User login
Retrospective Cohort Study of the Prevalence of Off-label Gabapentinoid Prescriptions in Hospitalized Medical Patients
In the1990s, gabapentin was licensed in the United States as an anticonvulsant and it became widely successful in the mid-2000s when marketed for the treatment of pain. Since then, prescriptions for gabapentinoids have accelerated dramatically.1,2 Between 2012 and 2016, the total spending on pregabalin in the United States increased from $1.9 to $4.4 billion, with pregabalin ranking eighth overall for specific drug spending.3
Despite a finite number of indications, there has been a steady rise in off-label use, with an increased risk of adverse drug events (ADEs).4,5 Several meta-analyses suggest either low-quality or no evidence of benefit for gabapentinoid use in settings including neuropathic pain in cancer, sciatica, and chronic low back pain.6-8 Lack of efficacy is compounded by adverse effects such as altered mental status, fluid retention, sedation, and increased risk of traumatic falls in older adults.6,9,10 Finally, dependency is a concern; opioids are coprescribed in up to 50% of patients,11 increasing the odds of opioid-related death by up to 60%.12
To better characterize gabapentinoid use in hospitalized patients, we analyzed a retrospective cohort of patients admitted to our tertiary care medical teaching unit, examining preadmission and in-hospital prescribing trends, off-label use, and deprescribing.
METHODS
Patient data were collected from a retrospective cohort, including all consecutive admissions to our 52-bed medical clinical teaching unit in Montréal, Canada, since December 2013.13 We reviewed admissions between December 17, 2013 and June 30, 2017 and identified three populations of gabapentinoid users from medication reconciliation documents: preadmission users continued at discharge, preadmission users deprescribed in hospital, and new in-hospital users continued at discharge. Deprescribing was defined as having the drug stopped at discharge or a prescribed taper that included stopping. The term “gabapentinoid users” refers to preadmission gabapentinoid use.
Gabapentinoid users were compared with nonusers with regard to demographic characteristics; select comorbidities; coprescription of opioids, benzodiazepines, and Z-drugs; length of stay (LOS); and inpatient mortality. Only the first eligible admission per patient was considered. Patients who had multiple admissions over the period of interest were classified as “users” in the patient-level analyses if they were taking a gabapentinoid at home or at discharge on at least one admission.
Doses and indications were collected from medication reconciliation performed by a clinical pharmacist, which included an interview with the patient or a proxy and a review of the indications for all drugs. These data were merged with any additional potential indications found in the admission notes (listing all chronic conditions from a detailed medical history) and review of the electronic medical record. The US Food and Drug Administration (FDA) approved the indications and the recommended doses were taken from product monographs and compared with doses prescribed to patients. When documented, the reason for new prescriptions and justification for deprescribing at discharge were manually abstracted from discharge summaries and medication reconciliation documents.
Continuous variables were expressed as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 test. Proportions of gabapentinoid use and deprescribing, including 95% confidence intervals around each proportion, were plotted and linear regression was performed versus fiscal quarter to evaluate for temporal trends. A two-sided α value of 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas). The McGill University Health Centre Research Ethics Board approved this study.
RESULTS
A total of 4,103 unique patients were admitted from December 2013 to July 2017, of whom 550 (13.4%) were receiving a gabapentinoid before admission. Two preadmission users were coprescribed gabapentin and pregabalin for a total of 552 prescriptions. The prevalence of preadmission gabapentinoid use remained steady during the period of interest (Appendix 1; P = .29 for temporal trend). There were no significant differences between gabapentinoid users and nonusers with regard to age or sex, but users had a higher prevalence of chronic disease (
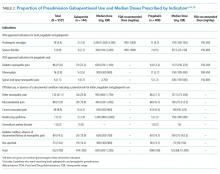
The indications for gabapentinoid use are presented in Table 2. Only a minority (17% or 94/552) had an approved indication. Among these 94 patients, 38 (40%) received FDA-recommended doses, 47 (50%) received doses below those demonstrated to be effective, and 9 (10%) received higher-than-recommended doses. New prescriptions at discharge were observed in 1.5% of patients, with the majority given for off-label indications (Appendix 2).
