User login
Tolerability of Tretinoin Lotion 0.05% for Moderate to Severe Acne Vulgaris: A Post Hoc Analysis in a Black Population
Acne vulgaris (acne) is the most common dermatologic condition in black patients.1,2 However, among outpatient visits, racial disparities exist in both the likelihood of seeing a dermatologist and being treated.3 Black patients are less likely to visit a dermatologist or receive any acne medication. Acne in black skin is frequently associated with postinflammatory hyperpigmentation (PIH), an important consideration in treatment choice and maintenance.
There is a paucity of clinical studies that specifically evaluate acne treatment in this patient population. An 8-week, vehicle-controlled study with tretinoin cream 0.025% in 27 black patients with acne reported notable decreases in papules, pustules, and hyperpigmented macules in 83% of patients treated with tretinoin compared to only 13% receiving vehicle.4 However, irritation and inflammation were problematic. An open-label study of adapalene gel 0.1% in 65 black South Africans also demonstrated significant improvement in inflammatory and noninflammatory lesions and PIH (P<.01), with seemingly better tolerability.5,6 A meta-analysis of 5 randomized studies from the United States and Europe (N=655) compared the efficacy and safety of adapalene gel 0.1% in black (n=46) and white patients.7 There was no significant difference in percentage reduction in comedonal (44%) or total (42%) lesion counts. The percentage reduction in inflammatory lesion counts (53%) was significantly greater in black patients (P=.012). Tolerability also was better; black patients experienced significantly less erythema and scaling (P<.001 and P=.026, respectively), though erythema can be underestimated in darker skin tones because of the masking effects of melanin.5,7 Dryness was more common, though a smaller percentage of black patients reported moderate or severe dryness compared to white patients (7% vs 18%).7
Black patients also are less likely to receive combination therapy, and again clinical data are limited.3 A more recent subgroup analysis evaluated the safety and efficacy of adapalene 0.1%–benzoyl peroxide 2.5% gel in black patients with moderate acne from 3 studies (n=238 out of a total of 3855 patients).8 Similar results were obtained as in the overall study populations, with 64.3% and 48.5% reductions in inflammatory and noninflammatory lesion counts, respectively, at week 12. The most common treatment-related adverse event (AE) in both treatment groups was dry skin (11.3%).8
Extensive clinical data in a predominantly white population have shown that topical retinoids (eg, tretinoin, adapalene, tazarotene) are highly effective in treating acne, and they are recommended as the cornerstone of topical therapy.9 However, there is a common perception that they are primarily effective in comedonal acne10 and that their use is associated with notable cutaneous irritation.11,12 Several attempts have been made to alleviate the tolerability issue using novel delivery systems. A new lotion formulation of tretinoin recently was developed and leveraged polymeric emulsion technology with the aim to improve both efficacy and tolerability of tretinoin. Herein, we performed a post hoc analysis of 2 large phase 3 clinical studies13 in patients with moderate or severe acne treated with tretinoin lotion 0.05% to evaluate its safety and tolerability in a black population.
METHODS
Study Design
We conducted a post hoc analysis of 2 identical multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies13 in black patients with moderate or severe acne. Protocols received approval from the appropriate institutional review board for each center before patient enrollment, and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as well as in compliance with local regulatory requirements. All patients were informed of the study details and provided written consent before entering the studies.
Patients were enrolled with an evaluator global severity score (EGSS) of 3 (moderate) or 4 (severe). Participants were randomized (1:1) to receive tretinoin lotion 0.05% or vehicle applied to the face once daily for 12 weeks.
Study Population
Eligible patients for the post hoc analysis included male and female patients with black skin who were 9 years and older and presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 or fewer nodules. A washout period of up to 1 month was required for patients who previously used prescription and over-the-counter acne treatments, and a washout period of 6 months was required for systemic retinoids.
Safety Evaluation
Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a 4-point scale (0=none; 3=severe). Severity of hypopigmentation and hyperpigmentation also was assessed using this 4-point scale. The investigator assessed erythema and scaling at the time of each study visit. Reports of itching, burning, and stinging were solicited from participants and recorded as an average score of their symptoms during the period since the prior visit.
Adverse events were evaluated throughout and summarized by treatment group, severity, and relationship to study medication.
Statistical Analysis
The safety analysis set comprised all randomized patients who were presumed to have used the study drug at least once and who provided at least 1 postbaseline evaluation. All AEs occurring during the studies were recorded and coded using the Medical Dictionary for Regulatory Activities version 18.0. Treatment group comparisons were made by tabulating the frequency of participants reporting 1 or more AEs during the study.
Cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and tolerability (itching, burning, and stinging) scores were presented by treatment group with descriptive statistics at baseline and weeks 4, 8, and 12. Frequencies and percentages for each outcome category were included in the statistics.
RESULTS
Baseline Characteristics
A total of 308 patients were included in the post hoc analysis. Overall, 257 (83.4%) patients completed the studies, including 138 (83.6%) patients receiving tretinoin lotion 0.05% and 119 (83.2%) receiving vehicle (Figure 1). Completion rates were similar in the female and male subgroups (83.3% and 83.8%, respectively). The most common reasons for study discontinuations were lost to follow-up (n=32; 10.4%) or participant request (n=13; 4.2%) and were similar irrespective of treatment or sex. There were no study discontinuations due to AEs.

Demographic data (Table) were similar across the 2 treatment arms. The mean age (standard deviation [SD]) of the participants was 22.1 (8.35) years (range, 9–58 years). Participants were predominantly female (209/308 [67.9%]) and tended to be a little older than the males (mean age, 23.6 vs 18.8 years).
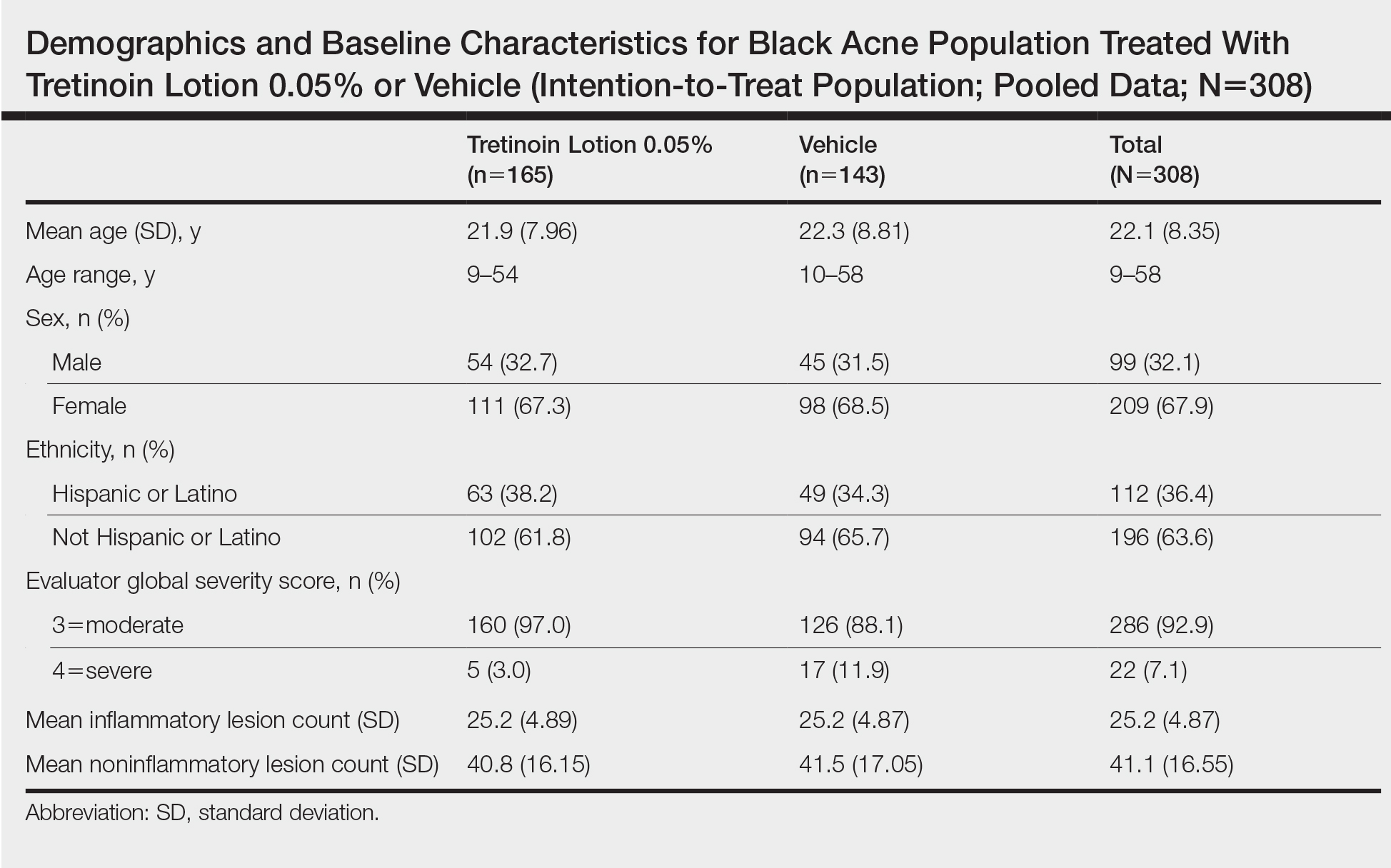
There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).
