSTF). Screening for Genital Herpes: Recommendation Statement. Rockville, Md: Agency for Healthcare Research and Quality (AHRQ); 2005. 11p. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed on February 20, 2007.
5. Guerry SI, Bauer HM, Klausner JD, et al. Recommendations for the selective use of herpes simplex virus type 2 serological tests. Clin Infect Dis 2005;40:38-45.
6. American Social Health Association. Herpes Blood Tests Quick Reference Guide. Herpes Resource Center 2005. Available at: www.ashastd.org. Accessed on February 20, 2007.
Enzyme-linked immunosorbent assay (ELISA) tests based on herpes simplex virus 2’s (HSV-2) glycoprotein G have demonstrated high sensitivity and specificity in determining seropositivity for HSV-2 antibodies (strength of recommendation [SOR]: C, based on cross-sectional studies).
ELISA tests not based on glycoprotein G are also highly sensitive, but they are less specific for HSV-2 and are prone to false-positive results because of cross-reactivity with HSV-1 antibodies (SOR: C, based on cross-sectional studies).
Random anogenital cultures are not sensitive for diagnosing HSV-2 infection (TABLE) (SOR: B, based on extrapolation from a well-designed prospective cohort study). No studies have found patient-oriented benefits to testing asymptomatic patients for HSV-2 infection.
Consider offering these tests to patients at high risk of coinfection with HSV
Manjula Julka, MD
University of Texas Southwestern, Dallas
An estimated 1.6 million new cases of genital herpes are diagnosed annually. The viral shedding among asymptomatic patients poses a great challenge in controlling its transmission. Older methods of detecting HSV infection by non–glycoprotein G-based ELISA tests are nonspecific and do not differentiate between HSV-1 and HSV-2. The newer serologic tests that detect antibodies to HSV glycoproteins G1 and G2 are available for rapid detection and typing of genital herpes. Sensitivity and specificity of these tests are also higher than older tests.
Although US Preventive Services Task Force (USPSTF) guidelines do not recommend routine screening of all patients for HSV, it’s important that you consider offering these tests to patients at high risk of coinfection with HSV, such as those who are HIV-positive. Good-quality evidence demonstrates that systemic antiviral therapy along with condom use effectively reduces the viral shedding and therefore reduces the risk of genital HSV transmission.
TABLE
Summary of HSV test characteristics
| TEST | SN (%) | SP (%) | LR+ | COST (EST.) | TIME TO RESULT |
|---|---|---|---|---|---|
| Genital culture3 | 5* | 100 | NA† | $90 | 24 hours |
| Western blot1,6 | 100 | 100 | >99‡ | $104 | 2 weeks |
| Glycoprotein G ELISA1,6 | 90–100 | 90–100 | 19‡ | $4–$20 | 1–2 weeks |
| Non–glycoprotein G ELISA1,6 | 95–100 | 60–85 | 3.5‡ | Not available | Not available |
| * Calculation using development of symptoms as gold standard. | |||||
| † Specificity=100%, so likelihood ratio=infinity and positive predictive value=1. | |||||
| ‡ LR calculated based on median sensitivity and specificity from sources cited in table. | |||||
| SN, sensitivity; SP, specificity; LR+, positive likelihood ratio; ELISA, enzyme-linked immunosorbent assay. | |||||
Evidence summary
Our literature search failed to find any randomized controlled trials comparing diagnostic tests for HSV-2 infection among asymptomatic populations. Data from cross-sectional studies, however, are available.
Glycoprotein G ELISA has better specificity
Using the Western blot technique as the gold standard, a total of 158 serum samples from patients with either HSV-1 or HSV-2 infection—without mention of symptomatology—were used to compare the performance of several commercially available ELISA assays.1 The glycoprotein G and non–glycoprotein G ELISA tests were both found to have sensitivities >90%, but the non–glycoprotein G ELISA tests had specificities under 90%.
In 47% to 82% of the samples tested with non–glycoprotein G ELISA, there was cross-reactivity between HSV-1 and HSV-2 antibodies.1 The College of American Pathologists found that 46% to 84% of laboratories using non–glycoprotein G ELISA tests incorrectly identified an HSV-1 sample as being HSV-2. All laboratories reporting use of glycoprotein G ELISA tests correctly identified the sample as containing only HSV-1 antibodies.2 Neither study included controls, delineated symptom status, or measured patient-oriented outcomes.
Genital culture has poor sensitivity
A prospective cohort study compared the viral shedding by Western blot among 52 asymptomatic seropositive patients with 90 seropositive and symptomatic patients.3 Daily genital swabs were done for 3 months for each patient. The asymptomatic individuals had HSV-2 positive cultures on 3% of culture days.3 Genital culture appears to have a very poor sensitivity (5%) for diagnosis of HSV-2 infection among asymptomatic individuals.
We found no studies that measured patient-oriented harms or benefits arising from testing asymptomatic individuals for HSV-2 infection.
Recommendations from others
The USPSTF recommends against routine serological screening for HSV in asymptomatic adolescents and adults (D recommendation, fair or good evidence that the service is ineffective or that the harms outweigh the benefits).4 The California Sexually Transmitted Diseases Controllers Association recommends that serologic testing is likely to benefit HIV-infected patients, those whose sexual partners have genital herpes, and those at high risk of STDs motivated to reduce their sexual risk behavior.5
Enzyme-linked immunosorbent assay (ELISA) tests based on herpes simplex virus 2’s (HSV-2) glycoprotein G have demonstrated high sensitivity and specificity in determining seropositivity for HSV-2 antibodies (strength of recommendation [SOR]: C, based on cross-sectional studies).
ELISA tests not based on glycoprotein G are also highly sensitive, but they are less specific for HSV-2 and are prone to false-positive results because of cross-reactivity with HSV-1 antibodies (SOR: C, based on cross-sectional studies).
Random anogenital cultures are not sensitive for diagnosing HSV-2 infection (TABLE) (SOR: B, based on extrapolation from a well-designed prospective cohort study). No studies have found patient-oriented benefits to testing asymptomatic patients for HSV-2 infection.
Consider offering these tests to patients at high risk of coinfection with HSV
Manjula Julka, MD
University of Texas Southwestern, Dallas
An estimated 1.6 million new cases of genital herpes are diagnosed annually. The viral shedding among asymptomatic patients poses a great challenge in controlling its transmission. Older methods of detecting HSV infection by non–glycoprotein G-based ELISA tests are nonspecific and do not differentiate between HSV-1 and HSV-2. The newer serologic tests that detect antibodies to HSV glycoproteins G1 and G2 are available for rapid detection and typing of genital herpes. Sensitivity and specificity of these tests are also higher than older tests.
Although US Preventive Services Task Force (USPSTF) guidelines do not recommend routine screening of all patients for HSV, it’s important that you consider offering these tests to patients at high risk of coinfection with HSV, such as those who are HIV-positive. Good-quality evidence demonstrates that systemic antiviral therapy along with condom use effectively reduces the viral shedding and therefore reduces the risk of genital HSV transmission.
TABLE
Summary of HSV test characteristics
| TEST | SN (%) | SP (%) | LR+ | COST (EST.) | TIME TO RESULT |
|---|---|---|---|---|---|
| Genital culture3 | 5* | 100 | NA† | $90 | 24 hours |
| Western blot1,6 | 100 | 100 | >99‡ | $104 | 2 weeks |
| Glycoprotein G ELISA1,6 | 90–100 | 90–100 | 19‡ | $4–$20 | 1–2 weeks |
| Non–glycoprotein G ELISA1,6 | 95–100 | 60–85 | 3.5‡ | Not available | Not available |
| * Calculation using development of symptoms as gold standard. | |||||
| † Specificity=100%, so likelihood ratio=infinity and positive predictive value=1. | |||||
| ‡ LR calculated based on median sensitivity and specificity from sources cited in table. | |||||
| SN, sensitivity; SP, specificity; LR+, positive likelihood ratio; ELISA, enzyme-linked immunosorbent assay. | |||||
Evidence summary
Our literature search failed to find any randomized controlled trials comparing diagnostic tests for HSV-2 infection among asymptomatic populations. Data from cross-sectional studies, however, are available.
Glycoprotein G ELISA has better specificity
Using the Western blot technique as the gold standard, a total of 158 serum samples from patients with either HSV-1 or HSV-2 infection—without mention of symptomatology—were used to compare the performance of several commercially available ELISA assays.1 The glycoprotein G and non–glycoprotein G ELISA tests were both found to have sensitivities >90%, but the non–glycoprotein G ELISA tests had specificities under 90%.
In 47% to 82% of the samples tested with non–glycoprotein G ELISA, there was cross-reactivity between HSV-1 and HSV-2 antibodies.1 The College of American Pathologists found that 46% to 84% of laboratories using non–glycoprotein G ELISA tests incorrectly identified an HSV-1 sample as being HSV-2. All laboratories reporting use of glycoprotein G ELISA tests correctly identified the sample as containing only HSV-1 antibodies.2 Neither study included controls, delineated symptom status, or measured patient-oriented outcomes.
Genital culture has poor sensitivity
A prospective cohort study compared the viral shedding by Western blot among 52 asymptomatic seropositive patients with 90 seropositive and symptomatic patients.3 Daily genital swabs were done for 3 months for each patient. The asymptomatic individuals had HSV-2 positive cultures on 3% of culture days.3 Genital culture appears to have a very poor sensitivity (5%) for diagnosis of HSV-2 infection among asymptomatic individuals.
We found no studies that measured patient-oriented harms or benefits arising from testing asymptomatic individuals for HSV-2 infection.
Recommendations from others
The USPSTF recommends against routine serological screening for HSV in asymptomatic adolescents and adults (D recommendation, fair or good evidence that the service is ineffective or that the harms outweigh the benefits).4 The California Sexually Transmitted Diseases Controllers Association recommends that serologic testing is likely to benefit HIV-infected patients, those whose sexual partners have genital herpes, and those at high risk of STDs motivated to reduce their sexual risk behavior.5
1. Martins TB, Woolstenhulme RD, Jaskowski TD, et al. Comparison of four enzyme immunoassays with a Western Blot assay for the determination of type-specific antibodies to herpes simplex virus. Am J Clin Pathol 2001;115:272-277.
2. Morrow RA, Brown ZA. Common use of inaccurate antibody assays to identify infection status with herpes simplex virus type 2. Am J Obstet Gynecol 2004;193:361-362.
3. Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 2000;342:844-850.
4. US Preventive Services Task Force (USPSTF). Screening for Genital Herpes: Recommendation Statement. Rockville, Md: Agency for Healthcare Research and Quality (AHRQ); 2005. 11p. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed on February 20, 2007.
5. Guerry SI, Bauer HM, Klausner JD, et al. Recommendations for the selective use of herpes simplex virus type 2 serological tests. Clin Infect Dis 2005;40:38-45.
6. American Social Health Association. Herpes Blood Tests Quick Reference Guide. Herpes Resource Center 2005. Available at: www.ashastd.org. Accessed on February 20, 2007.
1. Martins TB, Woolstenhulme RD, Jaskowski TD, et al. Comparison of four enzyme immunoassays with a Western Blot assay for the determination of type-specific antibodies to herpes simplex virus. Am J Clin Pathol 2001;115:272-277.
2. Morrow RA, Brown ZA. Common use of inaccurate antibody assays to identify infection status with herpes simplex virus type 2. Am J Obstet Gynecol 2004;193:361-362.
3. Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 2000;342:844-850.
4. US Preventive Services Task Force (USPSTF). Screening for Genital Herpes: Recommendation Statement. Rockville, Md: Agency for Healthcare Research and Quality (AHRQ); 2005. 11p. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed on February 20, 2007.
5. Guerry SI, Bauer HM, Klausner JD, et al. Recommendations for the selective use of herpes simplex virus type 2 serological tests. Clin Infect Dis 2005;40:38-45.
6. American Social Health Association. Herpes Blood Tests Quick Reference Guide. Herpes Resource Center 2005. Available at: www.ashastd.org. Accessed on February 20, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Which tests are the most useful for diagnosing PID?
No single test has adequate sensitivity and specificity to reliably identify pelvic inflammatory disease (PID) and thus help to spare women serious sequelae, including infertility (strength of recommendation [SOR]: B, based on systematic reviews of cohort studies and individual cohort studies).
A large multisite US study found that using adnexal tenderness as a minimum clinical criterion raises the sensitivity of the Centers for Disease Control and Prevention (CDC) criteria from 83% to 95%.1 However, even the modified 2002 CDC criteria fail to identify women with subclinical PID who are at roughly equivalent risk for PID sequelae as those with acute symptomatic disease2 (SOR: B, based on individual cohort studies).
