User login
Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
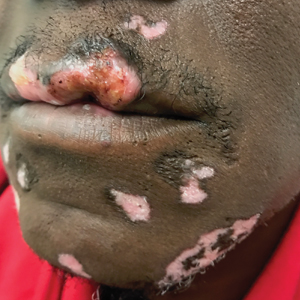
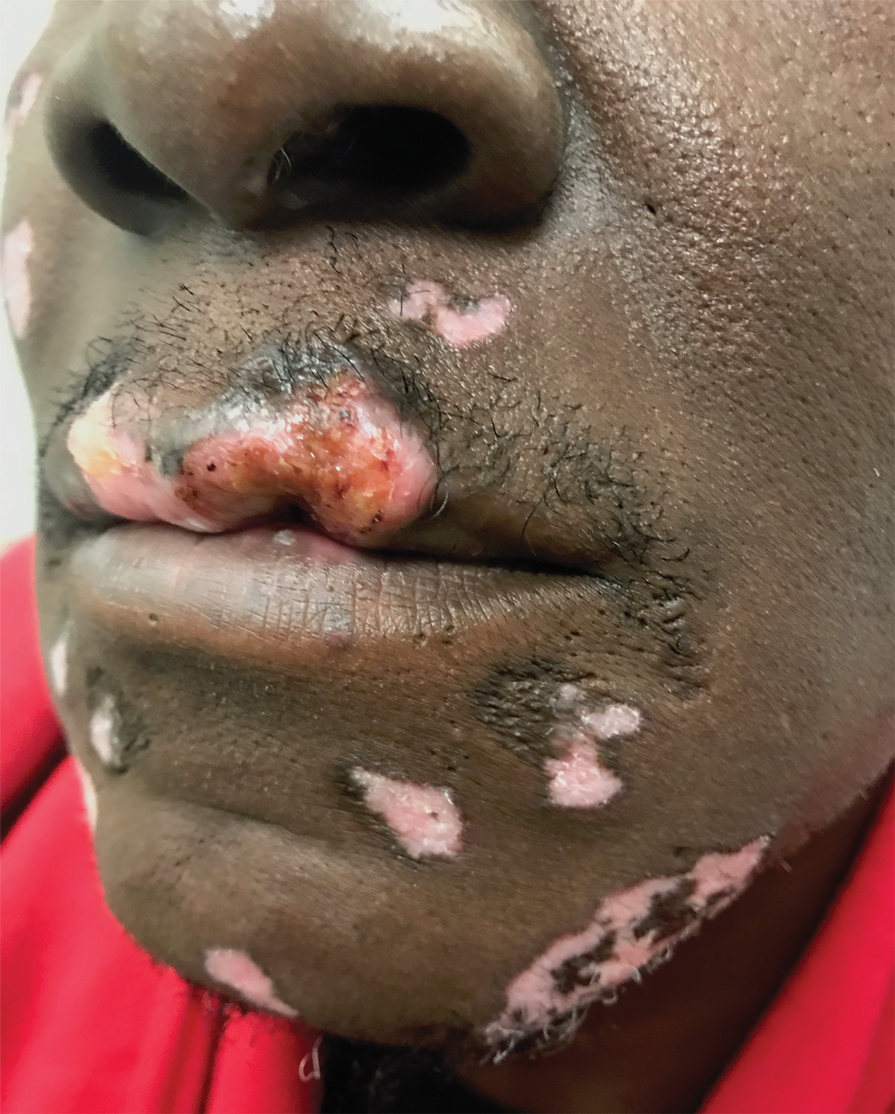
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10
Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
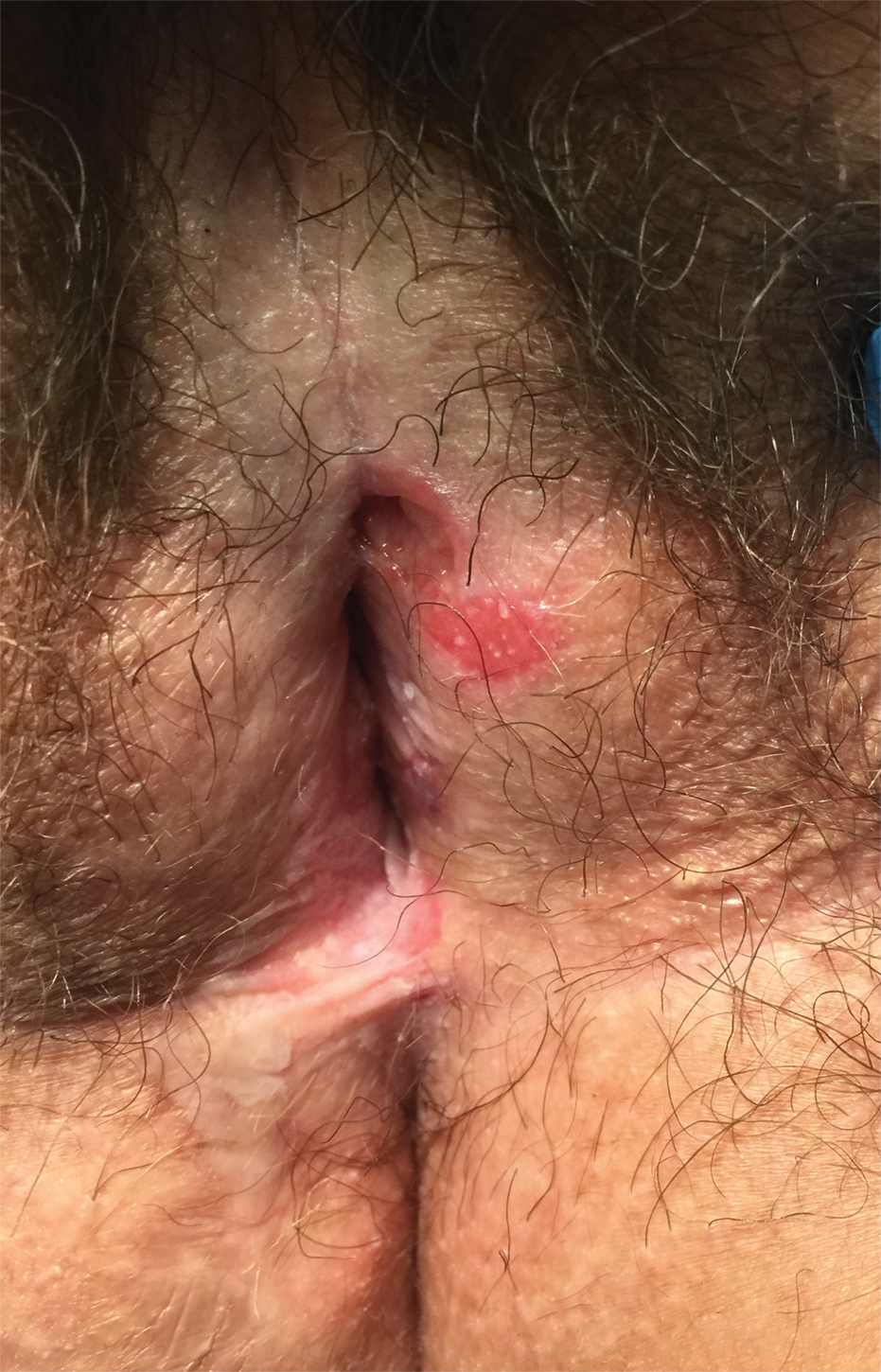
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28
In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
PRACTICE POINTS
- Squamous cell carcinoma can develop within chronic inflammatory dermatoses.
- Orolabial discoid lupus erythematosus (DLE), oral lichen planus, and lichen sclerosus can lead to relatively rapid tumorigenesis. Squamous cell carcinoma arising in cutaneous DLE, hidradenitis suppurativa (HS), necrobiosis lipoidica, chronic wounds, and hypertrophic lichen planus tends to appear after decades of inflammation.
- Be especially mindful of new orolabial DLE cases and chronic cases of HS and Marjolin ulcer because malignancies in these settings are particularly aggressive.