Safety
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).
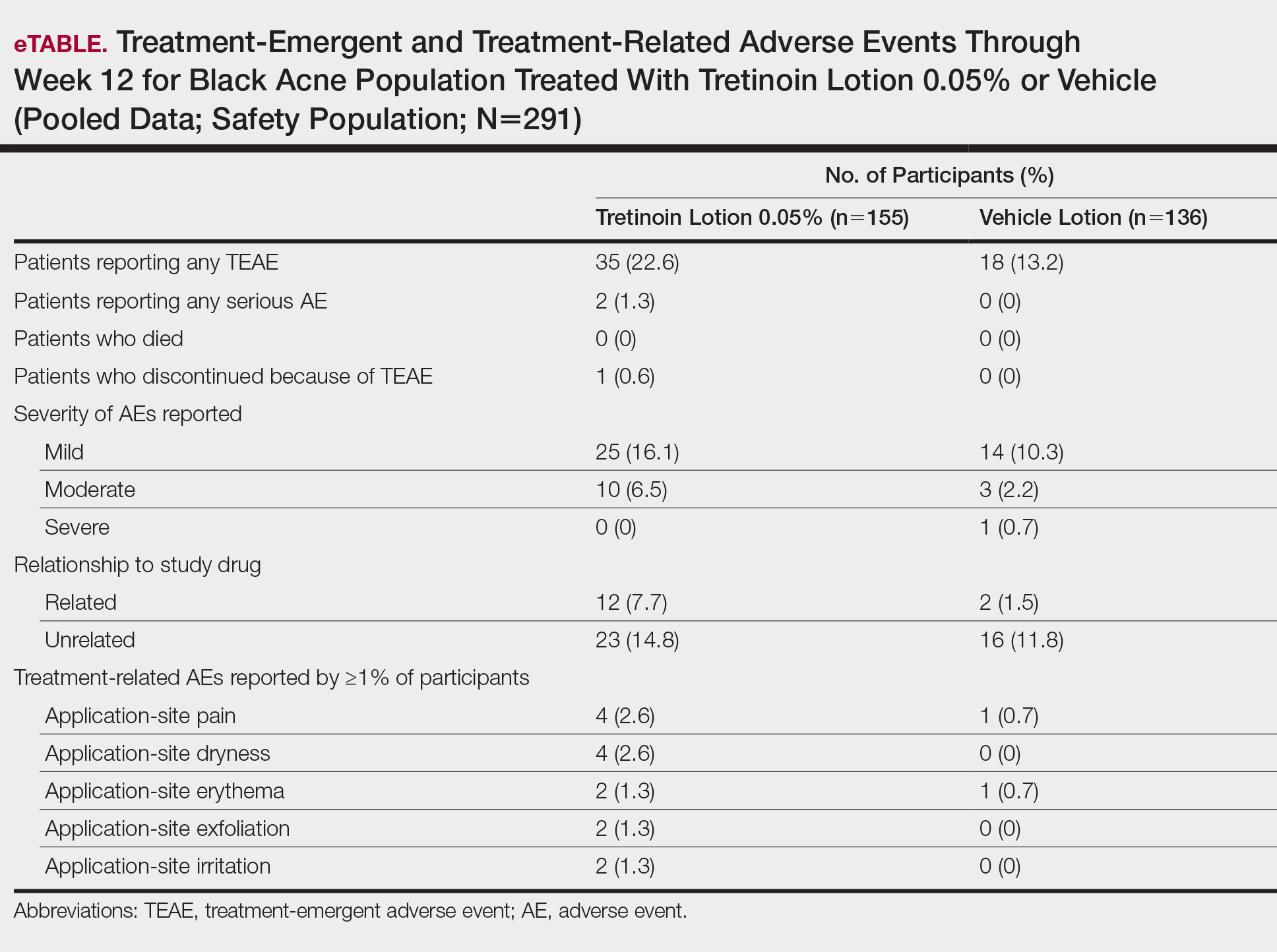
Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