It’s prudent to treat when there is a clinical diagnosis of PID
Kismet T. Roberts, MD
University of Nebraska Medical Center; Offutt Air Force Base Family Medicine Residency
Women presenting with acute pelvic pain need thorough evaluation to rule out ectopic pregnancy, cystitis, pyelonephritis, appendicitis, and ovarian torsion. In my experience, a likely history of a sexually transmitted disease along with adnexal pain or cervical motion tenderness on examination is the most helpful in diagnosing PID.
An elevated white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), or C-reactive protein (CRP) may help support the diagnosis. PID often becomes a diagnosis of exclusion if human chorionic gonadotropin (hCG), urine evaluation, and pelvic ultrasound are negative.
While PID is sometimes a frustrating diagnosis to make and is often viewed as a “wastebasket” diagnosis, empiric treatment may be beneficial. While we would love to know whether treating pending culture results reduces the risk of sepsis and infertility, it seems prudent to treat when we have made a clinical diagnosis of PID.
Evidence summary
Our search for articles that examined patient- and primary care–oriented PID diagnostic tests resulted in 2 systematic reviews, no randomized controlled trials, 4 data analyses, and 5 cohort studies, all of which were fair- to good-quality.
Systematic reviews don’t show consistent results
One systematic review of 12 fair- to good-quality studies, based in Europe and the US, included urban populations treated in Ob/Gyn departments, emergency rooms, and sexually transmitted disease clinics. This review supports a thorough evaluation when more severe disease is suspected and the use of sensitive diagnostic tests for suspected mild disease—eg, CRP (74%–93% sensitivity) and ESR (64%–81% sensitivity for value >20 or 15 mm/h).3
Another systematic review of 19 fair-to good-quality cohort studies found a sensitivity of only 64% for laparoscopy, 50% to 87% for endometrial biopsy, and up to 80% for microbiological tests. Results were not consistent for the reported sensitivity of WBC, ESR, or CRP.4
Multivariate analyses of Swedish data come to different conclusions
We identified no randomized controlled trials that addressed the diagnosis of PID. Two multivariate analyses of the same Swedish data from the 1960s came to different conclusions.
The Lund analysis includes data collected between 1960 and 1969 at Lund University Hospital in Sweden on women with suspected PID, with about 625 cases included for these analyses. Simms et al5 found insufficient evidence from these data for any existing diagnostic criteria.
Looking at the same data, Hagdu et al proposed the use of a clinical criteria model including low abdominal pain and 2 or more of the following other criteria: vaginal discharge, temperature greater than 38°C, vomiting, irregular menses, urologic or proctitis symptoms, pelvic tenderness, adnexal mass or swelling, and ESR ≥15.6 This model had a reported sensitivity of 87%, specificity of 52.5%, and false-positive and false-negative rates of 21.2% and 33.3%, respectively.
Looking at adnexal tenderness aids sensitivity of other tests
Cross-sectional analysis of a multisite US randomized treatment trial supported using adnexal tenderness as a minimum clinical criterion to increase sensitivity.1 Further analysis of that trial suggests that some asymptomatic women are at equivalent risk of developing sequelae compared with symptomatic women diagnosed with PID. Those asymptomatic women who met diagnostic criteria with a positive endometrial biopsy were more likely to have pelvic tenderness than asymptomatic women who were not diagnosed.2
Symptoms >1 week and elevated WBC also helpful
Two small, fair-quality cohort studies (N=61 and 176, respectively) investigated the use of clinical diagnostic criteria for PID. The smaller study compared clinical criteria to several reference standards (laparoscopy, histology, microbiological markers, and transvaginal ultrasound) and found clinical criteria, specifically adnexal tenderness, most sensitive (87%), and laparoscopy most specific (100%).7
In the second study, the authors evaluated 176 consecutive admissions for clinically diagnosed PID, 76% of which were laparoscopically confirmed. Reviewing clinical indicators, they found that a combination of adnexal tenderness, symptoms for <1 week, and elevated WBC was the most sensitive set of predictors (sensitivity 86.6%, specificity 45.7%) with positive predictive value of 0.84 and negative predictive value of 0.52.8
Useful lab indicators: C-reactive protein, serum CA-125
Three small cohort studies (N=50–152) of fair-quality evaluated various laboratory indicators in the diagnosis of PID. Each used a different reference standard: clinical criteria, laparoscopy, and endometrial biopsy, respectively.
One study found CRP >10 to be 93% sensitive and 83% specific in a cohort of women admitted to the emergency department with an acute gynecological disorder.9 This population had a high baseline incidence of PID, pregnancy, and intrauterine device use.
A study of serum CA-125 levels showed a predictive value of 97% for values >16 U/mL in diagnosing salpingitis. This test might therefore be useful in confirming peritoneal involvement when PID is suspected clinically.10
Another study developed a model using vaginal WBC (the single most sensitive factor at 78%), serum WBC (the single most specific factor at 88%), CRP, and ESR. The model was 100% sensitive if the diagnosis only required 1 positive test, although the specificity was only 18%. The positive predictive value was 65%. If all 4 were positive, specificity was 95%, with 29% sensitivity, a positive predictive value of 90%, and a negative predictive value of 47%. Prevalence was 60% in the group studied.11
Recommendations from others
The CDC recommends empiric treatment of women with lower abdominal or pelvic pain who are at risk for sexually transmitted diseases with uterine, adnexal, or cervical motion tenderness and no other identifiable cause.12
Clinical Evidence found no RCTs that compared empiric treatment of suspected PID with waiting for microbiological test results for guidance.13
The Agency for Healthcare Research and Quality recommends requiring the presence of lower abdominal, adnexal and cervical tenderness, without alternative diagnosis, for the diagnosis of PID. Temperature >101°F, cervical or vaginal discharge, elevated ESR, and positive gonococcal or chlamydia cultures all increase specificity of diagnosis.14
The United Kingdom’s national guideline recommends maintaining a low threshold for empirical treatment, citing a lack of definitive diagnostic criteria and potential for sequelae, but does recommend testing for gonorrhea and chlamydia.15
1. Piepert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol 2001;184:856-864.
2. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis 2005;32:400-405.
3. Kahn JG, Walker CK, Washington AE, et al. Diagnosing pelvic inflammatory disease: a comprehensive analysis and considerations for developing a new model. JAMA 1991;266:2594-2604.
4. Munday PE. Pelvic inflammatory disease: an evidence-based approach to diagnosis. J Infect 2000;40:31-41.
5. Simms I, Warburton F, Westrom L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sex Transm Infect 2003;79:491-494.
6. Hagdu A, Westrom L, Brooks CA, et al. Predicting acute pelvic inflammatory disease: a multivariate analysis. Am J Obstet Gynecol 1986;155:954-960.
7. Gaitan H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol 2002;10:171-180.
8. Morcos R, Frost N, Hnat M, et al. Laparoscopic versus clinical diagnosis of acute pelvic inflammatory disease. J Reprod Med 1993;38:53-56.
9. Hemila M, Henriksson L, Ylikorkala O. Serum CRP in the diagnosis and treatment of pelvic inflammatory disease. Arch Gynecol Obstet 1987;241:177-182.
10. Duk JM, Kauer FM, Fleuren GJ, et al. Serum CA 125 levels in patients with a provisional diagnosis of pelvic inflammatory disease. Acta Obstet Gynecol Scand 1989;68:637-641.
11. Peipert JF, Boardman L, Hogan JW, et al. Laboratory evaluation of acute upper genital tract infection. Obstet Gynecology 1996;87:730-736.
12. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):56-61.
13. Ross J. Pelvic infectious diseases. Clinical Evidence 2006. Web publication date December 1, 2005. Available at: www.clinicalevidence.com/ceweb/conditions/seh/1606/1606.jsp. Accessed on February 20, 2007.
14. Common Gynecologic Problems: A Guide to Diagnosis and Treatment. Boston, Mass: Brigham and Women’s Hospital; 2002.
15. United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease. London, England: British Association for Sexual Health and HIV; 2005.
No single test has adequate sensitivity and specificity to reliably identify pelvic inflammatory disease (PID) and thus help to spare women serious sequelae, including infertility (strength of recommendation [SOR]: B, based on systematic reviews of cohort studies and individual cohort studies).
A large multisite US study found that using adnexal tenderness as a minimum clinical criterion raises the sensitivity of the Centers for Disease Control and Prevention (CDC) criteria from 83% to 95%.1 However, even the modified 2002 CDC criteria fail to identify women with subclinical PID who are at roughly equivalent risk for PID sequelae as those with acute symptomatic disease2 (SOR: B, based on individual cohort studies).
It’s prudent to treat when there is a clinical diagnosis of PID
Kismet T. Roberts, MD
University of Nebraska Medical Center; Offutt Air Force Base Family Medicine Residency
Women presenting with acute pelvic pain need thorough evaluation to rule out ectopic pregnancy, cystitis, pyelonephritis, appendicitis, and ovarian torsion. In my experience, a likely history of a sexually transmitted disease along with adnexal pain or cervical motion tenderness on examination is the most helpful in diagnosing PID.
An elevated white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), or C-reactive protein (CRP) may help support the diagnosis. PID often becomes a diagnosis of exclusion if human chorionic gonadotropin (hCG), urine evaluation, and pelvic ultrasound are negative.
While PID is sometimes a frustrating diagnosis to make and is often viewed as a “wastebasket” diagnosis, empiric treatment may be beneficial. While we would love to know whether treating pending culture results reduces the risk of sepsis and infertility, it seems prudent to treat when we have made a clinical diagnosis of PID.
Evidence summary
Our search for articles that examined patient- and primary care–oriented PID diagnostic tests resulted in 2 systematic reviews, no randomized controlled trials, 4 data analyses, and 5 cohort studies, all of which were fair- to good-quality.
Systematic reviews don’t show consistent results
One systematic review of 12 fair- to good-quality studies, based in Europe and the US, included urban populations treated in Ob/Gyn departments, emergency rooms, and sexually transmitted disease clinics. This review supports a thorough evaluation when more severe disease is suspected and the use of sensitive diagnostic tests for suspected mild disease—eg, CRP (74%–93% sensitivity) and ESR (64%–81% sensitivity for value >20 or 15 mm/h).3
Another systematic review of 19 fair-to good-quality cohort studies found a sensitivity of only 64% for laparoscopy, 50% to 87% for endometrial biopsy, and up to 80% for microbiological tests. Results were not consistent for the reported sensitivity of WBC, ESR, or CRP.4
Multivariate analyses of Swedish data come to different conclusions
We identified no randomized controlled trials that addressed the diagnosis of PID. Two multivariate analyses of the same Swedish data from the 1960s came to different conclusions.
The Lund analysis includes data collected between 1960 and 1969 at Lund University Hospital in Sweden on women with suspected PID, with about 625 cases included for these analyses. Simms et al5 found insufficient evidence from these data for any existing diagnostic criteria.
Looking at the same data, Hagdu et al proposed the use of a clinical criteria model including low abdominal pain and 2 or more of the following other criteria: vaginal discharge, temperature greater than 38°C, vomiting, irregular menses, urologic or proctitis symptoms, pelvic tenderness, adnexal mass or swelling, and ESR ≥15.6 This model had a reported sensitivity of 87%, specificity of 52.5%, and false-positive and false-negative rates of 21.2% and 33.3%, respectively.
Looking at adnexal tenderness aids sensitivity of other tests
Cross-sectional analysis of a multisite US randomized treatment trial supported using adnexal tenderness as a minimum clinical criterion to increase sensitivity.1 Further analysis of that trial suggests that some asymptomatic women are at equivalent risk of developing sequelae compared with symptomatic women diagnosed with PID. Those asymptomatic women who met diagnostic criteria with a positive endometrial biopsy were more likely to have pelvic tenderness than asymptomatic women who were not diagnosed.2
Symptoms >1 week and elevated WBC also helpful
Two small, fair-quality cohort studies (N=61 and 176, respectively) investigated the use of clinical diagnostic criteria for PID. The smaller study compared clinical criteria to several reference standards (laparoscopy, histology, microbiological markers, and transvaginal ultrasound) and found clinical criteria, specifically adnexal tenderness, most sensitive (87%), and laparoscopy most specific (100%).7
In the second study, the authors evaluated 176 consecutive admissions for clinically diagnosed PID, 76% of which were laparoscopically confirmed. Reviewing clinical indicators, they found that a combination of adnexal tenderness, symptoms for <1 week, and elevated WBC was the most sensitive set of predictors (sensitivity 86.6%, specificity 45.7%) with positive predictive value of 0.84 and negative predictive value of 0.52.8
Useful lab indicators: C-reactive protein, serum CA-125
Three small cohort studies (N=50–152) of fair-quality evaluated various laboratory indicators in the diagnosis of PID. Each used a different reference standard: clinical criteria, laparoscopy, and endometrial biopsy, respectively.
