User login
Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
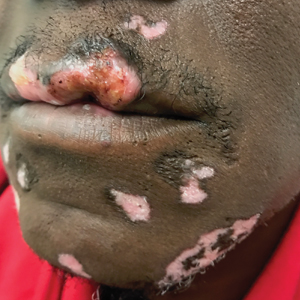
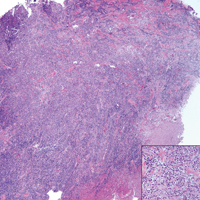
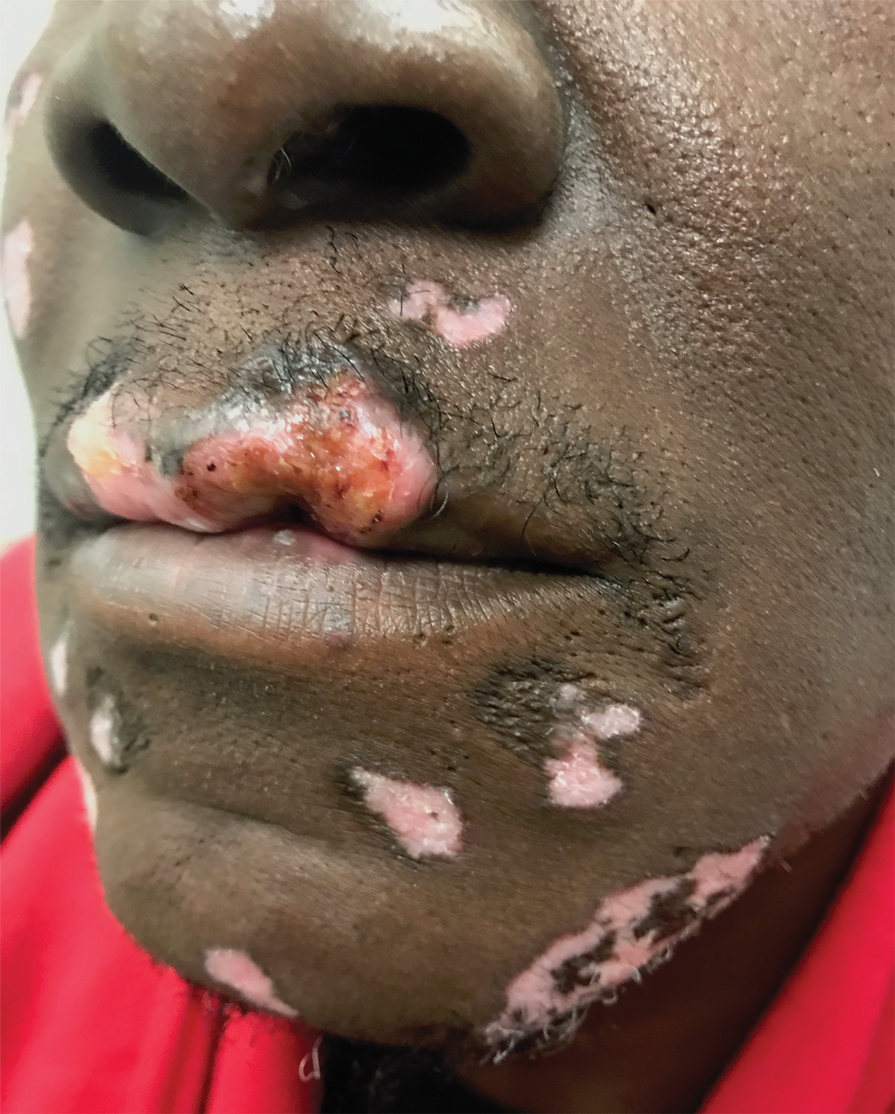
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10
Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
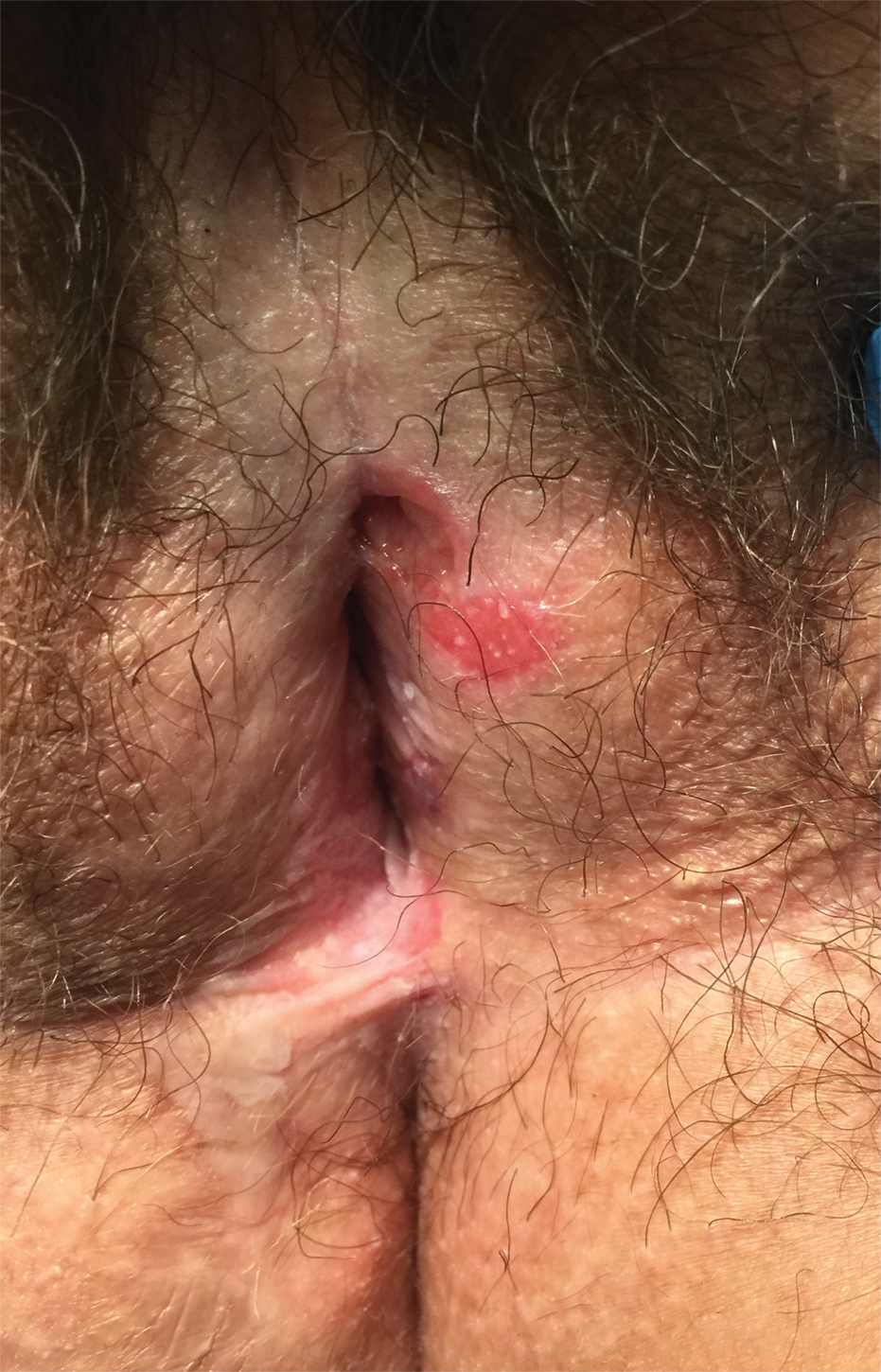
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28
In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
PRACTICE POINTS
- Squamous cell carcinoma can develop within chronic inflammatory dermatoses.
- Orolabial discoid lupus erythematosus (DLE), oral lichen planus, and lichen sclerosus can lead to relatively rapid tumorigenesis. Squamous cell carcinoma arising in cutaneous