Quiz
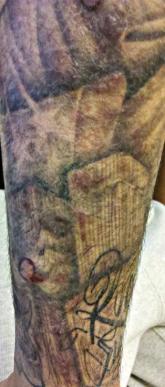
Indurated Erythematous Papules and Plaques on the Forearm
- Author:
- Fiona P. Blanco, MD
- Karan Lal, BS
- Sameera Husain, MD
A 21-year-old man presented with growing, mildly pruritic, cutaneous papules and plaques on the right extensor forearm of 3 weeks’ duration. The...