One study found CRP >10 to be 93% sensitive and 83% specific in a cohort of women admitted to the emergency department with an acute gynecological disorder.9 This population had a high baseline incidence of PID, pregnancy, and intrauterine device use.
A study of serum CA-125 levels showed a predictive value of 97% for values >16 U/mL in diagnosing salpingitis. This test might therefore be useful in confirming peritoneal involvement when PID is suspected clinically.10
Another study developed a model using vaginal WBC (the single most sensitive factor at 78%), serum WBC (the single most specific factor at 88%), CRP, and ESR. The model was 100% sensitive if the diagnosis only required 1 positive test, although the specificity was only 18%. The positive predictive value was 65%. If all 4 were positive, specificity was 95%, with 29% sensitivity, a positive predictive value of 90%, and a negative predictive value of 47%. Prevalence was 60% in the group studied.11
Recommendations from others
The CDC recommends empiric treatment of women with lower abdominal or pelvic pain who are at risk for sexually transmitted diseases with uterine, adnexal, or cervical motion tenderness and no other identifiable cause.12
Clinical Evidence found no RCTs that compared empiric treatment of suspected PID with waiting for microbiological test results for guidance.13
The Agency for Healthcare Research and Quality recommends requiring the presence of lower abdominal, adnexal and cervical tenderness, without alternative diagnosis, for the diagnosis of PID. Temperature >101°F, cervical or vaginal discharge, elevated ESR, and positive gonococcal or chlamydia cultures all increase specificity of diagnosis.14
The United Kingdom’s national guideline recommends maintaining a low threshold for empirical treatment, citing a lack of definitive diagnostic criteria and potential for sequelae, but does recommend testing for gonorrhea and chlamydia.15
No single test has adequate sensitivity and specificity to reliably identify pelvic inflammatory disease (PID) and thus help to spare women serious sequelae, including infertility (strength of recommendation [SOR]: B, based on systematic reviews of cohort studies and individual cohort studies).
A large multisite US study found that using adnexal tenderness as a minimum clinical criterion raises the sensitivity of the Centers for Disease Control and Prevention (CDC) criteria from 83% to 95%.1 However, even the modified 2002 CDC criteria fail to identify women with subclinical PID who are at roughly equivalent risk for PID sequelae as those with acute symptomatic disease2 (SOR: B, based on individual cohort studies).
It’s prudent to treat when there is a clinical diagnosis of PID
Kismet T. Roberts, MD
University of Nebraska Medical Center; Offutt Air Force Base Family Medicine Residency
Women presenting with acute pelvic pain need thorough evaluation to rule out ectopic pregnancy, cystitis, pyelonephritis, appendicitis, and ovarian torsion. In my experience, a likely history of a sexually transmitted disease along with adnexal pain or cervical motion tenderness on examination is the most helpful in diagnosing PID.
An elevated white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), or C-reactive protein (CRP) may help support the diagnosis. PID often becomes a diagnosis of exclusion if human chorionic gonadotropin (hCG), urine evaluation, and pelvic ultrasound are negative.
While PID is sometimes a frustrating diagnosis to make and is often viewed as a “wastebasket” diagnosis, empiric treatment may be beneficial. While we would love to know whether treating pending culture results reduces the risk of sepsis and infertility, it seems prudent to treat when we have made a clinical diagnosis of PID.
Evidence summary
Our search for articles that examined patient- and primary care–oriented PID diagnostic tests resulted in 2 systematic reviews, no randomized controlled trials, 4 data analyses, and 5 cohort studies, all of which were fair- to good-quality.
Systematic reviews don’t show consistent results
One systematic review of 12 fair- to good-quality studies, based in Europe and the US, included urban populations treated in Ob/Gyn departments, emergency rooms, and sexually transmitted disease clinics. This review supports a thorough evaluation when more severe disease is suspected and the use of sensitive diagnostic tests for suspected mild disease—eg, CRP (74%–93% sensitivity) and ESR (64%–81% sensitivity for value >20 or 15 mm/h).3
Another systematic review of 19 fair-to good-quality cohort studies found a sensitivity of only 64% for laparoscopy, 50% to 87% for endometrial biopsy, and up to 80% for microbiological tests. Results were not consistent for the reported sensitivity of WBC, ESR, or CRP.4
Multivariate analyses of Swedish data come to different conclusions
We identified no randomized controlled trials that addressed the diagnosis of PID. Two multivariate analyses of the same Swedish data from the 1960s came to different conclusions.
The Lund analysis includes data collected between 1960 and 1969 at Lund University Hospital in Sweden on women with suspected PID, with about 625 cases included for these analyses. Simms et al5 found insufficient evidence from these data for any existing diagnostic criteria.
Looking at the same data, Hagdu et al proposed the use of a clinical criteria model including low abdominal pain and 2 or more of the following other criteria: vaginal discharge, temperature greater than 38°C, vomiting, irregular menses, urologic or proctitis symptoms, pelvic tenderness, adnexal mass or swelling, and ESR ≥15.6 This model had a reported sensitivity of 87%, specificity of 52.5%, and false-positive and false-negative rates of 21.2% and 33.3%, respectively.
Looking at adnexal tenderness aids sensitivity of other tests
Cross-sectional analysis of a multisite US randomized treatment trial supported using adnexal tenderness as a minimum clinical criterion to increase sensitivity.1 Further analysis of that trial suggests that some asymptomatic women are at equivalent risk of developing sequelae compared with symptomatic women diagnosed with PID. Those asymptomatic women who met diagnostic criteria with a positive endometrial biopsy were more likely to have pelvic tenderness than asymptomatic women who were not diagnosed.2
Symptoms >1 week and elevated WBC also helpful
Two small, fair-quality cohort studies (N=61 and 176, respectively) investigated the use of clinical diagnostic criteria for PID. The smaller study compared clinical criteria to several reference standards (laparoscopy, histology, microbiological markers, and transvaginal ultrasound) and found clinical criteria, specifically adnexal tenderness, most sensitive (87%), and laparoscopy most specific (100%).7
In the second study, the authors evaluated 176 consecutive admissions for clinically diagnosed PID, 76% of which were laparoscopically confirmed. Reviewing clinical indicators, they found that a combination of adnexal tenderness, symptoms for <1 week, and elevated WBC was the most sensitive set of predictors (sensitivity 86.6%, specificity 45.7%) with positive predictive value of 0.84 and negative predictive value of 0.52.8
Useful lab indicators: C-reactive protein, serum CA-125
Three small cohort studies (N=50–152) of fair-quality evaluated various laboratory indicators in the diagnosis of PID. Each used a different reference standard: clinical criteria, laparoscopy, and endometrial biopsy, respectively.
One study found CRP >10 to be 93% sensitive and 83% specific in a cohort of women admitted to the emergency department with an acute gynecological disorder.9 This population had a high baseline incidence of PID, pregnancy, and intrauterine device use.
A study of serum CA-125 levels showed a predictive value of 97% for values >16 U/mL in diagnosing salpingitis. This test might therefore be useful in confirming peritoneal involvement when PID is suspected clinically.10
Another study developed a model using vaginal WBC (the single most sensitive factor at 78%), serum WBC (the single most specific factor at 88%), CRP, and ESR. The model was 100% sensitive if the diagnosis only required 1 positive test, although the specificity was only 18%. The positive predictive value was 65%. If all 4 were positive, specificity was 95%, with 29% sensitivity, a positive predictive value of 90%, and a negative predictive value of 47%. Prevalence was 60% in the group studied.11
Recommendations from others
The CDC recommends empiric treatment of women with lower abdominal or pelvic pain who are at risk for sexually transmitted diseases with uterine, adnexal, or cervical motion tenderness and no other identifiable cause.12
Clinical Evidence found no RCTs that compared empiric treatment of suspected PID with waiting for microbiological test results for guidance.13
The Agency for Healthcare Research and Quality recommends requiring the presence of lower abdominal, adnexal and cervical tenderness, without alternative diagnosis, for the diagnosis of PID. Temperature >101°F, cervical or vaginal discharge, elevated ESR, and positive gonococcal or chlamydia cultures all increase specificity of diagnosis.14
The United Kingdom’s national guideline recommends maintaining a low threshold for empirical treatment, citing a lack of definitive diagnostic criteria and potential for sequelae, but does recommend testing for gonorrhea and chlamydia.15
1. Piepert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol 2001;184:856-864.
2. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis 2005;32:400-405.
3. Kahn JG, Walker CK, Washington AE, et al. Diagnosing pelvic inflammatory disease: a comprehensive analysis and considerations for developing a new model. JAMA 1991;266:2594-2604.
4. Munday PE. Pelvic inflammatory disease: an evidence-based approach to diagnosis. J Infect 2000;40:31-41.
5. Simms I, Warburton F, Westrom L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sex Transm Infect 2003;79:491-494.
6. Hagdu A, Westrom L, Brooks CA, et al. Predicting acute pelvic inflammatory disease: a multivariate analysis. Am J Obstet Gynecol 1986;155:954-960.
7. Gaitan H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol 2002;10:171-180.
8. Morcos R, Frost N, Hnat M, et al. Laparoscopic versus clinical diagnosis of acute pelvic inflammatory disease. J Reprod Med 1993;38:53-56.
9. Hemila M, Henriksson L, Ylikorkala O. Serum CRP in the diagnosis and treatment of pelvic inflammatory disease. Arch Gynecol Obstet 1987;241:177-182.
10. Duk JM, Kauer FM, Fleuren GJ, et al. Serum CA 125 levels in patients with a provisional diagnosis of pelvic inflammatory disease. Acta Obstet Gynecol Scand 1989;68:637-641.
11. Peipert JF, Boardman L, Hogan JW, et al. Laboratory evaluation of acute upper genital tract infection. Obstet Gynecology 1996;87:730-736.
12. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):56-61.
13. Ross J. Pelvic infectious diseases. Clinical Evidence 2006. Web publication date December 1, 2005. Available at: www.clinicalevidence.com/ceweb/conditions/seh/1606/1606.jsp. Accessed on February 20, 2007.
14. Common Gynecologic Problems: A Guide to Diagnosis and Treatment. Boston, Mass: Brigham and Women’s Hospital; 2002.
15. United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease. London, England: British Association for Sexual Health and HIV; 2005.
1. Piepert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol 2001;184:856-864.
2. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis 2005;32:400-405.
3. Kahn JG, Walker CK, Washington AE, et al. Diagnosing pelvic inflammatory disease: a comprehensive analysis and considerations for developing a new model. JAMA 1991;266:2594-2604.
4. Munday PE. Pelvic inflammatory disease: an evidence-based approach to diagnosis. J Infect 2000;40:31-41.
5. Simms I, Warburton F, Westrom L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sex Transm Infect 2003;79:491-494.
6. Hagdu A, Westrom L, Brooks CA, et al. Predicting acute pelvic inflammatory disease: a multivariate analysis. Am J Obstet Gynecol 1986;155:954-960.
7. Gaitan H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol 2002;10:171-180.
8. Morcos R, Frost N, Hnat M, et al. Laparoscopic versus clinical diagnosis of acute pelvic inflammatory disease. J Reprod Med 1993;38:53-56.
9. Hemila M, Henriksson L, Ylikorkala O. Serum CRP in the diagnosis and treatment of pelvic inflammatory disease. Arch Gynecol Obstet 1987;241:177-182.
10. Duk JM, Kauer FM, Fleuren GJ, et al. Serum CA 125 levels in patients with a provisional diagnosis of pelvic inflammatory disease. Acta Obstet Gynecol Scand 1989;68:637-641.
11. Peipert JF, Boardman L, Hogan JW, et al. Laboratory evaluation of acute upper genital tract infection. Obstet Gynecology 1996;87:730-736.
12. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):56-61.
13. Ross J. Pelvic infectious diseases. Clinical Evidence 2006. Web publication date December 1, 2005. Available at: www.clinicalevidence.com/ceweb/conditions/seh/1606/1606.jsp. Accessed on February 20, 2007.
14. Common Gynecologic Problems: A Guide to Diagnosis and Treatment. Boston, Mass: Brigham and Women’s Hospital; 2002.
15. United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease. London, England: British Association for Sexual Health and HIV; 2005.