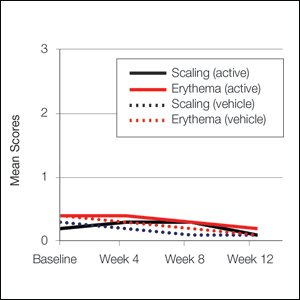
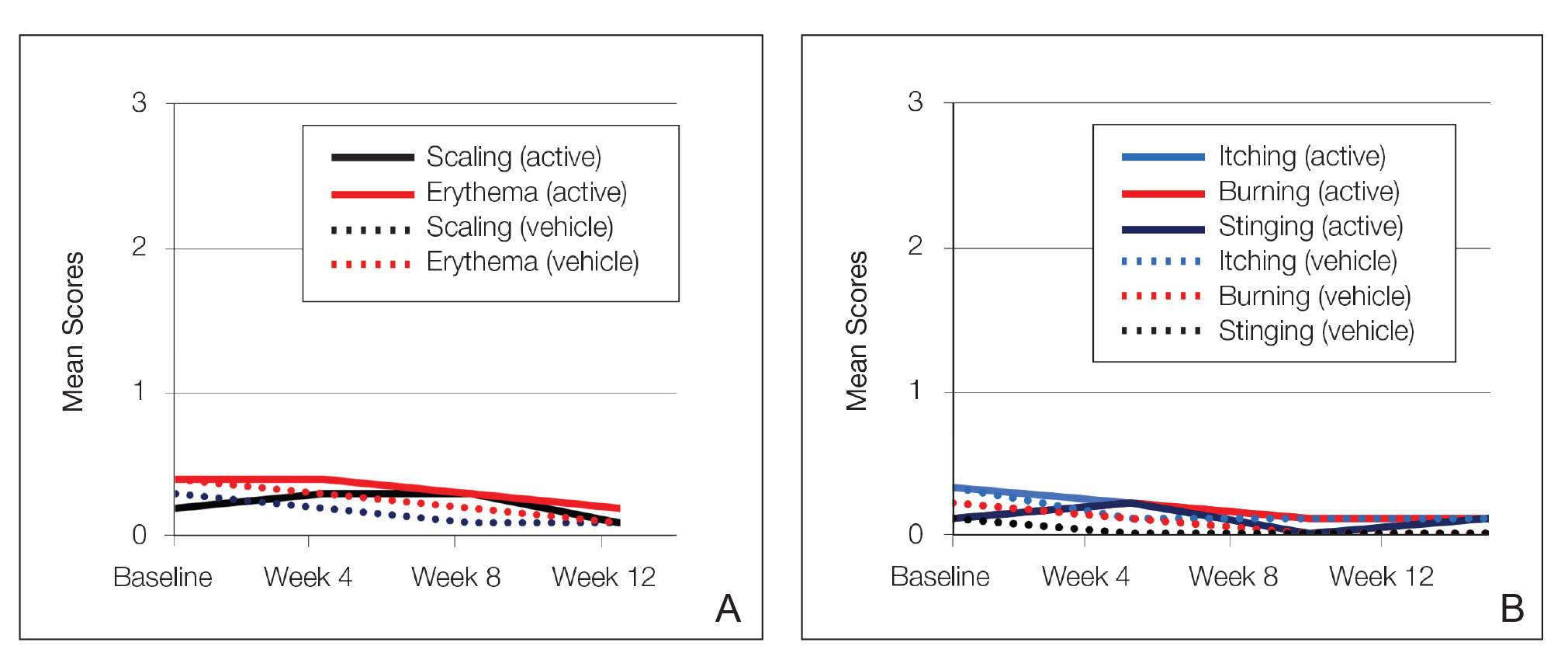
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.
