User login
Progressive, pruritic eruption of firm, skin-colored papules
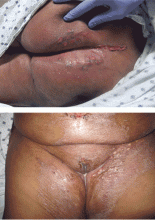
Scleromyxedema, or generalized lichen myxedematosus, is a primary cutaneous mucinosis with unknown pathogenesis characterized by generalized firm, skin-colored papules and is commonly associated with an underlying monoclonal gammopathy (usually Ig-gamma paraproteinemia).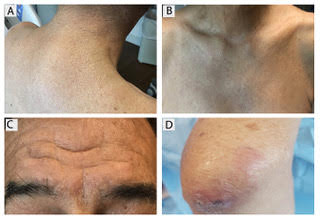
Scleromyxedema may have associated internal involvement, including neurologic, gastrointestinal, pulmonary, renal, cardiovascular, ophthalmological, or musculoskeletal. Histopathology demonstrates mucin in the dermis seen with Alcian blue staining, proliferation of fibroblasts, and increased collagen deposition.
The condition is chronic and progressive. Intravenous immunoglobulin is considered first-line treatment. Thalidomide and corticosteroids have been reported to also be efficacious.
It is associated with hematologic disorders, including IgA monoclonal gammopathy, as well as myeloproliferative disorders, leukemia, infections, and inflammatory bowel disease. Although its pathophysiology is not well understood, vascular immune complex deposition, repetitive inflammation, and subsequent fibrosis may play a role. On histology, there is leukocytoclastic vasculitis with polymorphonuclear cell infiltrate and fibrin deposition in the superficial and mid-dermis and onion-skin fibrosis.
EED often self-resolves within 5-10 years, although it can become chronic and recurrent. Dapsone, niacinamide, antimalarials, NSAIDs, tetracyclines, corticosteroids, colchicine, and plasmapheresis are reported treatments. This patient’s EED was recalcitrant to prednisone and responded to colchicine.
Scleromyxedema and EED are both rare, distinct cutaneous entities associated with different underlying paraproteinemias and to the best of our knowledge, have not been previously reported to coexist in a single patient.
This case and the photos were submitted by Rachel Fayne, BA; Yumeng Li, MD, MS; Fabrizio Galimberti, MD, PhD; and Brian Morrison, MD, of the department of dermatology and cutaneous surgery at the University of Miami.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Scleromyxedema, or generalized lichen myxedematosus, is a primary cutaneous mucinosis with unknown pathogenesis characterized by generalized firm, skin-colored papules and is commonly associated with an underlying monoclonal gammopathy (usually Ig-gamma paraproteinemia).
Scleromyxedema may have associated internal involvement, including neurologic, gastrointestinal, pulmonary, renal, cardiovascular, ophthalmological, or musculoskeletal. Histopathology demonstrates mucin in the dermis seen with Alcian blue staining, proliferation of fibroblasts, and increased collagen deposition.
The condition is chronic and progressive. Intravenous immunoglobulin is considered first-line treatment. Thalidomide and corticosteroids have been reported to also be efficacious.
It is associated with hematologic disorders, including IgA monoclonal gammopathy, as well as myeloproliferative disorders, leukemia, infections, and inflammatory bowel disease. Although its pathophysiology is not well understood, vascular immune complex deposition, repetitive inflammation, and subsequent fibrosis may play a role. On histology, there is leukocytoclastic vasculitis with polymorphonuclear cell infiltrate and fibrin deposition in the superficial and mid-dermis and onion-skin fibrosis.
EED often self-resolves within 5-10 years, although it can become chronic and recurrent. Dapsone, niacinamide, antimalarials, NSAIDs, tetracyclines, corticosteroids, colchicine, and plasmapheresis are reported treatments. This patient’s EED was recalcitrant to prednisone and responded to colchicine.
Scleromyxedema and EED are both rare, distinct cutaneous entities associated with different underlying paraproteinemias and to the best of our knowledge, have not been previously reported to coexist in a single patient.
This case and the photos were submitted by Rachel Fayne, BA; Yumeng Li, MD, MS; Fabrizio Galimberti, MD, PhD; and Brian Morrison, MD, of the department of dermatology and cutaneous surgery at the University of Miami.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Scleromyxedema, or generalized lichen myxedematosus, is a primary cutaneous mucinosis with unknown pathogenesis characterized by generalized firm, skin-colored papules and is commonly associated with an underlying monoclonal gammopathy (usually Ig-gamma paraproteinemia).
Scleromyxedema may have associated internal involvement, including neurologic, gastrointestinal, pulmonary, renal, cardiovascular, ophthalmological, or musculoskeletal. Histopathology demonstrates mucin in the dermis seen with Alcian blue staining, proliferation of fibroblasts, and increased collagen deposition.
The condition is chronic and progressive. Intravenous immunoglobulin is considered first-line treatment. Thalidomide and corticosteroids have been reported to also be efficacious.
It is associated with hematologic disorders, including IgA monoclonal gammopathy, as well as myeloproliferative disorders, leukemia, infections, and inflammatory bowel disease. Although its pathophysiology is not well understood, vascular immune complex deposition, repetitive inflammation, and subsequent fibrosis may play a role. On histology, there is leukocytoclastic vasculitis with polymorphonuclear cell infiltrate and fibrin deposition in the superficial and mid-dermis and onion-skin fibrosis.
EED often self-resolves within 5-10 years, although it can become chronic and recurrent. Dapsone, niacinamide, antimalarials, NSAIDs, tetracyclines, corticosteroids, colchicine, and plasmapheresis are reported treatments. This patient’s EED was recalcitrant to prednisone and responded to colchicine.
Scleromyxedema and EED are both rare, distinct cutaneous entities associated with different underlying paraproteinemias and to the best of our knowledge, have not been previously reported to coexist in a single patient.
This case and the photos were submitted by Rachel Fayne, BA; Yumeng Li, MD, MS; Fabrizio Galimberti, MD, PhD; and Brian Morrison, MD, of the department of dermatology and cutaneous surgery at the University of Miami.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.

Skin findings associated with nutritional deficiencies
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
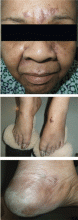
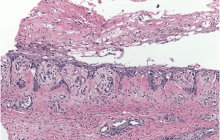
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
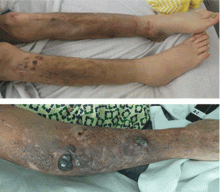
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
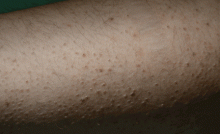
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
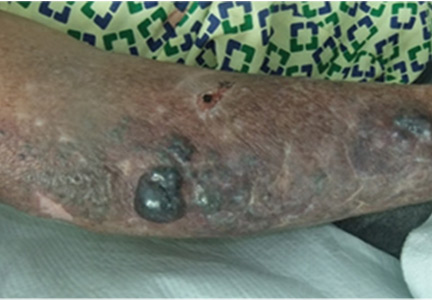
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
KEY POINTS
- Although nutritional deficiencies are relatively uncommon in the general population, certain groups have a higher risk, including infants, pregnant women, alcoholics, vegetarians, persons of poor socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery.
- Often, patients present with more than one deficiency.
- Zinc deficiency can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
- The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
- Manifestations of vitamin A deficiency include night-blindness, dry eyes, and phrynoderma (“toad skin”).
- The B-complex vitamins are linked. Vitamin B2 (riboflavin) deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12.