DISCUSSION
In this large cohort study of hospitalized medical patients, preadmission gabapentinoid use was present in one in every eight admitted patients. Most patients had off-label indications, including the small number of patients who had the drug started in hospital. Even for approved indications, the doses were often lower than what trials have suggested to be effective. Finally, although we have demonstrated that deprescribing occurred, it was uncommon and either precipitated by an adverse event or the justification was poorly documented.
To our knowledge, our study is one of the first to examine what happens to gabapentinoids in hospitalized patients and we present important new data with respect to dosing and prescribing patterns. The low rates of discontinuation, intent to taper, or dose decreases in our cohort represent a potential area of improvement in deprescribing.
Deprescribing should be considered for patients with serious adverse events, for whom less serious adverse effects preclude achieving clinically effective doses, and for those who do not perceive benefit. Given the magnitude of the problems presented by polypharmacy, we propose that stopping priority be given to off-label use (especially when clinically ineffective) and for patients coprescribed opioids or sedatives. Up to a third of users in our cohort were coprescribed opioids or benzodiazepines, which is particularly concerning given the association with increased opioid-related mortality.12,15 Although we did not observe a difference in inpatient mortality, such a study is underpowered for this outcome especially when considering the competing risks of death in hospital. Importantly, when deprescribing, the drug should be tapered over several weeks to limit symptoms of withdrawal and to prevent seizure.11
Presumed off-label use and subtherapeutic doses were common in our cohort, with only 17% of users having a clearly documented FDA-approved indication, in agreement with a previous study that reported only 5% on-label use.4 High doses of gabapentinoids required for efficacy in clinical trials may be difficult to achieve because of dose-limiting side effects, which may explain the relatively low median doses recorded in our real-world cohort. Another possibility is that frail, older patients with renal dysfunction experience effectiveness at lower median doses than those quoted from study populations. In our study, patients on lower doses of gabapentinoids had a higher prevalence of stage IV or V chronic kidney disease (CKD). Stage IV/V CKD was identified in 16/47 (34.0%) patients on lower doses of gabapentinoids, compared to 4/38 (10.5%) on doses within the FDA-recommended range.
Our study has limitations; findings from a single Canadian tertiary care hospital may not be generalizable to other hospitals or countries, particularly given the differences between the Canadian and US health systems. Indications were extracted from the patient chart and even with the best possible medication history and thorough review, sometimes they had to be inferred. Caution should also be exercised when interpreting the omission of an indication as equating to a lack of justifiable medication use; however, the rate of off-label use in our cohort is in agreement with prior research.4 Moreover, with a retrospective design, the effectiveness of the drug on an individual basis could not be assessed, which would have allowed a more precise estimate of the proportion of patients for whom deprescribing might have been appropriate. The strengths of this study include a large sample of real-world, heterogeneous, general medical patients spanning several years and our use of trained pharmacists and physicians to determine the drug indication as opposed to reliance on administrative data.
CONCLUSION
Gabapentinoid use was frequent in our cohort of hospitalized medical patients, with a high prevalence of off-label use, subtherapeutic doses, and coadministration with opioids and benzodiazepines. Deprescribing at discharge was uncommon and often triggered by an adverse event. The identification of gabapentinoids during hospitalization is an opportunity to reevaluate the indication for the drug, assess for effectiveness, and consider deprescribing to help reduce polypharmacy and ideally ADEs.
Acknowledgment
For the purposes of authorship, Dr. McDonald and Dr. Lee contributed equally.
Disclosures
Dr. Emily McDonald and Dr. Todd Lee have a patent pending for MedSafer, a deprescribing software, and both receive research salary support from the Fonds de Recherche Santé du Québec. Dr. Gingras, Dr. Lieu, and Dr. Papillon-Ferland have nothing to disclose.
1. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. https://doi.org/10.1001/jamainternmed.2017.7856.
2. Kwok H, Khuu W, Fernandes K, et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019-1026. https://doi.org/10.1093/pm/pnw351.