Hyperpigmentation and Hypopigmentation
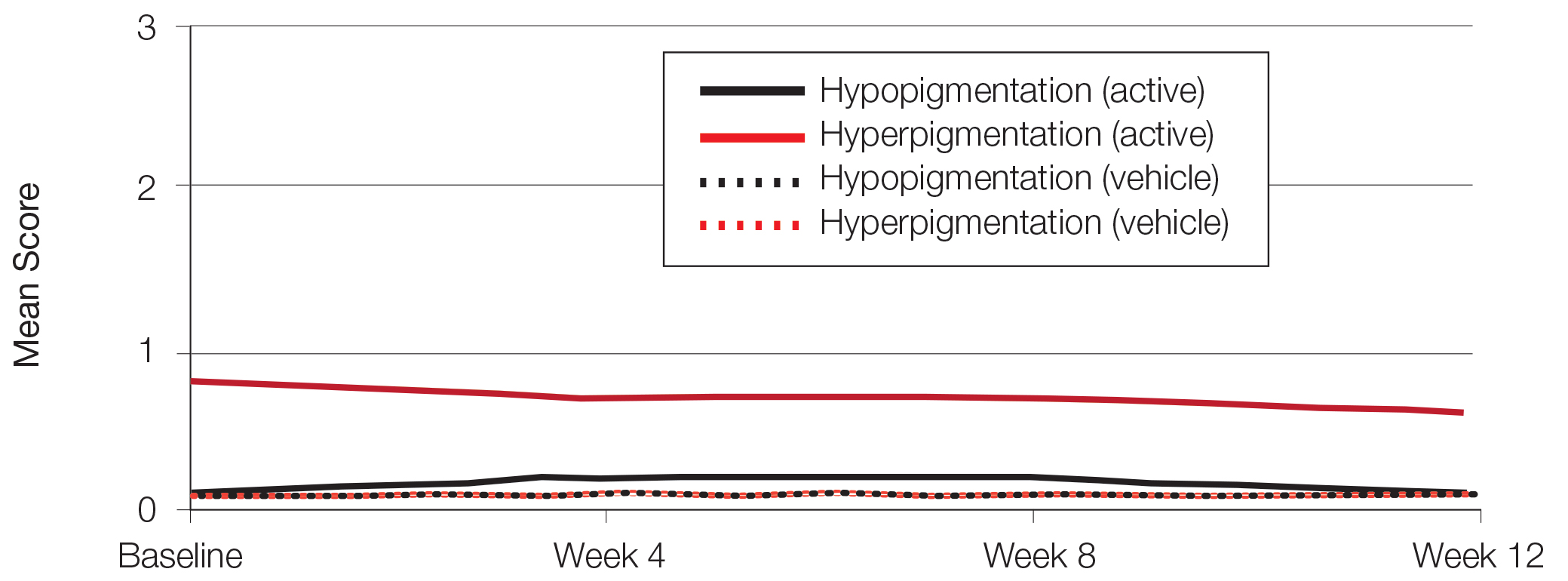
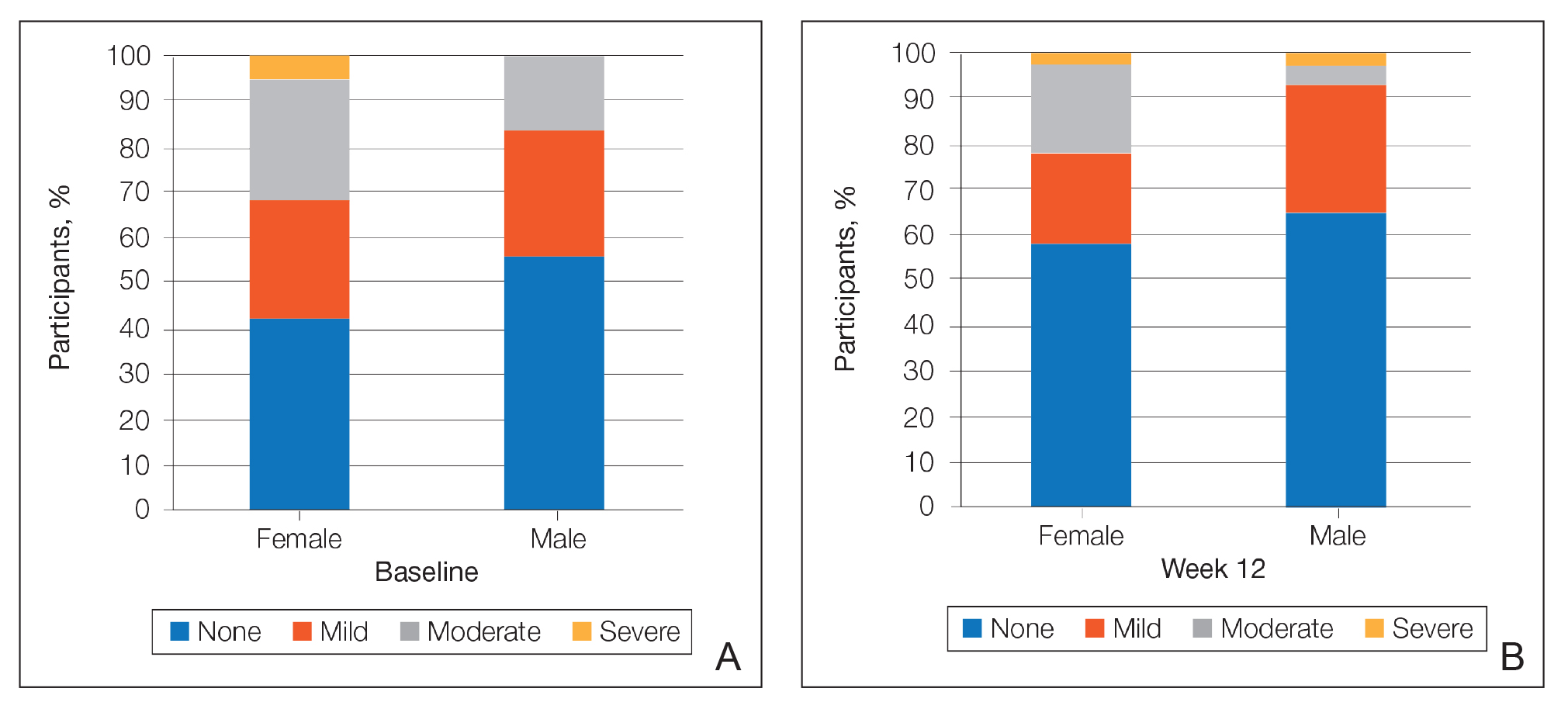
There was a progressive improvement in baseline hyperpigmentation severity in participants treated with tretinoin lotion 0.05%; mean scores reduced from 0.8 at baseline to 0.6 by week 12 (Figure 3), with a similar improvement in both sexes (Figure 4). Moderate to severe hyperpigmentation was reported in 24 (17.3%) participants by week 12 compared to 41 (26.4%) at baseline; the majority (n=21) were female at week 12. Moderate to severe hyperpigmentation was reported in 24 (19.7%) participants treated with vehicle at week 12.
Hypopigmentation at baseline was rare and mild, and again most common in female patients. There was no increase in hypopigmentation over the course of the study.
COMMENT
Topical retinoids (eg, tretinoin, adapalene, tazarotene) are recommended as the cornerstone of topical acne treatment, with safety and efficacy well documented in large pivotal trials.14 However, data in black patients are lacking. Acne is the most common dermatologic condition in these patients, and yet investigation into this important population is limited to small study populations or subgroup analyses.
Tretinoin lotion 0.05% is a novel topical treatment for moderate to severe acne that leverages polymeric emulsion technology. The development rationale was to provide a tretinoin formulation with improved efficacy and tolerability, features that could be especially suited to black patients with acne.
In our post hoc analysis of black patients with acne, tretinoin lotion 0.05% generally was considered safe and well tolerated. The most commonly reported treatment-related AEs were of low incidence and included application-site reactions and skin-related events attributed to the known properties of tretinoin. Most noteworthy was the extremely low irritation potential of this novel tretinoin formulation. Treatment-related AEs generally were mild, and interestingly, the majority occurred in female patients. The incidence of the most common treatment-related AEs—application-site dryness (2.6%) and application-site pain (2.6%)—was lower than that reported in the white populations in the 2 studies (3.8% and 3.4%, respectively).(unpublished data, Ortho Dermatologics), though the differences were not significant (P=.625 and P=.799).