DLE, hidradenitis suppurativa (HS), necrobiosis lipoidica, chronic wounds, and hypertrophic lichen planus tends to appear after decades of inflammation.
- Be especially mindful of new orolabial DLE cases and chronic cases of HS and Marjolin ulcer because malignancies in these settings are particularly aggressive.
Results From the First Annual Association of Professors of Dermatology Program Directors Survey
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).
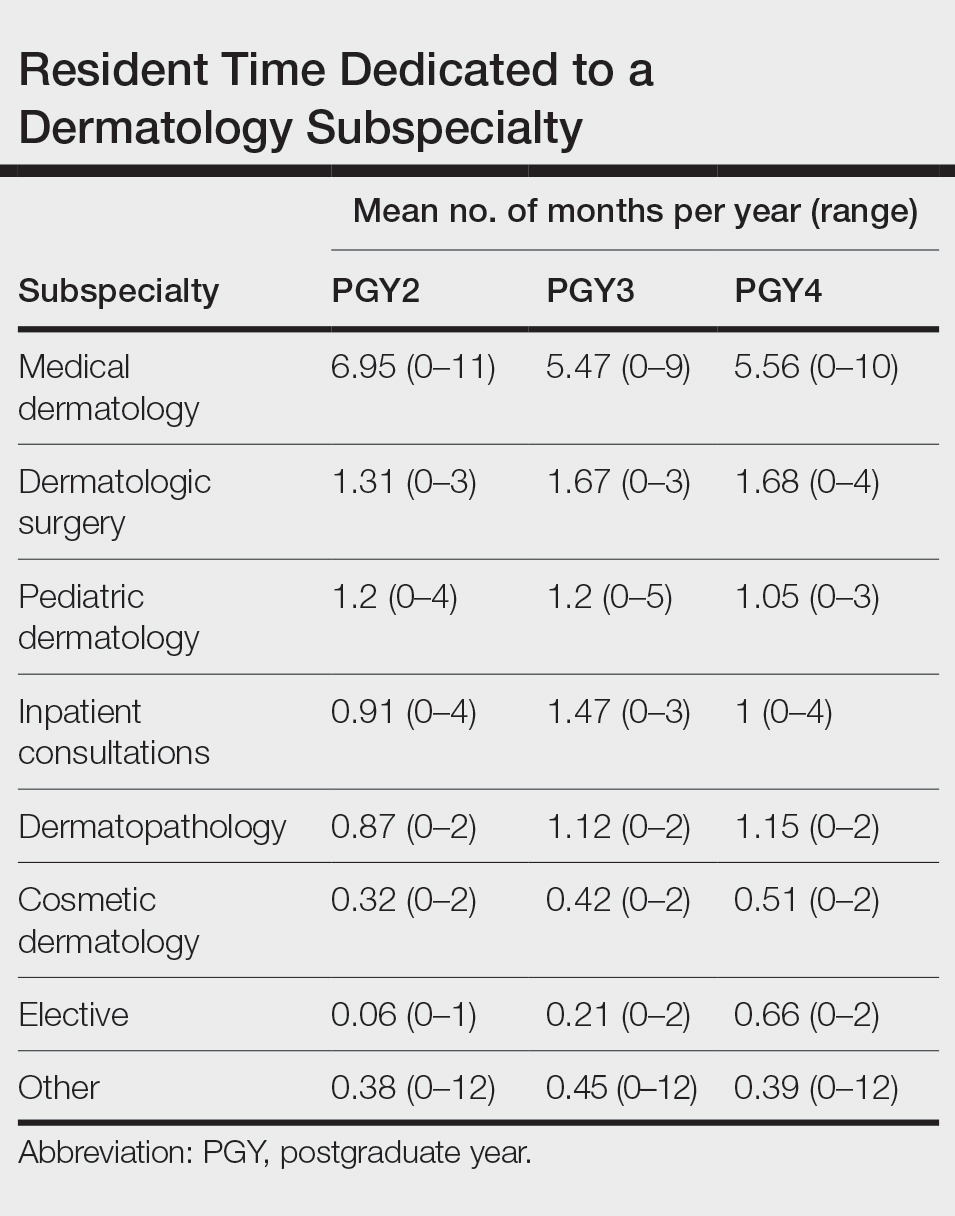
Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.
Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.
Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.
Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).

Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.

Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.

Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.

Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).

Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.

Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.

Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.

Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
Practice Points
- The first annual Association of Professors of Dermatology program directors survey allows faculty to compare their programs to other dermatology residency programs across the United States.
- The results should inspire opportunities for growth, improvement, and collaboration among dermatology residency programs.
Laser Safety: The Need for Protocols
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3
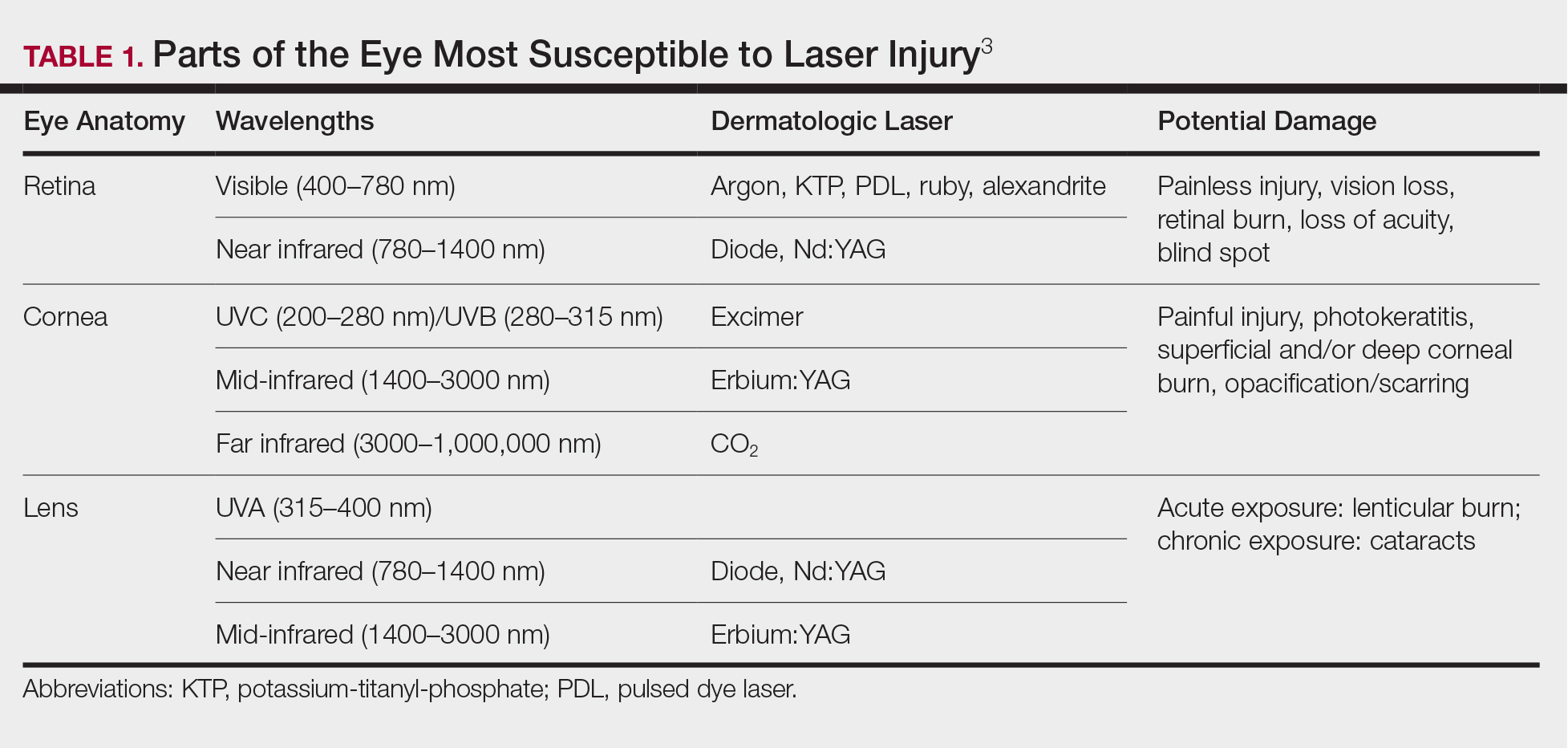
The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7
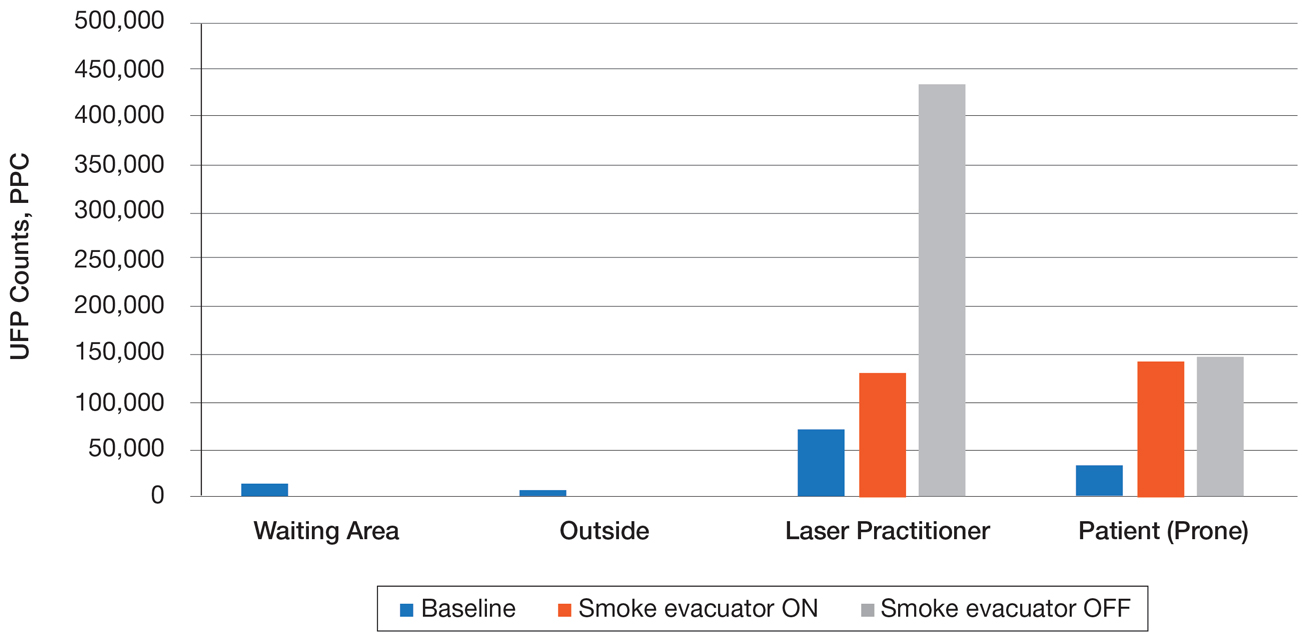
The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14
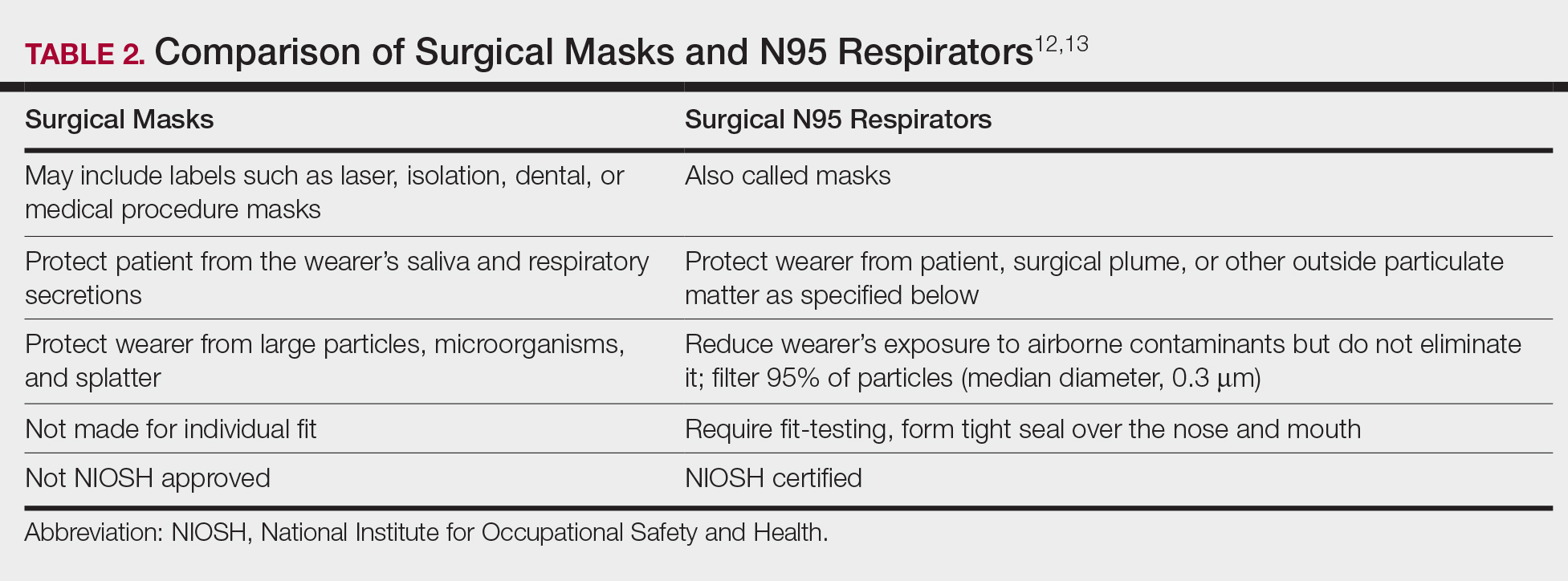
Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).
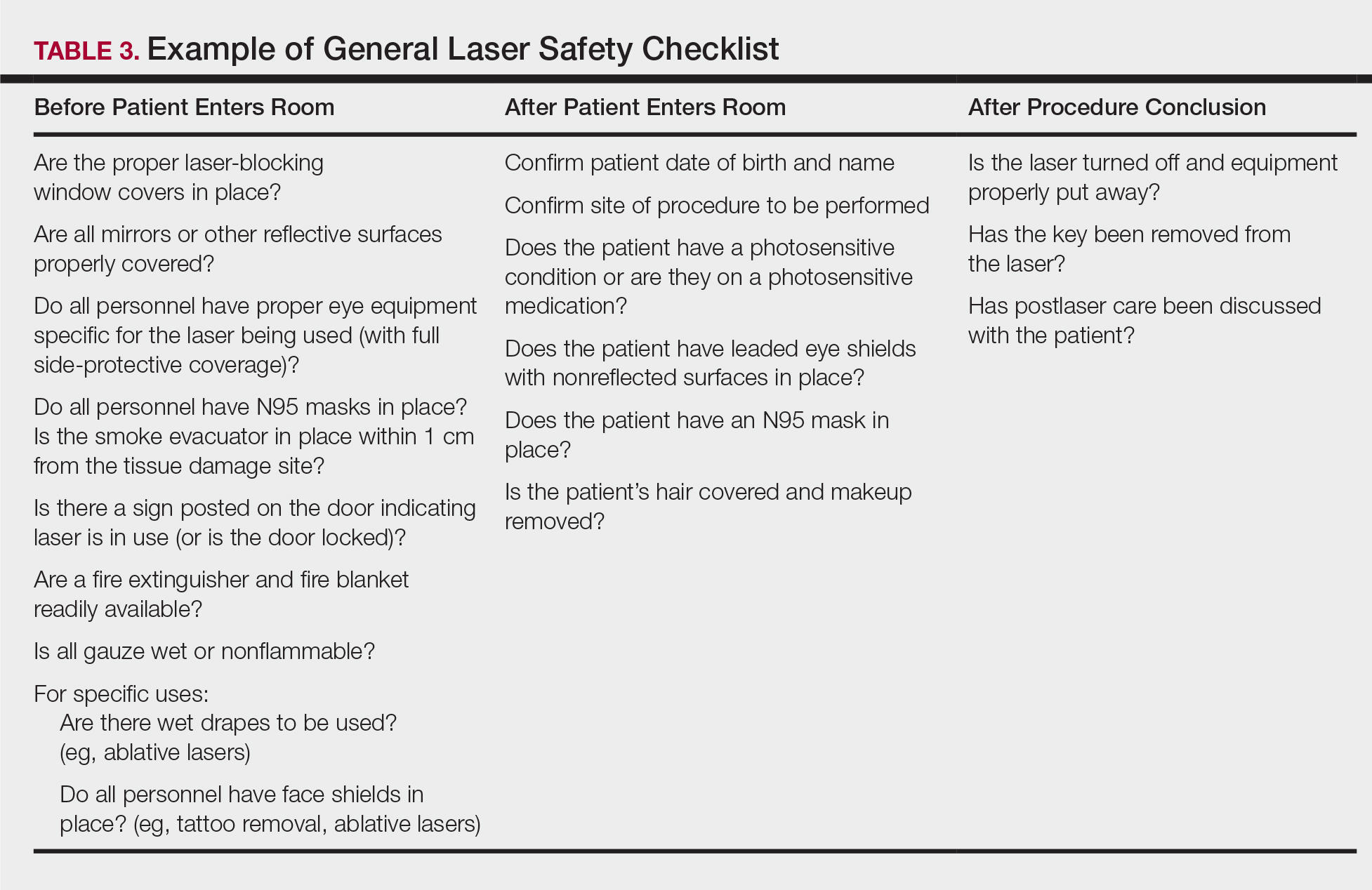
Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
Practice Points