Evidence-based answers from the Family Physicians Inquiries Network
Angiotensin blockade for diabetes: Monitor microalbuminuria?
No studies address whether continued screening for microalbuminuria once a patient is taking an angiotensin-converting enzyme (ACE) inhibitor or angiotensin-2 receptor blocker (ARB) improves outcomes. Indirect evidence and expert opinion suggest that it may be beneficial to continue microalbuminuria surveillance to assess response to therapy and monitor disease progression (strength of recommendation: C, based on expert opinion).
It is unclear whether microalbuminuria tests in these cases is money well spent
Chris Vincent, MD
Swedish Family Medicine Residency, University of Washington, Seattle
At the residency where I teach, clinicians routinely try to get to the evidence behind the expert opinions, and faculty are discouraged from giving off the cuff or experiential answers. When asked whether to monitor microalbuminuria for patients with diabetes receiving ACE inhibitors or ARBs, it is frustrating to discover that no direct evidence supports the experts’ advice.
The screening test for urine microalbuminuria at our hospital costs $90. Since most patients with diabetes are being treated with ACE inhibitors or ARBs, it would be nice to know that the money for the testing is well spent. Unfortunately, in this instance, we can only continue to practice the “standard of care” and hope for future research to definitively answer this question.
Evidence Summary
Our comprehensive literature search found no studies that provide direct evidence for or against continuing to monitor microalbuminuria among patients with diabetes already on ACE inhibitor or ARB therapy. We reviewed and included indirect evidence and expert opinion to answer this clinical question.
Diabetes mellitus is the leading cause of end-stage renal disease in the Western world.1 The prevalence of diabetic nephropathy continues to rise along with the rapidly rising prevalence of obesity and diabetes, with 40% of patients with diabetes at risk of developing nephropathy. One study that followed 5097 patients with type 2 diabetes over a median of 10.4 years found that 2% of patients progressed from normal levels of urinary protein to microalbuminuria; 2.8% changed from microalbuminuria to macroalbuminuria (urine albumin >300 mg in a 24-hour period); and 2.3% progressed to chronic renal disease (creatinine ≥2 mg/dL) from macroalbuminuria per year.2 Chronic renal disease is more likely to occur in those patients whose hypertension or hyperglycemia is poorly controlled.
For patients with diabetes who develop microalbuminuria, the evidence is good for starting ACE inhibitors or ARBs for renal protection.3,4 The American Diabetes Association (ADA) also recommends that patients with diabetes and hypertension and those aged >55 years with other cardiovascular risk factors (history of cardiovascular disease, dyslipidemia, or tobacco use) be started on ACE inhibitors or ARBs.1 There is also good evidence that screening for microalbuminuria can identify those who might benefit from treatments that delay the onset of nephropathy.5
Recent studies have raised the possibility of further benefit in prevention and treatment of diabetic nephropathy by maximizing doses of ACE inhibitors or ARBs, and even from the dual blockade attained from using both. For example, during a 2-year trial—in which 590 patients with diabetes and microalbuminuria were randomized to receive placebo, 150 mg irbesartan, or 300 mg irbesartan—14.9% of the placebo group, 9.7% of the 150-mg group, and only 5.2% of the 300-mg group progressed to overt nephropathy.6 In very small randomized trial of 20 patients with type 2 diabetes, adding 16 mg of candesartan to the maximal dose of an ACE inhibitor decreased albuminuria an additional 28%.7
Experts argue that it may benefit patients to continue regular surveillance for the presence or progression of microalbuminuria even if they are already taking an ACE inhibitor or ARB so that therapy can be maximized.1,8 However, no direct evidence supports this recommendation.
Recommendations from others
The ADA suggests that clinicians perform a test each year for microalbuminuria among patients with type 1 diabetes of ≥5 years duration, and in all patients with type 2 diabetes at diagnosis and during pregnancy. They also recommend continued surveillance of proteinuria to assess both response to therapy and progression of disease.1
The National Kidney Foundation recommends continued surveillance of microalbuminuria to assess progression of chronic kidney disease and response to therapy.8
1. American Diabetes Association. Standards of Medical Care in Diabetes—2006. Diabetes Care 2006;29:S4-S42.
2. Adler AI, Stevens RJ, Manley SE. Development and progression of nephropathy in type 2 diabetes: The united Kingdom Prospective Diabetes study (UKPDS 64). Kidney Intl 2003;63:225-232.
3. Sferra L, Kelsberg G. Do ACE inhibitors prevent nephropathy in type 2 diabetes without proteinuria? J Fam Pract 2004;53:68-69.
4. Foreman BH, Chambliss ML. Are ARBs or ACE inhibitors preferred for nephropathy in diabetes? J Fam Pract 2004;53:241-242.
5. Hale WA, Nashelsky J. Does microalbuminuria screening in diabetes prevent complications? J Fam Pract 2003;52:229-230.
6. Parving HH, Lehnart H, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870-878.
7. Rossing K, Jacobsen P, et al. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 2003;26:2268-2274.
8. Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other Markers of Chronic Kidney Disease: A Position statement of the National Kidney foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Am J Kidney Dis 2003;42:617-622.
No studies address whether continued screening for microalbuminuria once a patient is taking an angiotensin-converting enzyme (ACE) inhibitor or angiotensin-2 receptor blocker (ARB) improves outcomes. Indirect evidence and expert opinion suggest that it may be beneficial to continue microalbuminuria surveillance to assess response to therapy and monitor disease progression (strength of recommendation: C, based on expert opinion).
It is unclear whether microalbuminuria tests in these cases is money well spent
Chris Vincent, MD
Swedish Family Medicine Residency, University of Washington, Seattle
At the residency where I teach, clinicians routinely try to get to the evidence behind the expert opinions, and faculty are discouraged from giving off the cuff or experiential answers. When asked whether to monitor microalbuminuria for patients with diabetes receiving ACE inhibitors or ARBs, it is frustrating to discover that no direct evidence supports the experts’ advice.
The screening test for urine microalbuminuria at our hospital costs $90. Since most patients with diabetes are being treated with ACE inhibitors or ARBs, it would be nice to know that the money for the testing is well spent. Unfortunately, in this instance, we can only continue to practice the “standard of care” and hope for future research to definitively answer this question.
Evidence Summary
Our comprehensive literature search found no studies that provide direct evidence for or against continuing to monitor microalbuminuria among patients with diabetes already on ACE inhibitor or ARB therapy. We reviewed and included indirect evidence and expert opinion to answer this clinical question.
Diabetes mellitus is the leading cause of end-stage renal disease in the Western world.1 The prevalence of diabetic nephropathy continues to rise along with the rapidly rising prevalence of obesity and diabetes, with 40% of patients with diabetes at risk of developing nephropathy. One study that followed 5097 patients with type 2 diabetes over a median of 10.4 years found that 2% of patients progressed from normal levels of urinary protein to microalbuminuria; 2.8% changed from microalbuminuria to macroalbuminuria (urine albumin >300 mg in a 24-hour period); and 2.3% progressed to chronic renal disease (creatinine ≥2 mg/dL) from macroalbuminuria per year.2 Chronic renal disease is more likely to occur in those patients whose hypertension or hyperglycemia is poorly controlled.
For patients with diabetes who develop microalbuminuria, the evidence is good for starting ACE inhibitors or ARBs for renal protection.3,4 The American Diabetes Association (ADA) also recommends that patients with diabetes and hypertension and those aged >55 years with other cardiovascular risk factors (history of cardiovascular disease, dyslipidemia, or tobacco use) be started on ACE inhibitors or ARBs.1 There is also good evidence that screening for microalbuminuria can identify those who might benefit from treatments that delay the onset of nephropathy.5
Recent studies have raised the possibility of further benefit in prevention and treatment of diabetic nephropathy by maximizing doses of ACE inhibitors or ARBs, and even from the dual blockade attained from using both. For example, during a 2-year trial—in which 590 patients with diabetes and microalbuminuria were randomized to receive placebo, 150 mg irbesartan, or 300 mg irbesartan—14.9% of the placebo group, 9.7% of the 150-mg group, and only 5.2% of the 300-mg group progressed to overt nephropathy.6 In very small randomized trial of 20 patients with type 2 diabetes, adding 16 mg of candesartan to the maximal dose of an ACE inhibitor decreased albuminuria an additional 28%.7
Experts argue that it may benefit patients to continue regular surveillance for the presence or progression of microalbuminuria even if they are already taking an ACE inhibitor or ARB so that therapy can be maximized.1,8 However, no direct evidence supports this recommendation.
Recommendations from others
The ADA suggests that clinicians perform a test each year for microalbuminuria among patients with type 1 diabetes of ≥5 years duration, and in all patients with type 2 diabetes at diagnosis and during pregnancy. They also recommend continued surveillance of proteinuria to assess both response to therapy and progression of disease.1
The National Kidney Foundation recommends continued surveillance of microalbuminuria to assess progression of chronic kidney disease and response to therapy.8
No studies address whether continued screening for microalbuminuria once a patient is taking an angiotensin-converting enzyme (ACE) inhibitor or angiotensin-2 receptor blocker (ARB) improves outcomes. Indirect evidence and expert opinion suggest that it may be beneficial to continue microalbuminuria surveillance to assess response to therapy and monitor disease progression (strength of recommendation: C, based on expert opinion).
It is unclear whether microalbuminuria tests in these cases is money well spent
Chris Vincent, MD
Swedish Family Medicine Residency, University of Washington, Seattle
At the residency where I teach, clinicians routinely try to get to the evidence behind the expert opinions, and faculty are discouraged from giving off the cuff or experiential answers. When asked whether to monitor microalbuminuria for patients with diabetes receiving ACE inhibitors or ARBs, it is frustrating to discover that no direct evidence supports the experts’ advice.
The screening test for urine microalbuminuria at our hospital costs $90. Since most patients with diabetes are being treated with ACE inhibitors or ARBs, it would be nice to know that the money for the testing is well spent. Unfortunately, in this instance, we can only continue to practice the “standard of care” and hope for future research to definitively answer this question.
Evidence Summary
Our comprehensive literature search found no studies that provide direct evidence for or against continuing to monitor microalbuminuria among patients with diabetes already on ACE inhibitor or ARB therapy. We reviewed and included indirect evidence and expert opinion to answer this clinical question.
Diabetes mellitus is the leading cause of end-stage renal disease in the Western world.1 The prevalence of diabetic nephropathy continues to rise along with the rapidly rising prevalence of obesity and diabetes, with 40% of patients with diabetes at risk of developing nephropathy. One study that followed 5097 patients with type 2 diabetes over a median of 10.4 years found that 2% of patients progressed from normal levels of urinary protein to microalbuminuria; 2.8% changed from microalbuminuria to macroalbuminuria (urine albumin >300 mg in a 24-hour period); and 2.3% progressed to chronic renal disease (creatinine ≥2 mg/dL) from macroalbuminuria per year.2 Chronic renal disease is more likely to occur in those patients whose hypertension or hyperglycemia is poorly controlled.
For patients with diabetes who develop microalbuminuria, the evidence is good for starting ACE inhibitors or ARBs for renal protection.3,4 The American Diabetes Association (ADA) also recommends that patients with diabetes and hypertension and those aged >55 years with other cardiovascular risk factors (history of cardiovascular disease, dyslipidemia, or tobacco use) be started on ACE inhibitors or ARBs.1 There is also good evidence that screening for microalbuminuria can identify those who might benefit from treatments that delay the onset of nephropathy.5
Recent studies have raised the possibility of further benefit in prevention and treatment of diabetic nephropathy by maximizing doses of ACE inhibitors or ARBs, and even from the dual blockade attained from using both. For example, during a 2-year trial—in which 590 patients with diabetes and microalbuminuria were randomized to receive placebo, 150 mg irbesartan, or 300 mg irbesartan—14.9% of the placebo group, 9.7% of the 150-mg group, and only 5.2% of the 300-mg group progressed to overt nephropathy.6 In very small randomized trial of 20 patients with type 2 diabetes, adding 16 mg of candesartan to the maximal dose of an ACE inhibitor decreased albuminuria an additional 28%.7
Experts argue that it may benefit patients to continue regular surveillance for the presence or progression of microalbuminuria even if they are already taking an ACE inhibitor or ARB so that therapy can be maximized.1,8 However, no direct evidence supports this recommendation.