Approximately one-quarter of participants had mild to moderate erythema, scaling, itching, and stinging at baseline. All of these cutaneous symptoms improved with treatment. There were slight transient increases in scaling and stinging at week 4, with stinging more noticeable in the female population. There were no noticeable changes in mild to moderate burning during the study.
Postinflammatory hyperpigmentation is an important consideration in black patients with acne. It can arise from either acne-induced inflammation or injury. It can be of greater concern to the patient than the acne itself and often is the main reason black patients seek a dermatologist consultation. In a survey of adult female acne, nonwhite women experienced substantially more PIH than white women. In addition, clearance of PIH was most important for these nonwhite women (42% vs 8% for white women), whereas lesion clearance was the most important aspect for white women (58% vs 32% for nonwhite women).15 Erring on the side of increased tolerability is appropriate in black patients with acne, given that any irritant reactions can lead to pigmentary alterations—hyperpigmentation or hypopigmentation—that can cause considerable patient anxiety. The psychologic impact of PIH can be devastating, and an ideal acne treatment in these patients would be one that is effective against both PIH and acne. Tretinoin cream 0.1% monotherapy has been shown to be effective in reducing PIH.16 Postinflammatory hyperpigmentation lesions and normal skin were assessed by clinical and colorimetric evaluations and by analysis of biopsy specimens. Although facial PIH lesions in the 24 tretinoin-treated patients were significantly lighter after 40 weeks of treatment compared to vehicle in this study (P<.001), overall improvement was first noted after 4 weeks (P=.009). Normal skin also was minimally lightened by tretinoin; however, exuberant local skin reactions, including peeling, developed in 50% of patients. Mild to moderate PIH was present in the majority of tretinoin-treated patients at baseline in our post hoc analysis, severe in 3.2% of cases, and both more common and severe in females. Mean scores reduced over the 12-week study period, from 0.6 to 0.4 in male patients and 0.9 to 0.7 in female patients. Hypopigmentation was rare and mild at baseline and did not increase over the course of the study. A pilot study with a cream formulation of tazarotene in patients with acne from darker racial groups showed the retinoid to be effective in treating PIH following 18 weeks of once-daily application.17 Further longer-term studies on treating PIH with tretinoin lotion 0.05% are warranted given its tolerability profile.