3. Medicines use and spending in the U.S. — a review of 2016 and outlook to 2021: IMS Institute for Healthcare Informatics; 2017. https://structurecms-staging-psyclone.netdna-ssl.com/client_assets/dwonk/media/attachments/590c/6aa0/6970/2d2d/4182/0000/590c6aa069702d2d41820000.pdf?1493985952. Accessed March 21, 2019.
4. Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. J Manag Care Pharm. 2002;8(4):266-271. doi: 10.18553/jmcp.2002.8.4.266.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55-63. https://doi.org/10.1001/jamainternmed.2015.6058.
6. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. https://doi.org/10.1371/journal.pmed.1002369.
7. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793. https://doi.org/10.1503/cmaj.171333.
8. Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med. 2018;32(1):276-286. https://doi.org/10.1177/0269216317711826.
9. Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68(6):903-912. https://doi.org/10.1007/s00228-012-1213-x.
10. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359-376. https://doi.org/10.2165/11599460-000000000-00000.
11. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. https://doi.org/10.1007/s40265-017-0700-x.
12. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396.
13. McDonald EG, Saleh RR, Lee TC. Ezetimibe use remains common among medical inpatients. Am J Med. 2015;128(2):193-195. https://doi.org/10.1016/j.amjmed.2014.10.016.
14. U.S. Food and Drug Administration. LYRICA - Highlights of Prescribing Information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf. Accessed April 30, 2019.
15. U.S. Food and Drug Administration. NEURONTIN - Highlights of Prescribing Information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed April 30, 2019.
16. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732-734. https://doi.org/10.7326/M18-1136.
In the1990s, gabapentin was licensed in the United States as an anticonvulsant and it became widely successful in the mid-2000s when marketed for the treatment of pain. Since then, prescriptions for gabapentinoids have accelerated dramatically.1,2 Between 2012 and 2016, the total spending on pregabalin in the United States increased from $1.9 to $4.4 billion, with pregabalin ranking eighth overall for specific drug spending.3
Despite a finite number of indications, there has been a steady rise in off-label use, with an increased risk of adverse drug events (ADEs).4,5 Several meta-analyses suggest either low-quality or no evidence of benefit for gabapentinoid use in settings including neuropathic pain in cancer, sciatica, and chronic low back pain.6-8 Lack of efficacy is compounded by adverse effects such as altered mental status, fluid retention, sedation, and increased risk of traumatic falls in older adults.6,9,10 Finally, dependency is a concern; opioids are coprescribed in up to 50% of patients,11 increasing the odds of opioid-related death by up to 60%.12
To better characterize gabapentinoid use in hospitalized patients, we analyzed a retrospective cohort of patients admitted to our tertiary care medical teaching unit, examining preadmission and in-hospital prescribing trends, off-label use, and deprescribing.
METHODS
Patient data were collected from a retrospective cohort, including all consecutive admissions to our 52-bed medical clinical teaching unit in Montréal, Canada, since December 2013.13 We reviewed admissions between December 17, 2013 and June 30, 2017 and identified three populations of gabapentinoid users from medication reconciliation documents: preadmission users continued at discharge, preadmission users deprescribed in hospital, and new in-hospital users continued at discharge. Deprescribing was defined as having the drug stopped at discharge or a prescribed taper that included stopping. The term “gabapentinoid users” refers to preadmission gabapentinoid use.
Gabapentinoid users were compared with nonusers with regard to demographic characteristics; select comorbidities; coprescription of opioids, benzodiazepines, and Z-drugs; length of stay (LOS); and inpatient mortality. Only the first eligible admission per patient was considered. Patients who had multiple admissions over the period of interest were classified as “users” in the patient-level analyses if they were taking a gabapentinoid at home or at discharge on at least one admission.
Doses and indications were collected from medication reconciliation performed by a clinical pharmacist, which included an interview with the patient or a proxy and a review of the indications for all drugs. These data were merged with any additional potential indications found in the admission notes (listing all chronic conditions from a detailed medical history) and review of the electronic medical record. The US Food and Drug Administration (FDA) approved the indications and the recommended doses were taken from product monographs and compared with doses prescribed to patients. When documented, the reason for new prescriptions and justification for deprescribing at discharge were manually abstracted from discharge summaries and medication reconciliation documents.