- Laser therapy has evolved and expanded since its first cutaneous use in 1963.
- The 4 regulatory agencies for laser safety in the United States establish standards and guidelines, but implementation is voluntary.
- Ocular hazards, laser-generated airborne contaminants, fires, and unintended laser beam injuries constitute the main safety concerns.
- Safety protocols with a laser checklist can reduce adverse outcomes.
Growing Nodule on the Arm
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.
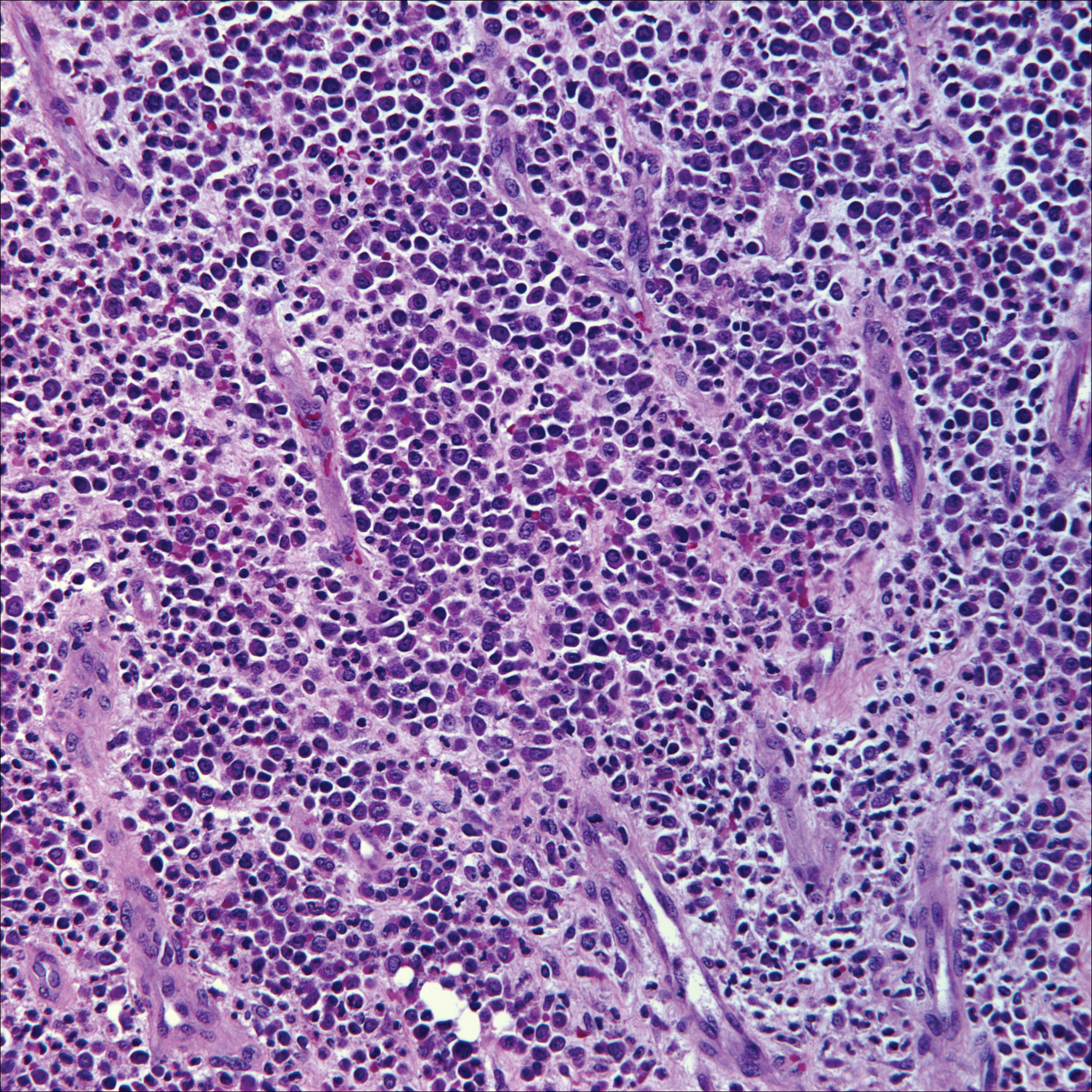
Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.
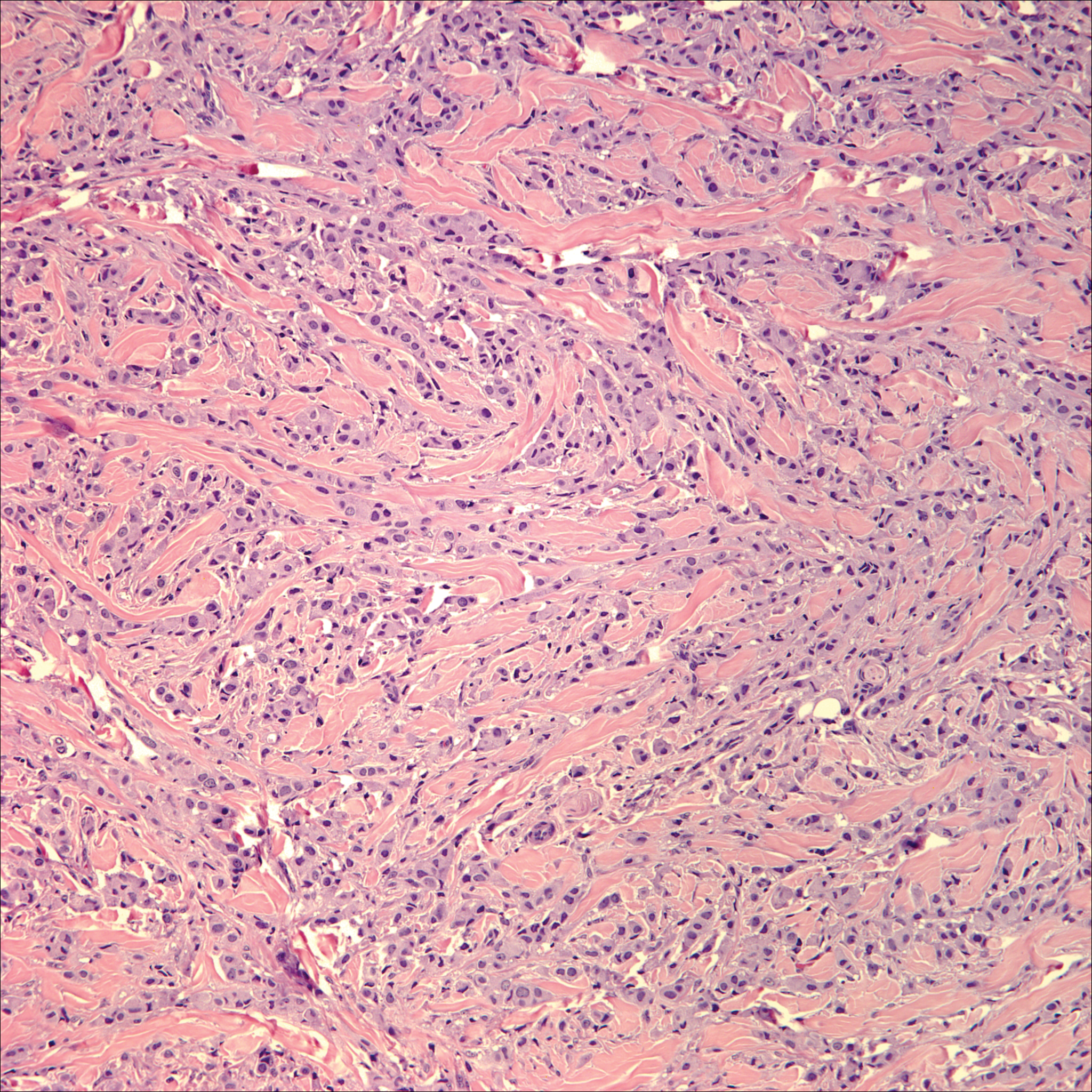
Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.
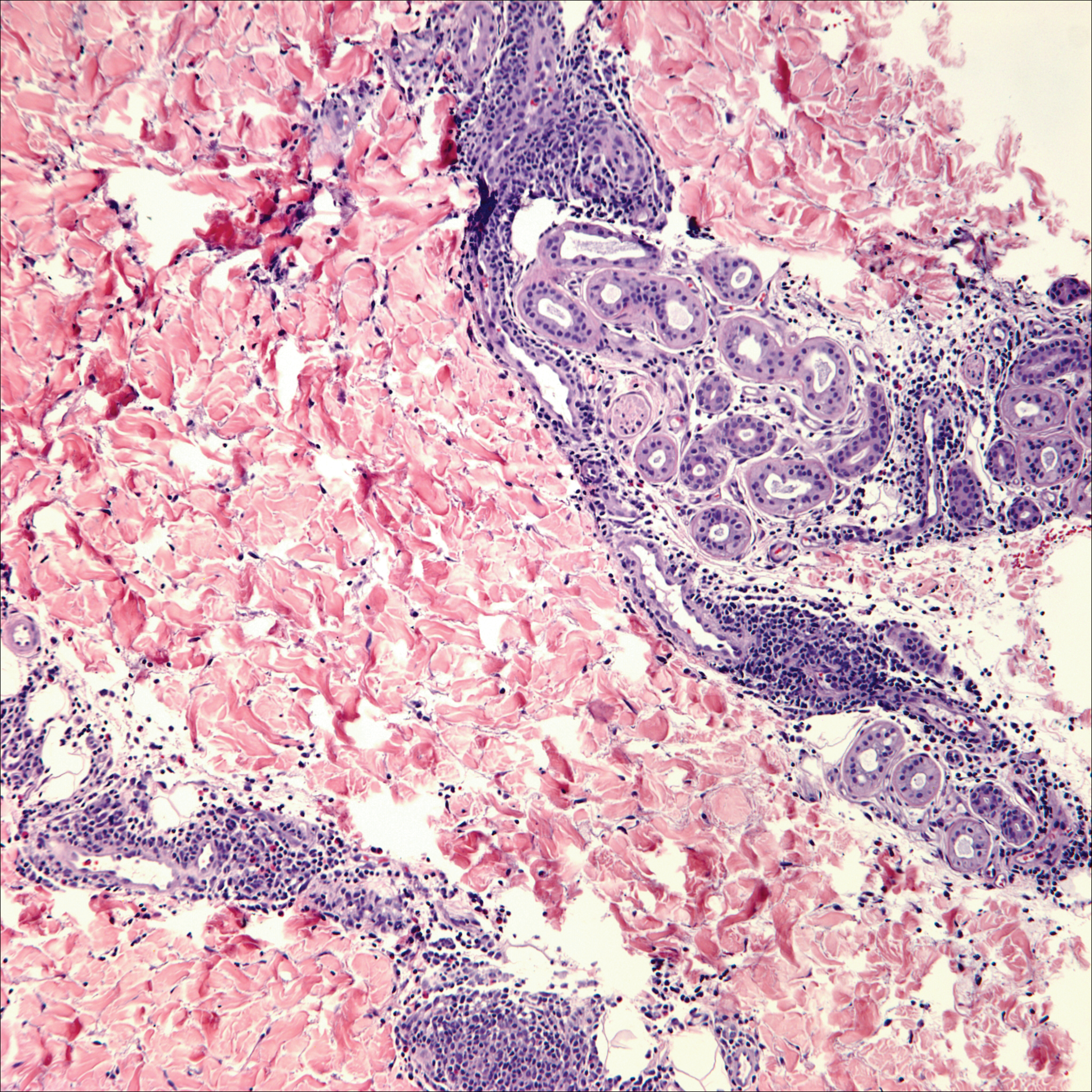
Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8
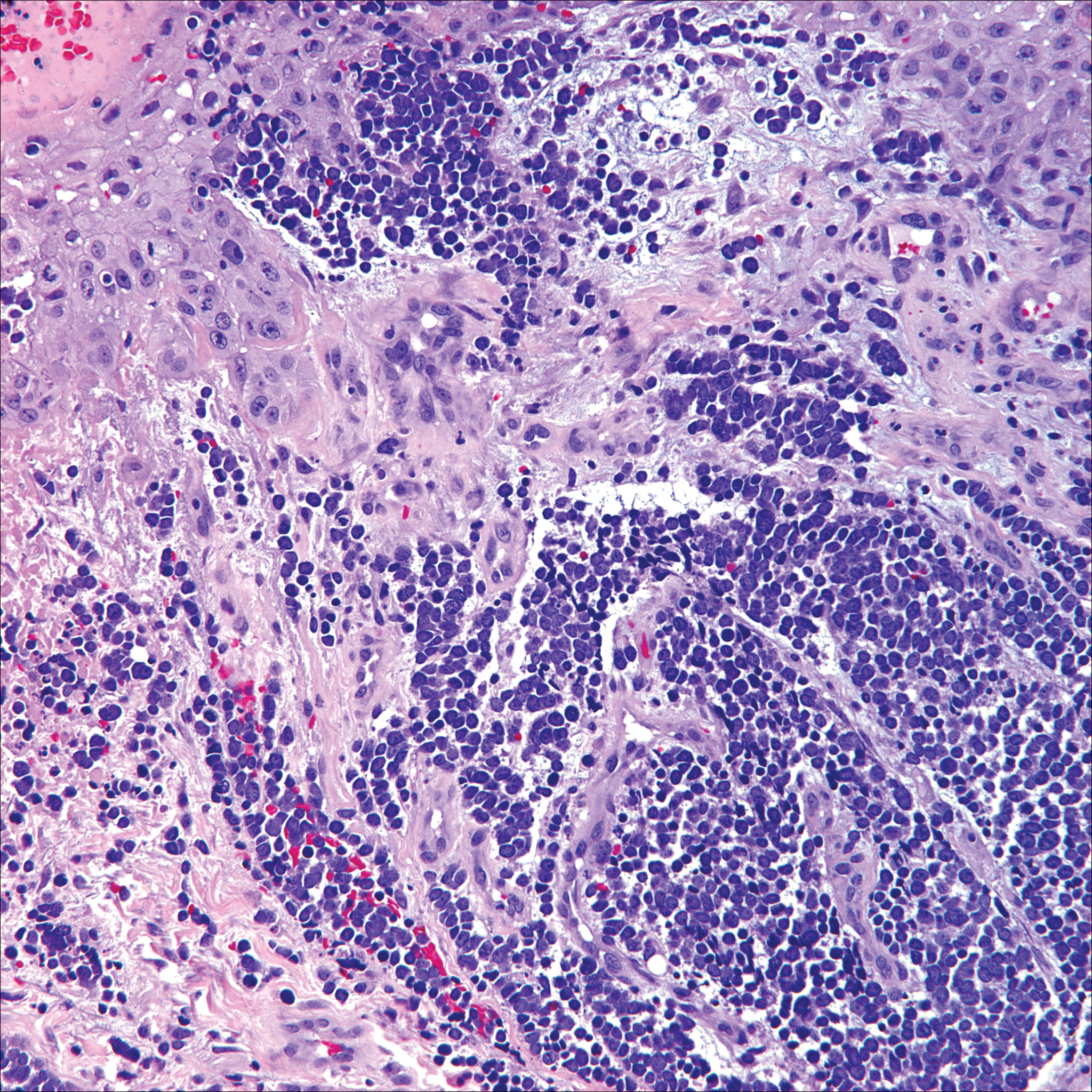
Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.

Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.

Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.

Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8

Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.

Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.

Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.

Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8

Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
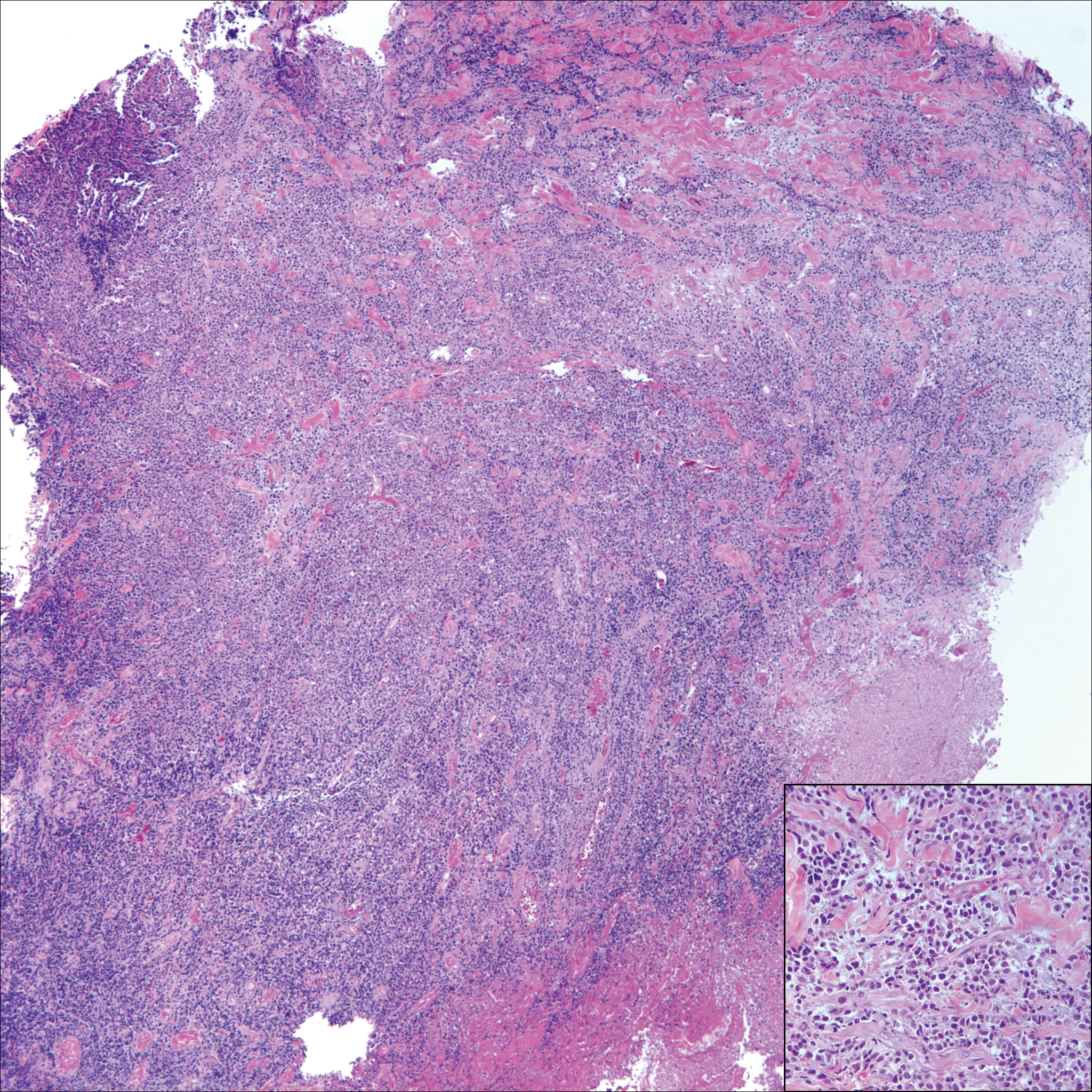
A 65-year-old white woman presented with an asymptomatic bump on the left upper arm of 4 months' duration that arose following a cat scratch. Physical examination was notable for a 35×30-mm, firm, ulcerated, exophytic nodule. Histologic examination demonstrated an ulcerated epidermis and a dense basophilic infiltrate occupying the entire dermis and extending to the subcutaneous tissue. Higher magnification (inset) demonstrated a pleomorphic population of medium- to large-sized discohesive round cells containing variable amounts of slightly eosinophilic cytoplasm, irregular nuclear contours, and prominent nucleoli. Scattered atypical mitotic figures were identified. CD30, CD4, leukocyte common antigen, and Ki-67 immunostains were strongly and diffusely positive. Notable negative stains included anaplastic lymphoma kinase, synaptophysin, epithelial membrane antigen, neuron-specific enolase, CD20, and S-100.