CONCLUSION
This novel tretinoin lotion 0.05% formulation is a safe and well-tolerated topical treatment for moderate to severe comedonal and inflammatory acne in black patients. Tretinoin lotion 0.05% does not appear to induce PIH and may afford an effective, well-tolerated, dual-treatment option.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for medical writing support. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388,390.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(suppl 2):S98-S106.
- Rogers AT, Semenov YR, Kwatra SG, et al. Racial disparities in the management of acne: evidence from the National Ambulatory Medial Care Survey, 2005-2014. J Dermatolog Treat. 2018;29:287-289.
- Halder RM. The role of retinoids in the management of cutaneous conditions in blacks. J Am Acad Dermatol. 1998;39(suppl 2):S98-S103.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Jacyk WK, Mpofu P. Adapalene gel 0.1% for topical treatment of acne vulgaris in African patients. Cutis. 2001;68(suppl 4):48-54.
- Czernielewski J, Poncet M, Mizzi F. Efficacy and cutaneous safety of adapalene in black patients versus white patients with acne vulgaris. Cutis. 2002;70:243-248.
- Alexis AF, Johnson LA, Kerrouche N, et al. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170-174.
- Leyden JJ, Shalita A, Thiboutot D, et al. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216-224.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids?J Cutan Med Surg. 2015;19:530-538.
- Kircik LH. Evaluating tretinoin formulations in the treatment of acne. J Drugs Dermatol. 2014;13:466-470.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Leyden J, Stein-Gold l, Weiss J. Why topical retionoids are the mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017;7:293-304.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
Acne vulgaris (acne) is the most common dermatologic condition in black patients.1,2 However, among outpatient visits, racial disparities exist in both the likelihood of seeing a dermatologist and being treated.3 Black patients are less likely to visit a dermatologist or receive any acne medication. Acne in black skin is frequently associated with postinflammatory hyperpigmentation (PIH), an important consideration in treatment choice and maintenance.
There is a paucity of clinical studies that specifically evaluate acne treatment in this patient population. An 8-week, vehicle-controlled study with tretinoin cream 0.025% in 27 black patients with acne reported notable decreases in papules, pustules, and hyperpigmented macules in 83% of patients treated with tretinoin compared to only 13% receiving vehicle.4 However, irritation and inflammation were problematic. An open-label study of adapalene gel 0.1% in 65 black South Africans also demonstrated significant improvement in inflammatory and noninflammatory lesions and PIH (P<.01), with seemingly better tolerability.5,6 A meta-analysis of 5 randomized studies from the United States and Europe (N=655) compared the efficacy and safety of adapalene gel 0.1% in black (n=46) and white patients.7 There was no significant difference in percentage reduction in comedonal (44%) or total (42%) lesion counts. The percentage reduction in inflammatory lesion counts (53%) was significantly greater in black patients (P=.012). Tolerability also was better; black patients experienced significantly less erythema and scaling (P<.001 and P=.026, respectively), though erythema can be underestimated in darker skin tones because of the masking effects of melanin.5,7 Dryness was more common, though a smaller percentage of black patients reported moderate or severe dryness compared to white patients (7% vs 18%).7
Black patients also are less likely to receive combination therapy, and again clinical data are limited.3 A more recent subgroup analysis evaluated the safety and efficacy of adapalene 0.1%–benzoyl peroxide 2.5% gel in black patients with moderate acne from 3 studies (n=238 out of a total of 3855 patients).8 Similar results were obtained as in the overall study populations, with 64.3% and 48.5% reductions in inflammatory and noninflammatory lesion counts, respectively, at week 12. The most common treatment-related adverse event (AE) in both treatment groups was dry skin (11.3%).8
Extensive clinical data in a predominantly white population have shown that topical retinoids (eg, tretinoin, adapalene, tazarotene) are highly effective in treating acne, and they are recommended as the cornerstone of topical therapy.9 However, there is a common perception that they are primarily effective in comedonal acne10 and that their use is associated with notable cutaneous irritation.11,12 Several attempts have been made to alleviate the tolerability issue using novel delivery systems. A new lotion formulation of tretinoin recently was developed and leveraged polymeric emulsion technology with the aim to improve both efficacy and tolerability of tretinoin. Herein, we performed a post hoc analysis of 2 large phase 3 clinical studies13 in patients with moderate or severe acne treated with tretinoin lotion 0.05% to evaluate its safety and tolerability in a black population.