Continuous variables were expressed as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 test. Proportions of gabapentinoid use and deprescribing, including 95% confidence intervals around each proportion, were plotted and linear regression was performed versus fiscal quarter to evaluate for temporal trends. A two-sided α value of 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas). The McGill University Health Centre Research Ethics Board approved this study.
RESULTS
A total of 4,103 unique patients were admitted from December 2013 to July 2017, of whom 550 (13.4%) were receiving a gabapentinoid before admission. Two preadmission users were coprescribed gabapentin and pregabalin for a total of 552 prescriptions. The prevalence of preadmission gabapentinoid use remained steady during the period of interest (Appendix 1; P = .29 for temporal trend). There were no significant differences between gabapentinoid users and nonusers with regard to age or sex, but users had a higher prevalence of chronic disease (
The indications for gabapentinoid use are presented in Table 2. Only a minority (17% or 94/552) had an approved indication. Among these 94 patients, 38 (40%) received FDA-recommended doses, 47 (50%) received doses below those demonstrated to be effective, and 9 (10%) received higher-than-recommended doses. New prescriptions at discharge were observed in 1.5% of patients, with the majority given for off-label indications (Appendix 2).
DISCUSSION
In this large cohort study of hospitalized medical patients, preadmission gabapentinoid use was present in one in every eight admitted patients. Most patients had off-label indications, including the small number of patients who had the drug started in hospital. Even for approved indications, the doses were often lower than what trials have suggested to be effective. Finally, although we have demonstrated that deprescribing occurred, it was uncommon and either precipitated by an adverse event or the justification was poorly documented.
To our knowledge, our study is one of the first to examine what happens to gabapentinoids in hospitalized patients and we present important new data with respect to dosing and prescribing patterns. The low rates of discontinuation, intent to taper, or dose decreases in our cohort represent a potential area of improvement in deprescribing.
Deprescribing should be considered for patients with serious adverse events, for whom less serious adverse effects preclude achieving clinically effective doses, and for those who do not perceive benefit. Given the magnitude of the problems presented by polypharmacy, we propose that stopping priority be given to off-label use (especially when clinically ineffective) and for patients coprescribed opioids or sedatives. Up to a third of users in our cohort were coprescribed opioids or benzodiazepines, which is particularly concerning given the association with increased opioid-related mortality.12,15 Although we did not observe a difference in inpatient mortality, such a study is underpowered for this outcome especially when considering the competing risks of death in hospital. Importantly, when deprescribing, the drug should be tapered over several weeks to limit symptoms of withdrawal and to prevent seizure.11
Presumed off-label use and subtherapeutic doses were common in our cohort, with only 17% of users having a clearly documented FDA-approved indication, in agreement with a previous study that reported only 5% on-label use.4 High doses of gabapentinoids required for efficacy in clinical trials may be difficult to achieve because of dose-limiting side effects, which may explain the relatively low median doses recorded in our real-world cohort. Another possibility is that frail, older patients with renal dysfunction experience effectiveness at lower median doses than those quoted from study populations. In our study, patients on lower doses of gabapentinoids had a higher prevalence of stage IV or V chronic kidney disease (CKD). Stage IV/V CKD was identified in 16/47 (34.0%) patients on lower doses of gabapentinoids, compared to 4/38 (10.5%) on doses within the FDA-recommended range.
Our study has limitations; findings from a single Canadian tertiary care hospital may not be generalizable to other hospitals or countries, particularly given the differences between the Canadian and US health systems. Indications were extracted from the patient chart and even with the best possible medication history and thorough review, sometimes they had to be inferred. Caution should also be exercised when interpreting the omission of an indication as equating to a lack of justifiable medication use; however, the rate of off-label use in our cohort is in agreement with prior research.4 Moreover, with a retrospective design, the effectiveness of the drug on an individual basis could not be assessed, which would have allowed a more precise estimate of the proportion of patients for whom deprescribing might have been appropriate. The strengths of this study include a large sample of real-world, heterogeneous, general medical patients spanning several years and our use of trained pharmacists and physicians to determine the drug indication as opposed to reliance on administrative data.
