Article
Spontaneous, Chronic Expanding Posterior Thigh Hematoma Mimicking Soft-Tissue Sarcoma in a Morbidly Obese Pregnant Woman
- Author:
- Everhart JS
- Fajolu OK
- Mayerson JL
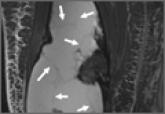
Soft-tissue sarcomas are rare and often confused for more common and benign disorders during diagnosis. Chronic expanding hematomas are...