Recommendations from others
The ADA suggests that clinicians perform a test each year for microalbuminuria among patients with type 1 diabetes of ≥5 years duration, and in all patients with type 2 diabetes at diagnosis and during pregnancy. They also recommend continued surveillance of proteinuria to assess both response to therapy and progression of disease.1
The National Kidney Foundation recommends continued surveillance of microalbuminuria to assess progression of chronic kidney disease and response to therapy.8
1. American Diabetes Association. Standards of Medical Care in Diabetes—2006. Diabetes Care 2006;29:S4-S42.
2. Adler AI, Stevens RJ, Manley SE. Development and progression of nephropathy in type 2 diabetes: The united Kingdom Prospective Diabetes study (UKPDS 64). Kidney Intl 2003;63:225-232.
3. Sferra L, Kelsberg G. Do ACE inhibitors prevent nephropathy in type 2 diabetes without proteinuria? J Fam Pract 2004;53:68-69.
4. Foreman BH, Chambliss ML. Are ARBs or ACE inhibitors preferred for nephropathy in diabetes? J Fam Pract 2004;53:241-242.
5. Hale WA, Nashelsky J. Does microalbuminuria screening in diabetes prevent complications? J Fam Pract 2003;52:229-230.
6. Parving HH, Lehnart H, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870-878.
7. Rossing K, Jacobsen P, et al. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 2003;26:2268-2274.
8. Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other Markers of Chronic Kidney Disease: A Position statement of the National Kidney foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Am J Kidney Dis 2003;42:617-622.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2006. Diabetes Care 2006;29:S4-S42.
2. Adler AI, Stevens RJ, Manley SE. Development and progression of nephropathy in type 2 diabetes: The united Kingdom Prospective Diabetes study (UKPDS 64). Kidney Intl 2003;63:225-232.
3. Sferra L, Kelsberg G. Do ACE inhibitors prevent nephropathy in type 2 diabetes without proteinuria? J Fam Pract 2004;53:68-69.
4. Foreman BH, Chambliss ML. Are ARBs or ACE inhibitors preferred for nephropathy in diabetes? J Fam Pract 2004;53:241-242.
5. Hale WA, Nashelsky J. Does microalbuminuria screening in diabetes prevent complications? J Fam Pract 2003;52:229-230.
6. Parving HH, Lehnart H, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870-878.
7. Rossing K, Jacobsen P, et al. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 2003;26:2268-2274.
8. Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other Markers of Chronic Kidney Disease: A Position statement of the National Kidney foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Am J Kidney Dis 2003;42:617-622.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best approach to the evaluation of resting tachycardia for an adult?
The best evidence about the diagnostic evaluation of resting tachycardias in adults is currently outlined by practice guidelines.1 Initial evaluation includes clinical history, physical examination, and 12-lead electrocardiogram (ECG). If the initial evaluation suggests a sinus tachycardia with narrow QRS complexes and no identifiable secondary cause, a 24-hour Holter monitor is usually recommended (strength of recommendation: C, based on expert opinion).
Wide-complex tachycardias and irregular heartbeats should be urgently managed
Laurel Woods, MD
Group Health Family Medicine Residency Program, Seattle, Wash
This Clinical Inquiry organizes a rational approach to tachycardia, which is frequently an incidental and asymptomatic finding on patient intake. The recommendation of evaluating a 12-lead ECG for sinus vs non-sinus tachycardia, then further investigating underlying causes, helps frame the workup in an approachable manner. Particularly helpful is the pointer that the wide-complex tachycardias and irregular heartbeats should be urgently managed, whereas the rest can be assessed at a more relaxed pace. For nonurgent cases it is important to keep in mind the differential diagnosis and rationally evaluate the likely causes. In my patient population, I tend to see sinus tachycardias in young healthy patients in whom no secondary cause aside from anxiety is identified. Oftentimes I follow up after initiating treatment for anxiety or its underlying cause and find that the tachycardia has resolved. In these cases, I have been less aggressive about ordering a 24-hour Holter monitor.
Evidence Summary
Heart rate varies by age; however, tachycardia in adults is usually defined as a rate exceeding 100 beats/minute.1 Tachycardia at rest requires a diagnostic evaluation. However, our search found no systematic reviews, randomized trials, or prospective cohort studies relevant to this question. The highest level of evidence we located was an international practice guideline developed by the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the European Society of Cardiology Committee for Practice Guidelines.1
This joint guideline recommends that the diagnostic evaluation of a hemodynamically stable patient should begin with a clinical history, physical examination with relevant labs, and 12-lead ECG.1 Many patients with tachycardia are asymptomatic; however, common symptoms and complaints include palpitations, fatigue, lightheadedness, chest discomfort, dyspnea, presyncope, or syncope.1 If the patient has experienced symptoms, it is of crucial importance to obtain a clinical history describing the pattern in terms of the number of episodes, duration, frequency, mode of onset, and possible triggers.1
The main goals of the physical examination, labs, and the 12-lead ECG are to determine if the patient has a sinus or non-sinus tachycardia and to look for other findings that may suggest either a cause for the tachycardia or any complications resulting from the tachycardia.
First, determine if the patient’s heartbeat is regular or irregular. Atrial flutter and atrial fibrillation are common causes of an irregular heartbeat that can easily be diagnosed with a 12-lead ECG. Second, determine the width of the QRS interval: narrow QRS complex tachycardias are supraventricular (from the sinus node, the atria, and the atrioventricular junction) in origin, and wide QRS complex tachycardias are usually ventricular (from all sites below the AV junction).2,3 If an irregular heartbeat or wide-complex tachycardia is detected, appropriate management (including possible urgent referrals) should begin immediately.1 A stable patient with a regular rhythm and a narrow QRS complex can be further investigated at a more relaxed pace.
Refer to the TABLE for a listing of common secondary causes for sinus tachycardia, which should direct lab investigations. If no secondary cause is easily identifiable, a 24-hour monitor is recommended as the next step.
TABLE
Potential secondary causes of resting sinus tachycardia5-7
| Hyperthyroidism |
| Fever |
| Sepsis |
| Anxiety |
| Pheochromocytoma |
| Anemia |
| Hypotension and shock |
| Pulmonary embolism |
| Acute coronary ischemia and myocardial infarction |
| Heart failure |
| Chronic pulmonary disease |
| Hypoxia exposure to medications, stimulants, or illicit drugs |
| Malignancy |
| Pregnancy |
Recommendations from others
Textbook chapters and other review articles regarding this topic describe a similar initial evaluation and provide further details about interpreting the 12-lead ECG.2-7 The most relevant and recent review article suggests that further investigation of narrow QRS complex tachycardias with a regular rate currently involves 4 diagnostic categories: normal sinus tachycardia (ie, secondary cause can be identified), inappropriate sinus tachycardia (IST), postural orthostatic tachycardia syndrome (POTS), and sinus node reentry tachycardia (SNRT).4
If a secondary cause is identified, it should be treated appropriately. If no underlying cause is discovered, a 24-hour Holter monitor is recommended.
Persistent sinus tachycardia (sometimes with nocturnal normalization of heart rate) is diagnosed as IST.4 If the monitor shows paroxysmal episodes of sinus tachycardia, determine if they are triggered by orthostasis and relieved by recumbency (confirm with head upright tilt test) to make the diagnosis of POTS. If it is not POTS, the recordings from the 24-hour Holter monitor help make the diagnosis of SNRT, which consists of sudden, paroxysmal, and usually nonsustained tachycardia.4 The FIGURE shows an algorithm of one common diagnostic strategy for evaluation of tachycardia.2-7
FIGURE
Diagnostic algorithm for evaluating tachycardias4
1. American College of Cardiology/American Heart association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (ACC/AHA/ESC). ACC/AHA/ESC guidelines for the management of patients with surpraventricular arrythmias-executive summary. J Am Coll Cardiol 2003;42:1493-1531.
2. Yusuf S, Camm JA. Deciphering the sinus tachycardias. Clin Cardiol 2005;28:267-276.
3. Martin DT. Arrhythmias. In: Noble J, Greene HL, eds. Textbook of Primary Care Medicine. 3rd ed. St. Louis, Mo: Mosby; 2001:528–537.
4. Stoenescu ML, Kowey PR. Tachycardias. In: Rakel RE, ed. Conn’s Current Therapy. 57th ed. Philadelphia, Pa: Saunders; 2005;354-355.
5. Olgin JE, Zipes DP. Specific arrhythmias: diagnosis and treatment. In: Braunwald E, ed. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pa: Saunders; 2005;803-806.
6. Castellanos A, Moleiro F, Chakko S, et al. Heart rate variability in inappropriate sinus tachycardia. Am J Cardiol 1998;82:531-534.
7. Krahn AD, yee R, Klein GJ, Morillo C. Inappropriate sinus tachycardia: evaluation and treatment. J Cardiovasc Electrophysiol 1995;6:1124-1128.
The best evidence about the diagnostic evaluation of resting tachycardias in adults is currently outlined by practice guidelines.1 Initial evaluation includes clinical history, physical examination, and 12-lead electrocardiogram (ECG). If the initial evaluation suggests a sinus tachycardia with narrow QRS complexes and no identifiable secondary cause, a 24-hour Holter monitor is usually recommended (strength of recommendation: C, based on expert opinion).
Wide-complex tachycardias and irregular heartbeats should be urgently managed
Laurel Woods, MD
Group Health Family Medicine Residency Program, Seattle, Wash
This Clinical Inquiry organizes a rational approach to tachycardia, which is frequently an incidental and asymptomatic finding on patient intake. The recommendation of evaluating a 12-lead ECG for sinus vs non-sinus tachycardia, then further investigating underlying causes, helps frame the workup in an approachable manner. Particularly helpful is the pointer that the wide-complex tachycardias and irregular heartbeats should be urgently managed, whereas the rest can be assessed at a more relaxed pace. For nonurgent cases it is important to keep in mind the differential diagnosis and rationally evaluate the likely causes. In my patient population, I tend to see sinus tachycardias in young healthy patients in whom no secondary cause aside from anxiety is identified. Oftentimes I follow up after initiating treatment for anxiety or its underlying cause and find that the tachycardia has resolved. In these cases, I have been less aggressive about ordering a 24-hour Holter monitor.
Evidence Summary
Heart rate varies by age; however, tachycardia in adults is usually defined as a rate exceeding 100 beats/minute.1 Tachycardia at rest requires a diagnostic evaluation. However, our search found no systematic reviews, randomized trials, or prospective cohort studies relevant to this question. The highest level of evidence we located was an international practice guideline developed by the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the European Society of Cardiology Committee for Practice Guidelines.1
This joint guideline recommends that the diagnostic evaluation of a hemodynamically stable patient should begin with a clinical history, physical examination with relevant labs, and 12-lead ECG.1 Many patients with tachycardia are asymptomatic; however, common symptoms and complaints include palpitations, fatigue, lightheadedness, chest discomfort, dyspnea, presyncope, or syncope.1 If the patient has experienced symptoms, it is of crucial importance to obtain a clinical history describing the pattern in terms of the number of episodes, duration, frequency, mode of onset, and possible triggers.1
The main goals of the physical examination, labs, and the 12-lead ECG are to determine if the patient has a sinus or non-sinus tachycardia and to look for other findings that may suggest either a cause for the tachycardia or any complications resulting from the tachycardia.
First, determine if the patient’s heartbeat is regular or irregular. Atrial flutter and atrial fibrillation are common causes of an irregular heartbeat that can easily be diagnosed with a 12-lead ECG. Second, determine the width of the QRS interval: narrow QRS complex tachycardias are supraventricular (from the sinus node, the atria, and the atrioventricular junction) in origin, and wide QRS complex tachycardias are usually ventricular (from all sites below the AV junction).2,3 If an irregular heartbeat or wide-complex tachycardia is detected, appropriate management (including possible urgent referrals) should begin immediately.1 A stable patient with a regular rhythm and a narrow QRS complex can be further investigated at a more relaxed pace.
Refer to the TABLE for a listing of common secondary causes for sinus tachycardia, which should direct lab investigations. If no secondary cause is easily identifiable, a 24-hour monitor is recommended as the next step.
TABLE
Potential secondary causes of resting sinus tachycardia5-7
| Hyperthyroidism |
| Fever |
| Sepsis |
| Anxiety |
| Pheochromocytoma |
| Anemia |
| Hypotension and shock |
| Pulmonary embolism |
| Acute coronary ischemia and myocardial infarction |
| Heart failure |
| Chronic pulmonary disease |
| Hypoxia exposure to medications, stimulants, or illicit drugs |
| Malignancy |
| Pregnancy |
Recommendations from others
Textbook chapters and other review articles regarding this topic describe a similar initial evaluation and provide further details about interpreting the 12-lead ECG.2-7 The most relevant and recent review article suggests that further investigation of narrow QRS complex tachycardias with a regular rate currently involves 4 diagnostic categories: normal sinus tachycardia (ie, secondary cause can be identified), inappropriate sinus tachycardia (IST), postural orthostatic tachycardia syndrome (POTS), and sinus node reentry tachycardia (SNRT).4
If a secondary cause is identified, it should be treated appropriately. If no underlying cause is discovered, a 24-hour Holter monitor is recommended.