CONCLUSION
Gabapentinoid use was frequent in our cohort of hospitalized medical patients, with a high prevalence of off-label use, subtherapeutic doses, and coadministration with opioids and benzodiazepines. Deprescribing at discharge was uncommon and often triggered by an adverse event. The identification of gabapentinoids during hospitalization is an opportunity to reevaluate the indication for the drug, assess for effectiveness, and consider deprescribing to help reduce polypharmacy and ideally ADEs.
Acknowledgment
For the purposes of authorship, Dr. McDonald and Dr. Lee contributed equally.
Disclosures
Dr. Emily McDonald and Dr. Todd Lee have a patent pending for MedSafer, a deprescribing software, and both receive research salary support from the Fonds de Recherche Santé du Québec. Dr. Gingras, Dr. Lieu, and Dr. Papillon-Ferland have nothing to disclose.
In the1990s, gabapentin was licensed in the United States as an anticonvulsant and it became widely successful in the mid-2000s when marketed for the treatment of pain. Since then, prescriptions for gabapentinoids have accelerated dramatically.1,2 Between 2012 and 2016, the total spending on pregabalin in the United States increased from $1.9 to $4.4 billion, with pregabalin ranking eighth overall for specific drug spending.3
Despite a finite number of indications, there has been a steady rise in off-label use, with an increased risk of adverse drug events (ADEs).4,5 Several meta-analyses suggest either low-quality or no evidence of benefit for gabapentinoid use in settings including neuropathic pain in cancer, sciatica, and chronic low back pain.6-8 Lack of efficacy is compounded by adverse effects such as altered mental status, fluid retention, sedation, and increased risk of traumatic falls in older adults.6,9,10 Finally, dependency is a concern; opioids are coprescribed in up to 50% of patients,11 increasing the odds of opioid-related death by up to 60%.12
To better characterize gabapentinoid use in hospitalized patients, we analyzed a retrospective cohort of patients admitted to our tertiary care medical teaching unit, examining preadmission and in-hospital prescribing trends, off-label use, and deprescribing.
METHODS
Patient data were collected from a retrospective cohort, including all consecutive admissions to our 52-bed medical clinical teaching unit in Montréal, Canada, since December 2013.13 We reviewed admissions between December 17, 2013 and June 30, 2017 and identified three populations of gabapentinoid users from medication reconciliation documents: preadmission users continued at discharge, preadmission users deprescribed in hospital, and new in-hospital users continued at discharge. Deprescribing was defined as having the drug stopped at discharge or a prescribed taper that included stopping. The term “gabapentinoid users” refers to preadmission gabapentinoid use.
Gabapentinoid users were compared with nonusers with regard to demographic characteristics; select comorbidities; coprescription of opioids, benzodiazepines, and Z-drugs; length of stay (LOS); and inpatient mortality. Only the first eligible admission per patient was considered. Patients who had multiple admissions over the period of interest were classified as “users” in the patient-level analyses if they were taking a gabapentinoid at home or at discharge on at least one admission.
Doses and indications were collected from medication reconciliation performed by a clinical pharmacist, which included an interview with the patient or a proxy and a review of the indications for all drugs. These data were merged with any additional potential indications found in the admission notes (listing all chronic conditions from a detailed medical history) and review of the electronic medical record. The US Food and Drug Administration (FDA) approved the indications and the recommended doses were taken from product monographs and compared with doses prescribed to patients. When documented, the reason for new prescriptions and justification for deprescribing at discharge were manually abstracted from discharge summaries and medication reconciliation documents.
Continuous variables were expressed as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 test. Proportions of gabapentinoid use and deprescribing, including 95% confidence intervals around each proportion, were plotted and linear regression was performed versus fiscal quarter to evaluate for temporal trends. A two-sided α value of 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas). The McGill University Health Centre Research Ethics Board approved this study.
