Quiz
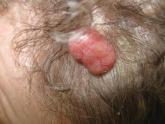
Lobular-Appearing Nodule on the Scalp
- Author:
- E. Eugene Bain Iii, MD
- Melissa Hoffman, BS
- Ilene L. Rothman, MD
A 79-year-old woman presented with a lesion on the left side of the scalp of several years’ duration that had slowly increased in size. Despite...
Article
9 tips to help prevent derm biopsy mistakes
- Author:
- Jayson Miedema, MD
- Daniel C. Zedek, MD
- Brian Z. Rayala, MD
- E. Eugene Bain Iii, MD
The authors—with expertise in dermatology and pathology—provide pointers that can help you improve your approach to skin biopsy.