Persistent sinus tachycardia (sometimes with nocturnal normalization of heart rate) is diagnosed as IST.4 If the monitor shows paroxysmal episodes of sinus tachycardia, determine if they are triggered by orthostasis and relieved by recumbency (confirm with head upright tilt test) to make the diagnosis of POTS. If it is not POTS, the recordings from the 24-hour Holter monitor help make the diagnosis of SNRT, which consists of sudden, paroxysmal, and usually nonsustained tachycardia.4 The FIGURE shows an algorithm of one common diagnostic strategy for evaluation of tachycardia.2-7
FIGURE
Diagnostic algorithm for evaluating tachycardias4
The best evidence about the diagnostic evaluation of resting tachycardias in adults is currently outlined by practice guidelines.1 Initial evaluation includes clinical history, physical examination, and 12-lead electrocardiogram (ECG). If the initial evaluation suggests a sinus tachycardia with narrow QRS complexes and no identifiable secondary cause, a 24-hour Holter monitor is usually recommended (strength of recommendation: C, based on expert opinion).
Wide-complex tachycardias and irregular heartbeats should be urgently managed
Laurel Woods, MD
Group Health Family Medicine Residency Program, Seattle, Wash
This Clinical Inquiry organizes a rational approach to tachycardia, which is frequently an incidental and asymptomatic finding on patient intake. The recommendation of evaluating a 12-lead ECG for sinus vs non-sinus tachycardia, then further investigating underlying causes, helps frame the workup in an approachable manner. Particularly helpful is the pointer that the wide-complex tachycardias and irregular heartbeats should be urgently managed, whereas the rest can be assessed at a more relaxed pace. For nonurgent cases it is important to keep in mind the differential diagnosis and rationally evaluate the likely causes. In my patient population, I tend to see sinus tachycardias in young healthy patients in whom no secondary cause aside from anxiety is identified. Oftentimes I follow up after initiating treatment for anxiety or its underlying cause and find that the tachycardia has resolved. In these cases, I have been less aggressive about ordering a 24-hour Holter monitor.
Evidence Summary
Heart rate varies by age; however, tachycardia in adults is usually defined as a rate exceeding 100 beats/minute.1 Tachycardia at rest requires a diagnostic evaluation. However, our search found no systematic reviews, randomized trials, or prospective cohort studies relevant to this question. The highest level of evidence we located was an international practice guideline developed by the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the European Society of Cardiology Committee for Practice Guidelines.1
This joint guideline recommends that the diagnostic evaluation of a hemodynamically stable patient should begin with a clinical history, physical examination with relevant labs, and 12-lead ECG.1 Many patients with tachycardia are asymptomatic; however, common symptoms and complaints include palpitations, fatigue, lightheadedness, chest discomfort, dyspnea, presyncope, or syncope.1 If the patient has experienced symptoms, it is of crucial importance to obtain a clinical history describing the pattern in terms of the number of episodes, duration, frequency, mode of onset, and possible triggers.1
The main goals of the physical examination, labs, and the 12-lead ECG are to determine if the patient has a sinus or non-sinus tachycardia and to look for other findings that may suggest either a cause for the tachycardia or any complications resulting from the tachycardia.
First, determine if the patient’s heartbeat is regular or irregular. Atrial flutter and atrial fibrillation are common causes of an irregular heartbeat that can easily be diagnosed with a 12-lead ECG. Second, determine the width of the QRS interval: narrow QRS complex tachycardias are supraventricular (from the sinus node, the atria, and the atrioventricular junction) in origin, and wide QRS complex tachycardias are usually ventricular (from all sites below the AV junction).2,3 If an irregular heartbeat or wide-complex tachycardia is detected, appropriate management (including possible urgent referrals) should begin immediately.1 A stable patient with a regular rhythm and a narrow QRS complex can be further investigated at a more relaxed pace.
Refer to the TABLE for a listing of common secondary causes for sinus tachycardia, which should direct lab investigations. If no secondary cause is easily identifiable, a 24-hour monitor is recommended as the next step.
TABLE
Potential secondary causes of resting sinus tachycardia5-7
| Hyperthyroidism |
| Fever |
| Sepsis |
| Anxiety |
| Pheochromocytoma |
| Anemia |
| Hypotension and shock |
| Pulmonary embolism |
| Acute coronary ischemia and myocardial infarction |
| Heart failure |
| Chronic pulmonary disease |
| Hypoxia exposure to medications, stimulants, or illicit drugs |
| Malignancy |
| Pregnancy |
Recommendations from others
Textbook chapters and other review articles regarding this topic describe a similar initial evaluation and provide further details about interpreting the 12-lead ECG.2-7 The most relevant and recent review article suggests that further investigation of narrow QRS complex tachycardias with a regular rate currently involves 4 diagnostic categories: normal sinus tachycardia (ie, secondary cause can be identified), inappropriate sinus tachycardia (IST), postural orthostatic tachycardia syndrome (POTS), and sinus node reentry tachycardia (SNRT).4
If a secondary cause is identified, it should be treated appropriately. If no underlying cause is discovered, a 24-hour Holter monitor is recommended.
Persistent sinus tachycardia (sometimes with nocturnal normalization of heart rate) is diagnosed as IST.4 If the monitor shows paroxysmal episodes of sinus tachycardia, determine if they are triggered by orthostasis and relieved by recumbency (confirm with head upright tilt test) to make the diagnosis of POTS. If it is not POTS, the recordings from the 24-hour Holter monitor help make the diagnosis of SNRT, which consists of sudden, paroxysmal, and usually nonsustained tachycardia.4 The FIGURE shows an algorithm of one common diagnostic strategy for evaluation of tachycardia.2-7
FIGURE
Diagnostic algorithm for evaluating tachycardias4
1. American College of Cardiology/American Heart association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (ACC/AHA/ESC). ACC/AHA/ESC guidelines for the management of patients with surpraventricular arrythmias-executive summary. J Am Coll Cardiol 2003;42:1493-1531.
2. Yusuf S, Camm JA. Deciphering the sinus tachycardias. Clin Cardiol 2005;28:267-276.
3. Martin DT. Arrhythmias. In: Noble J, Greene HL, eds. Textbook of Primary Care Medicine. 3rd ed. St. Louis, Mo: Mosby; 2001:528–537.
4. Stoenescu ML, Kowey PR. Tachycardias. In: Rakel RE, ed. Conn’s Current Therapy. 57th ed. Philadelphia, Pa: Saunders; 2005;354-355.
5. Olgin JE, Zipes DP. Specific arrhythmias: diagnosis and treatment. In: Braunwald E, ed. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pa: Saunders; 2005;803-806.
6. Castellanos A, Moleiro F, Chakko S, et al. Heart rate variability in inappropriate sinus tachycardia. Am J Cardiol 1998;82:531-534.
7. Krahn AD, yee R, Klein GJ, Morillo C. Inappropriate sinus tachycardia: evaluation and treatment. J Cardiovasc Electrophysiol 1995;6:1124-1128.
1. American College of Cardiology/American Heart association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (ACC/AHA/ESC). ACC/AHA/ESC guidelines for the management of patients with surpraventricular arrythmias-executive summary. J Am Coll Cardiol 2003;42:1493-1531.
2. Yusuf S, Camm JA. Deciphering the sinus tachycardias. Clin Cardiol 2005;28:267-276.
3. Martin DT. Arrhythmias. In: Noble J, Greene HL, eds. Textbook of Primary Care Medicine. 3rd ed. St. Louis, Mo: Mosby; 2001:528–537.
4. Stoenescu ML, Kowey PR. Tachycardias. In: Rakel RE, ed. Conn’s Current Therapy. 57th ed. Philadelphia, Pa: Saunders; 2005;354-355.
5. Olgin JE, Zipes DP. Specific arrhythmias: diagnosis and treatment. In: Braunwald E, ed. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pa: Saunders; 2005;803-806.
6. Castellanos A, Moleiro F, Chakko S, et al. Heart rate variability in inappropriate sinus tachycardia. Am J Cardiol 1998;82:531-534.
7. Krahn AD, yee R, Klein GJ, Morillo C. Inappropriate sinus tachycardia: evaluation and treatment. J Cardiovasc Electrophysiol 1995;6:1124-1128.
Evidence-based answers from the Family Physicians Inquiries Network
What GI stress ulcer prophylaxis should we provide hospitalized patients?
Patients in intensive care unit (ICE) settings who are receiving prolonged mechanical ventilation (for >48 hours) or who have a coagulopathy or multiple organ dysfunction (especially renal failure) should receive stress ulcer prophylaxis. Current evidence does not support prophylaxis for non-ICU patients1,2 (strength of recommendation [SOR]: B, based on multiple systematic reviews).
Prophylaxis with H2 receptor antagonists (H2RAs) and sucralfate are equally efficacious in lowering mortality and length of hospital stay.3 No randomized controlled trials demonstrate that proton pump inhibitors (PPIs) are superior to H2RAs or sucralfate (SOR: B, based on multiple systematic reviews.)
Consider a protocol to identify patients needing prophylaxis in the ICU
Julia Fashner, MD
Wright State University Boonshoft School of Medicine, Detroit, Mich
Many patients may enter the hospital already on a PPI for reflux disease or prevention of gastrointestinal side effects from other medications. This Clinical Inquiry shows that only certain patients in the hospital will benefit from prophylaxis for stress ulcers and have less bleeding. Therefore, consider using a protocol to identify those specific patients in the ICU and place them on an H2 blocker, PPI or sucralfate automatically.
Evidence summary
Critically ill patients are at increased risk of bleeding from stress-induced gastroduodenal ulceration. Decades ago, ICUs began using pharmacologic prophylaxis on most patients to prevent gastrointestinal bleeding, which had a mortality rate as high as 80%. Before the advent of prophylaxis, the incidence of upper gastro-intestinal bleeding was 6% to 25%.4 Since then, improvements in ICU management have decreased this incidence to 0% to 2.8%.5 Recent studies suggest that only ICU patients with certain risk factors benefit from ulcer prophylaxis (TABLE).1
Our search retrieved 20 randomized controlled trials and 6 systematic reviews with meta-analyses from the Medline database since 1990. It was difficult to find a consensus on the matter of stress ulcer prophylaxis because of inconsistencies in the outcomes measured in these studies. We focused on studies examining clinically important bleeding, but even in these studies definitions and measurements vary. Few studies addressed mortality or length of stay; those that did reported no significant difference in either outcome with prophylaxis.
Medications used to prevent gastrointestinal bleeding have included antacids, sucralfate, H2RAs, and PPIs. Sucralfate and H2RAs have been studied most frequently, and both agents significantly reduce the incidence of clinically important bleeding in high-risk patients. Compared with placebo, the odds ratio for clinically important bleeding was 0.44 with ranitidine (95% confidence interval [CI], 0.22–0.88) and 0.58 with sucralfate (95% CI, 0.34–0.99).6 In a population with a clinically important bleeding incidence of 3% to 6%, a range consistent with the most recent studies we reviewed, the number needed to treat to prevent 1 bleeding episode is 30 to 60 for ranitidine and 40 to 79 for sucralfate.
Some studies suggest that pharmacologic prophylaxis may increase the incidence of aspiration pneumonia in ventilator-dependent patients. The largest randomized trial addressing this issue (N=1200) found no significant difference between H2RAs and sucralfate in ventilator-associated pneumonia.3 Improved ICU management, such as frequent suctioning, upright positioning, and use of enteral nutrition may help prevent nosocomial pneumonia due to aspiration.
TABLE
Risk factors for stress ulcers
| STRESS ULCER RISK FACTORS | ODDS RATIOS FOR CLINICALLY IMPORTANT BLEEDING (95% CI) |
|---|---|
| Mechanical ventilation >48 hours5 | 3.4 (1.0–11) |
| Platelet count <50,0001,2 | 2.58 (1.19–5.57) |
| Maximum serum creatinine1 | 1.16 (1.02–1.32) |
Recommendation from others
In the American Journal of Health-System Pharmacy, Allen et al5 state “the frequency of clinically important bleeding is low … the majority of recently published prospective studies and meta-analyses have been unable to demonstrate a reduction in clinically important bleeding with pharmacologic agents.” A 2001 Agency for Healthcare Research and Quality evidence report7 states that the evidence is not conclusive that all intensive care patients benefit from stress ulcer prophylaxis and that clinicians “may consider use of prophylactic agents in very high risk patients.”
1. Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med 1999;27:2812-2817.
2. Cook DJ, Reeve BK, Scholes LC. Histamine-2-receptor antagonists and antacids in the critically ill population: stress ulceration versus nosocomial pneumonia. Infect Control Hosp Epidemiol 1994;15:437-442.
3. Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 1998;338:791-797.
4. Zinner MJ, Rypins EB, Martin LR, et al. Misoprostel versus antacid titration for preventing stress Ulcers in postoperative surgical ICU patients. Ann Surg 1989;210:590-595.
5. Allen ME, Kopp BJ, Erstad BL. Stress ulcer prophylaxis in the postoperative period. Am J Health Syst Pharm 2004;61:588-596.
6. Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA 1996;275:308-314.
7. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment #43. Contract no. 290-97-0013, Chapter 43, AHRQ 2001, Rockville, Md.
Patients in intensive care unit (ICE) settings who are receiving prolonged mechanical ventilation (for >48 hours) or who have a coagulopathy or multiple organ dysfunction (especially renal failure) should receive stress ulcer prophylaxis. Current evidence does not support prophylaxis for non-ICU patients1,2 (strength of recommendation [SOR]: B, based on multiple systematic reviews).
Prophylaxis with H2 receptor antagonists (H2RAs) and sucralfate are equally efficacious in lowering mortality and length of hospital stay.3 No randomized controlled trials demonstrate that proton pump inhibitors (PPIs) are superior to H2RAs or sucralfate (SOR: B, based on multiple systematic reviews.)
Consider a protocol to identify patients needing prophylaxis in the ICU
Julia Fashner, MD
Wright State University Boonshoft School of Medicine, Detroit, Mich
Many patients may enter the hospital already on a PPI for reflux disease or prevention of gastrointestinal side effects from other medications. This Clinical Inquiry shows that only certain patients in the hospital will benefit from prophylaxis for stress ulcers and have less bleeding. Therefore, consider using a protocol to identify those specific patients in the ICU and place them on an H2 blocker, PPI or sucralfate automatically.
Evidence summary
Critically ill patients are at increased risk of bleeding from stress-induced gastroduodenal ulceration. Decades ago, ICUs began using pharmacologic prophylaxis on most patients to prevent gastrointestinal bleeding, which had a mortality rate as high as 80%. Before the advent of prophylaxis, the incidence of upper gastro-intestinal bleeding was 6% to 25%.4 Since then, improvements in ICU management have decreased this incidence to 0% to 2.8%.5 Recent studies suggest that only ICU patients with certain risk factors benefit from ulcer prophylaxis (TABLE).1
Our search retrieved 20 randomized controlled trials and 6 systematic reviews with meta-analyses from the Medline database since 1990. It was difficult to find a consensus on the matter of stress ulcer prophylaxis because of inconsistencies in the outcomes measured in these studies. We focused on studies examining clinically important bleeding, but even in these studies definitions and measurements vary. Few studies addressed mortality or length of stay; those that did reported no significant difference in either outcome with prophylaxis.
Medications used to prevent gastrointestinal bleeding have included antacids, sucralfate, H2RAs, and PPIs. Sucralfate and H2RAs have been studied most frequently, and both agents significantly reduce the incidence of clinically important bleeding in high-risk patients. Compared with placebo, the odds ratio for clinically important bleeding was 0.44 with ranitidine (95% confidence interval [CI], 0.22–0.88) and 0.58 with sucralfate (95% CI, 0.34–0.99).6 In a population with a clinically important bleeding incidence of 3% to 6%, a range consistent with the most recent studies we reviewed, the number needed to treat to prevent 1 bleeding episode is 30 to 60 for ranitidine and 40 to 79 for sucralfate.
Some studies suggest that pharmacologic prophylaxis may increase the incidence of aspiration pneumonia in ventilator-dependent patients. The largest randomized trial addressing this issue (N=1200) found no significant difference between H2RAs and sucralfate in ventilator-associated pneumonia.3 Improved ICU management, such as frequent suctioning, upright positioning, and use of enteral nutrition may help prevent nosocomial pneumonia due to aspiration.
TABLE
Risk factors for stress ulcers
| STRESS ULCER RISK FACTORS | ODDS RATIOS FOR CLINICALLY IMPORTANT BLEEDING (95% CI) |
|---|---|
| Mechanical ventilation >48 hours5 | 3.4 (1.0–11) |
| Platelet count <50,0001,2 | 2.58 (1.19–5.57) |
| Maximum serum creatinine1 | 1.16 (1.02–1.32) |
Recommendation from others
In the American Journal of Health-System Pharmacy, Allen et al5 state “the frequency of clinically important bleeding is low … the majority of recently published prospective studies and meta-analyses have been unable to demonstrate a reduction in clinically important bleeding with pharmacologic agents.” A 2001 Agency for Healthcare Research and Quality evidence report7 states that the evidence is not conclusive that all intensive care patients benefit from stress ulcer prophylaxis and that clinicians “may consider use of prophylactic agents in very high risk patients.”
Patients in intensive care unit (ICE) settings who are receiving prolonged mechanical ventilation (for >48 hours) or who have a coagulopathy or multiple organ dysfunction (especially renal failure) should receive stress ulcer prophylaxis. Current evidence does not support prophylaxis for non-ICU patients1,2 (strength of recommendation [SOR]: B, based on multiple systematic reviews).
Prophylaxis with H2 receptor antagonists (H2RAs) and sucralfate are equally efficacious in lowering mortality and length of hospital stay.3 No randomized controlled trials demonstrate that proton pump inhibitors (PPIs) are superior to H2RAs or sucralfate (SOR: B, based on multiple systematic reviews.)
Consider a protocol to identify patients needing prophylaxis in the ICU
Julia Fashner, MD
Wright State University Boonshoft School of Medicine, Detroit, Mich
Many patients may enter the hospital already on a PPI for reflux disease or prevention of gastrointestinal side effects from other medications. This Clinical Inquiry shows that only certain patients in the hospital will benefit from prophylaxis for stress ulcers and have less bleeding. Therefore, consider using a protocol to identify those specific patients in the ICU and place them on an H2 blocker, PPI or sucralfate automatically.
Evidence summary
Critically ill patients are at increased risk of bleeding from stress-induced gastroduodenal ulceration. Decades ago, ICUs began using pharmacologic prophylaxis on most patients to prevent gastrointestinal bleeding, which had a mortality rate as high as 80%. Before the advent of prophylaxis, the incidence of upper gastro-intestinal bleeding was 6% to 25%.4 Since then, improvements in ICU management have decreased this incidence to 0% to 2.8%.5 Recent studies suggest that only ICU patients with certain risk factors benefit from ulcer prophylaxis (TABLE).1
Our search retrieved 20 randomized controlled trials and 6 systematic reviews with meta-analyses from the Medline database since 1990. It was difficult to find a consensus on the matter of stress ulcer prophylaxis because of inconsistencies in the outcomes measured in these studies. We focused on studies examining clinically important bleeding, but even in these studies definitions and measurements vary. Few studies addressed mortality or length of stay; those that did reported no significant difference in either outcome with prophylaxis.
Medications used to prevent gastrointestinal bleeding have included antacids, sucralfate, H2RAs, and PPIs. Sucralfate and H2RAs have been studied most frequently, and both agents significantly reduce the incidence of clinically important bleeding in high-risk patients. Compared with placebo, the odds ratio for clinically important bleeding was 0.44 with ranitidine (95% confidence interval [CI], 0.22–0.88) and 0.58 with sucralfate (95% CI, 0.34–0.99).6 In a population with a clinically important bleeding incidence of 3% to 6%, a range consistent with the most recent studies we reviewed, the number needed to treat to prevent 1 bleeding episode is 30 to 60 for ranitidine and 40 to 79 for sucralfate.
Some studies suggest that pharmacologic prophylaxis may increase the incidence of aspiration pneumonia in ventilator-dependent patients. The largest randomized trial addressing this issue (N=1200) found no significant difference between H2RAs and sucralfate in ventilator-associated pneumonia.3 Improved ICU management, such as frequent suctioning, upright positioning, and use of enteral nutrition may help prevent nosocomial pneumonia due to aspiration.
TABLE
Risk factors for stress ulcers
| STRESS ULCER RISK FACTORS | ODDS RATIOS FOR CLINICALLY IMPORTANT BLEEDING (95% CI) |
|---|---|
| Mechanical ventilation >48 hours5 | 3.4 (1.0–11) |
| Platelet count <50,0001,2 | 2.58 (1.19–5.57) |
| Maximum serum creatinine1 | 1.16 (1.02–1.32) |
Recommendation from others
In the American Journal of Health-System Pharmacy, Allen et al5 state “the frequency of clinically important bleeding is low … the majority of recently published prospective studies and meta-analyses have been unable to demonstrate a reduction in clinically important bleeding with pharmacologic agents.” A 2001 Agency for Healthcare Research and Quality evidence report7 states that the evidence is not conclusive that all intensive care patients benefit from stress ulcer prophylaxis and that clinicians “may consider use of prophylactic agents in very high risk patients.”
1. Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med 1999;27:2812-2817.
2. Cook DJ, Reeve BK, Scholes LC. Histamine-2-receptor antagonists and antacids in the critically ill population: stress ulceration versus nosocomial pneumonia. Infect Control Hosp Epidemiol 1994;15:437-442.
3. Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 1998;338:791-797.
4. Zinner MJ, Rypins EB, Martin LR, et al. Misoprostel versus antacid titration for preventing stress Ulcers in postoperative surgical ICU patients. Ann Surg 1989;210:590-595.
5. Allen ME, Kopp BJ, Erstad BL. Stress ulcer prophylaxis in the postoperative period. Am J Health Syst Pharm 2004;61:588-596.
6. Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA 1996;275:308-314.
7. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment #43. Contract no. 290-97-0013, Chapter 43, AHRQ 2001, Rockville, Md.
1. Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med 1999;27:2812-2817.
2. Cook DJ, Reeve BK, Scholes LC. Histamine-2-receptor antagonists and antacids in the critically ill population: stress ulceration versus nosocomial pneumonia. Infect Control Hosp Epidemiol 1994;15:437-442.
3. Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 1998;338:791-797.
4. Zinner MJ, Rypins EB, Martin LR, et al. Misoprostel versus antacid titration for preventing stress Ulcers in postoperative surgical ICU patients. Ann Surg 1989;210:590-595.
5. Allen ME, Kopp BJ, Erstad BL. Stress ulcer prophylaxis in the postoperative period. Am J Health Syst Pharm 2004;61:588-596.
6. Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA 1996;275:308-314.
7. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment #43. Contract no. 290-97-0013, Chapter 43, AHRQ 2001, Rockville, Md.
Evidence-based answers from the Family Physicians Inquiries Network
Do allergy shots help seasonal allergies more than antihistamines and nasal steroids?
Multiple randomized controlled trials (RCTs) demonstrate the effectiveness of both allergen immunotherapy and antihistamines, with or without nasal steroids, in the treatment of seasonal allergic rhinitis (strength of recommendation [SOR]: A). No RCTs directly compare immunotherapy with conservative management. Treatment decisions are driven by the clinical presentation, patient and physician preferences, practice guidelines, and expert opinion1 (SOR: C, based on expert opinion). In standard practice, immunotherapy is not recommended for most patients with seasonal allergic rhinitis.
Usually there’s an acceptable treatment alternative with better symptom control or fewer side effects
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
When patients ask me about allergy shots, I ask them to tell me about their concerns about their allergies and experiences with previous treatments. Often I find that they do not really want shots, but just want to feel better! Usually you can find an acceptable treatment alternative, one with better symptom control or fewer side effects.
When patients are referred for immunotherapy, it’s important for them to have realistic expectations. The initial process involves weekly visits, and it may take years to gain adequate symptom control. For patients with the commitment, time, and insurance coverage, however, the outcomes can be very positive.