RESULTS
A total of 4,103 unique patients were admitted from December 2013 to July 2017, of whom 550 (13.4%) were receiving a gabapentinoid before admission. Two preadmission users were coprescribed gabapentin and pregabalin for a total of 552 prescriptions. The prevalence of preadmission gabapentinoid use remained steady during the period of interest (Appendix 1; P = .29 for temporal trend). There were no significant differences between gabapentinoid users and nonusers with regard to age or sex, but users had a higher prevalence of chronic disease (
The indications for gabapentinoid use are presented in Table 2. Only a minority (17% or 94/552) had an approved indication. Among these 94 patients, 38 (40%) received FDA-recommended doses, 47 (50%) received doses below those demonstrated to be effective, and 9 (10%) received higher-than-recommended doses. New prescriptions at discharge were observed in 1.5% of patients, with the majority given for off-label indications (Appendix 2).
DISCUSSION
In this large cohort study of hospitalized medical patients, preadmission gabapentinoid use was present in one in every eight admitted patients. Most patients had off-label indications, including the small number of patients who had the drug started in hospital. Even for approved indications, the doses were often lower than what trials have suggested to be effective. Finally, although we have demonstrated that deprescribing occurred, it was uncommon and either precipitated by an adverse event or the justification was poorly documented.
To our knowledge, our study is one of the first to examine what happens to gabapentinoids in hospitalized patients and we present important new data with respect to dosing and prescribing patterns. The low rates of discontinuation, intent to taper, or dose decreases in our cohort represent a potential area of improvement in deprescribing.
Deprescribing should be considered for patients with serious adverse events, for whom less serious adverse effects preclude achieving clinically effective doses, and for those who do not perceive benefit. Given the magnitude of the problems presented by polypharmacy, we propose that stopping priority be given to off-label use (especially when clinically ineffective) and for patients coprescribed opioids or sedatives. Up to a third of users in our cohort were coprescribed opioids or benzodiazepines, which is particularly concerning given the association with increased opioid-related mortality.12,15 Although we did not observe a difference in inpatient mortality, such a study is underpowered for this outcome especially when considering the competing risks of death in hospital. Importantly, when deprescribing, the drug should be tapered over several weeks to limit symptoms of withdrawal and to prevent seizure.11
Presumed off-label use and subtherapeutic doses were common in our cohort, with only 17% of users having a clearly documented FDA-approved indication, in agreement with a previous study that reported only 5% on-label use.4 High doses of gabapentinoids required for efficacy in clinical trials may be difficult to achieve because of dose-limiting side effects, which may explain the relatively low median doses recorded in our real-world cohort. Another possibility is that frail, older patients with renal dysfunction experience effectiveness at lower median doses than those quoted from study populations. In our study, patients on lower doses of gabapentinoids had a higher prevalence of stage IV or V chronic kidney disease (CKD). Stage IV/V CKD was identified in 16/47 (34.0%) patients on lower doses of gabapentinoids, compared to 4/38 (10.5%) on doses within the FDA-recommended range.
Our study has limitations; findings from a single Canadian tertiary care hospital may not be generalizable to other hospitals or countries, particularly given the differences between the Canadian and US health systems. Indications were extracted from the patient chart and even with the best possible medication history and thorough review, sometimes they had to be inferred. Caution should also be exercised when interpreting the omission of an indication as equating to a lack of justifiable medication use; however, the rate of off-label use in our cohort is in agreement with prior research.4 Moreover, with a retrospective design, the effectiveness of the drug on an individual basis could not be assessed, which would have allowed a more precise estimate of the proportion of patients for whom deprescribing might have been appropriate. The strengths of this study include a large sample of real-world, heterogeneous, general medical patients spanning several years and our use of trained pharmacists and physicians to determine the drug indication as opposed to reliance on administrative data.
CONCLUSION
Gabapentinoid use was frequent in our cohort of hospitalized medical patients, with a high prevalence of off-label use, subtherapeutic doses, and coadministration with opioids and benzodiazepines. Deprescribing at discharge was uncommon and often triggered by an adverse event. The identification of gabapentinoids during hospitalization is an opportunity to reevaluate the indication for the drug, assess for effectiveness, and consider deprescribing to help reduce polypharmacy and ideally ADEs.
Acknowledgment
For the purposes of authorship, Dr. McDonald and Dr. Lee contributed equally.
Disclosures
Dr. Emily McDonald and Dr. Todd Lee have a patent pending for MedSafer, a deprescribing software, and both receive research salary support from the Fonds de Recherche Santé du Québec. Dr. Gingras, Dr. Lieu, and Dr. Papillon-Ferland have nothing to disclose.
1. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. https://doi.org/10.1001/jamainternmed.2017.7856.
2. Kwok H, Khuu W, Fernandes K, et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019-1026. https://doi.org/10.1093/pm/pnw351.
3. Medicines use and spending in the U.S. — a review of 2016 and outlook to 2021: IMS Institute for Healthcare Informatics; 2017. https://structurecms-staging-psyclone.netdna-ssl.com/client_assets/dwonk/media/attachments/590c/6aa0/6970/2d2d/4182/0000/590c6aa069702d2d41820000.pdf?1493985952. Accessed March 21, 2019.
4. Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. J Manag Care Pharm. 2002;8(4):266-271. doi: 10.18553/jmcp.2002.8.4.266.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55-63. https://doi.org/10.1001/jamainternmed.2015.6058.
6. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. https://doi.org/10.1371/journal.pmed.1002369.
7. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793. https://doi.org/10.1503/cmaj.171333.
8. Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med. 2018;32(1):276-286. https://doi.org/10.1177/0269216317711826.
9. Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68(6):903-912. https://doi.org/10.1007/s00228-012-1213-x.
10. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359-376. https://doi.org/10.2165/11599460-000000000-00000.
11. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. https://doi.org/10.1007/s40265-017-0700-x.
12. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396.
13. McDonald EG, Saleh RR, Lee TC. Ezetimibe use remains common among medical inpatients. Am J Med. 2015;128(2):193-195. https://doi.org/10.1016/j.amjmed.2014.10.016.
14. U.S. Food and Drug Administration. LYRICA - Highlights of Prescribing Information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf. Accessed April 30, 2019.
15. U.S. Food and Drug Administration. NEURONTIN - Highlights of Prescribing Information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed April 30, 2019.
16. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732-734. https://doi.org/10.7326/M18-1136.
1. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. https://doi.org/10.1001/jamainternmed.2017.7856.
2. Kwok H, Khuu W, Fernandes K, et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019-1026. https://doi.org/10.1093/pm/pnw351.
3. Medicines use and spending in the U.S. — a review of 2016 and outlook to 2021: IMS Institute for Healthcare Informatics; 2017. https://structurecms-staging-psyclone.netdna-ssl.com/client_assets/dwonk/media/attachments/590c/6aa0/6970/2d2d/4182/0000/590c6aa069702d2d41820000.pdf?1493985952. Accessed March 21, 2019.
4. Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. J Manag Care Pharm. 2002;8(4):266-271. doi: 10.18553/jmcp.2002.8.4.266.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55-63. https://doi.org/10.1001/jamainternmed.2015.6058.
6. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. https://doi.org/10.1371/journal.pmed.1002369.
7. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793. https://doi.org/10.1503/cmaj.171333.
8. Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med. 2018;32(1):276-286. https://doi.org/10.1177/0269216317711826.
9. Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68(6):903-912. https://doi.org/10.1007/s00228-012-1213-x.
10. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359-376. https://doi.org/10.2165/11599460-000000000-00000.
11. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. https://doi.org/10.1007/s40265-017-0700-x.
12. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396.
13. McDonald EG, Saleh RR, Lee TC. Ezetimibe use remains common among medical inpatients. Am J Med. 2015;128(2):193-195. https://doi.org/10.1016/j.amjmed.2014.10.016.
14. U.S. Food and Drug Administration. LYRICA - Highlights of Prescribing Information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf. Accessed April 30, 2019.
15. U.S. Food and Drug Administration. NEURONTIN - Highlights of Prescribing Information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed April 30, 2019.
16. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732-734. https://doi.org/10.7326/M18-1136.
© 2019 Society of Hospital Medicine