Evidence summary
A 2002 Agency for Healthcare Research and Quality systematic review on the diagnosis and treatment of allergic rhinitis found no RCTs comparing antihistamines or nasal corticosteroids with immunotherapy.2 Our literature review found 4 studies not included in this report that compared immunotherapy with nasal steroids or oral antihistamines.3-6 Only 2 of these examined patient-oriented outcomes and both are of poor quality.3,6 One study reported that inhaled nasal steroid therapy was superior to a nonstandard immunotherapy for ragweed pollen–induced rhinitis.3 The second study allowed patients to choose a treatment arm; it found that immunotherapy was superior to treatment with antihistamines and nasal steroids for patients who chose it.6
For patients requiring medication, studies comparing antihistamines with nasal corticosteroids have documented the superiority of intranasal steroids for symptom control of allergic rhinitis.2,7
The effectiveness of immunotherapy has been documented in more than 40 placebo-controlled trials. However, the patients involved in these trials were often concurrently treated with allergy medications.8 In standard practice, immunotherapy is not recommended for most patients with seasonal allergic rhinitis unless avoidance measures and symptomatic therapy are ineffective, have adverse effects, or are not feasible.9 Studies indicate that immunotherapy is effective for several years after treatment is discontinued.10
A review of recent placebo-controlled trials indicates that the risk of developing asthma among patients with allergic rhinoconjunctivitis is significantly reduced when patients receive specific immunotherapy.11 However, allergy immunotherapy presents risk of systemic reactions, with one study reporting a 0.5% risk of systemic reactions per year of therapy.12
Recommendations from others
The American College of Allergy, Asthma, and Immunology recommends that effective management of allergic rhinitis may require combinations of medications—antihistamines, decongestants, nasal corticosteroids, and anticholinergic agents as well as aggressive avoidance of rhinitis triggers. Consider allergen immunotherapy in carefully selected patients in consultation with an allergist-immunologist.10
1. Rachelefsky GS. National guidelines needed to manage rhinitis and prevent complications. Ann Allergy Asthma Immunol 1999;82:296-305.
2. Long A, et al. Management of allergic and nonallergic rhinitis. Evid Rep Technol Assess (Summ) 2002;54:1-6.
3. Juniper EF, et al. Comparison of the efficacy and side effects of aqueous steroid nasal spray (budesonide) and allergen-injection therapy (Pollinex-R) in the treatment of seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 1990;85:606-611.
4. Rak S, et al. A double-blinded, comparative study of the effects of short preseason specific immunotherapy and topical steroids in patients with allergic rhinoconjunctivitis and asthma. J Allergy Clin Immunol 2001;108:921-928.
5. Rak S, Heinrich C, Scheynius A. Comparison of nasal immunohistology in patients with seasonal rhinoconjunctivitis treated with topical steroids or specific allergen immunotherapy. Allergy 2005;60:643-649.
6. Giovannini M, et al. Comparison of allergen immunotherapy and drug treatment in seasonal rhinoconjunctivitis: a 3-years study. Allerg Immunol (Paris) 2005;37:69-71.
7. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998;317:1624-1629.
8. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998;102:558-562.
9. Naclerio R, Solomon W. Rhinitis and inhalant allergens. JAMA 1997;278:1842-1848.
10. American Academy of Allergy, Asthma and Immunology and American College of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter. Ann Allergy Asthma Immunol 2003;90(1 Suppl 1):1-40.
11. Dinakar C, Portnoy JM. Allergen immunotherapy in the prevention of asthma. Curr Opin Allergy Clin Immunol 2004;4:131-136.
12. Matloff SM, et al. Systemic reactions to immunotherapy. Allergy Proc 1993;14:347-350.
Multiple randomized controlled trials (RCTs) demonstrate the effectiveness of both allergen immunotherapy and antihistamines, with or without nasal steroids, in the treatment of seasonal allergic rhinitis (strength of recommendation [SOR]: A). No RCTs directly compare immunotherapy with conservative management. Treatment decisions are driven by the clinical presentation, patient and physician preferences, practice guidelines, and expert opinion1 (SOR: C, based on expert opinion). In standard practice, immunotherapy is not recommended for most patients with seasonal allergic rhinitis.
Usually there’s an acceptable treatment alternative with better symptom control or fewer side effects
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
When patients ask me about allergy shots, I ask them to tell me about their concerns about their allergies and experiences with previous treatments. Often I find that they do not really want shots, but just want to feel better! Usually you can find an acceptable treatment alternative, one with better symptom control or fewer side effects.
When patients are referred for immunotherapy, it’s important for them to have realistic expectations. The initial process involves weekly visits, and it may take years to gain adequate symptom control. For patients with the commitment, time, and insurance coverage, however, the outcomes can be very positive.
Evidence summary
A 2002 Agency for Healthcare Research and Quality systematic review on the diagnosis and treatment of allergic rhinitis found no RCTs comparing antihistamines or nasal corticosteroids with immunotherapy.2 Our literature review found 4 studies not included in this report that compared immunotherapy with nasal steroids or oral antihistamines.3-6 Only 2 of these examined patient-oriented outcomes and both are of poor quality.3,6 One study reported that inhaled nasal steroid therapy was superior to a nonstandard immunotherapy for ragweed pollen–induced rhinitis.3 The second study allowed patients to choose a treatment arm; it found that immunotherapy was superior to treatment with antihistamines and nasal steroids for patients who chose it.6
For patients requiring medication, studies comparing antihistamines with nasal corticosteroids have documented the superiority of intranasal steroids for symptom control of allergic rhinitis.2,7
The effectiveness of immunotherapy has been documented in more than 40 placebo-controlled trials. However, the patients involved in these trials were often concurrently treated with allergy medications.8 In standard practice, immunotherapy is not recommended for most patients with seasonal allergic rhinitis unless avoidance measures and symptomatic therapy are ineffective, have adverse effects, or are not feasible.9 Studies indicate that immunotherapy is effective for several years after treatment is discontinued.10
A review of recent placebo-controlled trials indicates that the risk of developing asthma among patients with allergic rhinoconjunctivitis is significantly reduced when patients receive specific immunotherapy.11 However, allergy immunotherapy presents risk of systemic reactions, with one study reporting a 0.5% risk of systemic reactions per year of therapy.12
Recommendations from others
The American College of Allergy, Asthma, and Immunology recommends that effective management of allergic rhinitis may require combinations of medications—antihistamines, decongestants, nasal corticosteroids, and anticholinergic agents as well as aggressive avoidance of rhinitis triggers. Consider allergen immunotherapy in carefully selected patients in consultation with an allergist-immunologist.10
Multiple randomized controlled trials (RCTs) demonstrate the effectiveness of both allergen immunotherapy and antihistamines, with or without nasal steroids, in the treatment of seasonal allergic rhinitis (strength of recommendation [SOR]: A). No RCTs directly compare immunotherapy with conservative management. Treatment decisions are driven by the clinical presentation, patient and physician preferences, practice guidelines, and expert opinion1 (SOR: C, based on expert opinion). In standard practice, immunotherapy is not recommended for most patients with seasonal allergic rhinitis.
Usually there’s an acceptable treatment alternative with better symptom control or fewer side effects
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
When patients ask me about allergy shots, I ask them to tell me about their concerns about their allergies and experiences with previous treatments. Often I find that they do not really want shots, but just want to feel better! Usually you can find an acceptable treatment alternative, one with better symptom control or fewer side effects.
When patients are referred for immunotherapy, it’s important for them to have realistic expectations. The initial process involves weekly visits, and it may take years to gain adequate symptom control. For patients with the commitment, time, and insurance coverage, however, the outcomes can be very positive.
Evidence summary
A 2002 Agency for Healthcare Research and Quality systematic review on the diagnosis and treatment of allergic rhinitis found no RCTs comparing antihistamines or nasal corticosteroids with immunotherapy.2 Our literature review found 4 studies not included in this report that compared immunotherapy with nasal steroids or oral antihistamines.3-6 Only 2 of these examined patient-oriented outcomes and both are of poor quality.3,6 One study reported that inhaled nasal steroid therapy was superior to a nonstandard immunotherapy for ragweed pollen–induced rhinitis.3 The second study allowed patients to choose a treatment arm; it found that immunotherapy was superior to treatment with antihistamines and nasal steroids for patients who chose it.6
For patients requiring medication, studies comparing antihistamines with nasal corticosteroids have documented the superiority of intranasal steroids for symptom control of allergic rhinitis.2,7
The effectiveness of immunotherapy has been documented in more than 40 placebo-controlled trials. However, the patients involved in these trials were often concurrently treated with allergy medications.8 In standard practice, immunotherapy is not recommended for most patients with seasonal allergic rhinitis unless avoidance measures and symptomatic therapy are ineffective, have adverse effects, or are not feasible.9 Studies indicate that immunotherapy is effective for several years after treatment is discontinued.10
A review of recent placebo-controlled trials indicates that the risk of developing asthma among patients with allergic rhinoconjunctivitis is significantly reduced when patients receive specific immunotherapy.11 However, allergy immunotherapy presents risk of systemic reactions, with one study reporting a 0.5% risk of systemic reactions per year of therapy.12
Recommendations from others
The American College of Allergy, Asthma, and Immunology recommends that effective management of allergic rhinitis may require combinations of medications—antihistamines, decongestants, nasal corticosteroids, and anticholinergic agents as well as aggressive avoidance of rhinitis triggers. Consider allergen immunotherapy in carefully selected patients in consultation with an allergist-immunologist.10
1. Rachelefsky GS. National guidelines needed to manage rhinitis and prevent complications. Ann Allergy Asthma Immunol 1999;82:296-305.
2. Long A, et al. Management of allergic and nonallergic rhinitis. Evid Rep Technol Assess (Summ) 2002;54:1-6.
3. Juniper EF, et al. Comparison of the efficacy and side effects of aqueous steroid nasal spray (budesonide) and allergen-injection therapy (Pollinex-R) in the treatment of seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 1990;85:606-611.
4. Rak S, et al. A double-blinded, comparative study of the effects of short preseason specific immunotherapy and topical steroids in patients with allergic rhinoconjunctivitis and asthma. J Allergy Clin Immunol 2001;108:921-928.
5. Rak S, Heinrich C, Scheynius A. Comparison of nasal immunohistology in patients with seasonal rhinoconjunctivitis treated with topical steroids or specific allergen immunotherapy. Allergy 2005;60:643-649.
6. Giovannini M, et al. Comparison of allergen immunotherapy and drug treatment in seasonal rhinoconjunctivitis: a 3-years study. Allerg Immunol (Paris) 2005;37:69-71.
7. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998;317:1624-1629.
8. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998;102:558-562.
9. Naclerio R, Solomon W. Rhinitis and inhalant allergens. JAMA 1997;278:1842-1848.
10. American Academy of Allergy, Asthma and Immunology and American College of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter. Ann Allergy Asthma Immunol 2003;90(1 Suppl 1):1-40.
11. Dinakar C, Portnoy JM. Allergen immunotherapy in the prevention of asthma. Curr Opin Allergy Clin Immunol 2004;4:131-136.
12. Matloff SM, et al. Systemic reactions to immunotherapy. Allergy Proc 1993;14:347-350.
1. Rachelefsky GS. National guidelines needed to manage rhinitis and prevent complications. Ann Allergy Asthma Immunol 1999;82:296-305.
2. Long A, et al. Management of allergic and nonallergic rhinitis. Evid Rep Technol Assess (Summ) 2002;54:1-6.
3. Juniper EF, et al. Comparison of the efficacy and side effects of aqueous steroid nasal spray (budesonide) and allergen-injection therapy (Pollinex-R) in the treatment of seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 1990;85:606-611.
4. Rak S, et al. A double-blinded, comparative study of the effects of short preseason specific immunotherapy and topical steroids in patients with allergic rhinoconjunctivitis and asthma. J Allergy Clin Immunol 2001;108:921-928.
5. Rak S, Heinrich C, Scheynius A. Comparison of nasal immunohistology in patients with seasonal rhinoconjunctivitis treated with topical steroids or specific allergen immunotherapy. Allergy 2005;60:643-649.
6. Giovannini M, et al. Comparison of allergen immunotherapy and drug treatment in seasonal rhinoconjunctivitis: a 3-years study. Allerg Immunol (Paris) 2005;37:69-71.
7. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998;317:1624-1629.
8. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998;102:558-562.
9. Naclerio R, Solomon W. Rhinitis and inhalant allergens. JAMA 1997;278:1842-1848.
10. American Academy of Allergy, Asthma and Immunology and American College of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter. Ann Allergy Asthma Immunol 2003;90(1 Suppl 1):1-40.
11. Dinakar C, Portnoy JM. Allergen immunotherapy in the prevention of asthma. Curr Opin Allergy Clin Immunol 2004;4:131-136.
12. Matloff SM, et al. Systemic reactions to immunotherapy. Allergy Proc 1993;14:347-350.
Evidence-based answers from the Family Physicians Inquiries Network