METHODS
Study Design
We conducted a post hoc analysis of 2 identical multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies13 in black patients with moderate or severe acne. Protocols received approval from the appropriate institutional review board for each center before patient enrollment, and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as well as in compliance with local regulatory requirements. All patients were informed of the study details and provided written consent before entering the studies.
Patients were enrolled with an evaluator global severity score (EGSS) of 3 (moderate) or 4 (severe). Participants were randomized (1:1) to receive tretinoin lotion 0.05% or vehicle applied to the face once daily for 12 weeks.
Study Population
Eligible patients for the post hoc analysis included male and female patients with black skin who were 9 years and older and presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 or fewer nodules. A washout period of up to 1 month was required for patients who previously used prescription and over-the-counter acne treatments, and a washout period of 6 months was required for systemic retinoids.
Safety Evaluation
Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a 4-point scale (0=none; 3=severe). Severity of hypopigmentation and hyperpigmentation also was assessed using this 4-point scale. The investigator assessed erythema and scaling at the time of each study visit. Reports of itching, burning, and stinging were solicited from participants and recorded as an average score of their symptoms during the period since the prior visit.
Adverse events were evaluated throughout and summarized by treatment group, severity, and relationship to study medication.
Statistical Analysis
The safety analysis set comprised all randomized patients who were presumed to have used the study drug at least once and who provided at least 1 postbaseline evaluation. All AEs occurring during the studies were recorded and coded using the Medical Dictionary for Regulatory Activities version 18.0. Treatment group comparisons were made by tabulating the frequency of participants reporting 1 or more AEs during the study.
Cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and tolerability (itching, burning, and stinging) scores were presented by treatment group with descriptive statistics at baseline and weeks 4, 8, and 12. Frequencies and percentages for each outcome category were included in the statistics.
RESULTS
Baseline Characteristics
A total of 308 patients were included in the post hoc analysis. Overall, 257 (83.4%) patients completed the studies, including 138 (83.6%) patients receiving tretinoin lotion 0.05% and 119 (83.2%) receiving vehicle (Figure 1). Completion rates were similar in the female and male subgroups (83.3% and 83.8%, respectively). The most common reasons for study discontinuations were lost to follow-up (n=32; 10.4%) or participant request (n=13; 4.2%) and were similar irrespective of treatment or sex. There were no study discontinuations due to AEs.

Demographic data (Table) were similar across the 2 treatment arms. The mean age (standard deviation [SD]) of the participants was 22.1 (8.35) years (range, 9–58 years). Participants were predominantly female (209/308 [67.9%]) and tended to be a little older than the males (mean age, 23.6 vs 18.8 years).

There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).

Safety
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).

Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.
Hyperpigmentation and Hypopigmentation
There was a progressive improvement in baseline hyperpigmentation severity in participants treated with tretinoin lotion 0.05%; mean scores reduced from 0.8 at baseline to 0.6 by week 12 (Figure 3), with a similar improvement in both sexes (Figure 4). Moderate to severe hyperpigmentation was reported in 24 (17.3%) participants by week 12 compared to 41 (26.4%) at baseline; the majority (n=21) were female at week 12. Moderate to severe hyperpigmentation was reported in 24 (19.7%) participants treated with vehicle at week 12.


Hypopigmentation at baseline was rare and mild, and again most common in female patients. There was no increase in hypopigmentation over the course of the study.
COMMENT
Topical retinoids (eg, tretinoin, adapalene, tazarotene) are recommended as the cornerstone of topical acne treatment, with safety and efficacy well documented in large pivotal trials.14 However, data in black patients are lacking. Acne is the most common dermatologic condition in these patients, and yet investigation into this important population is limited to small study populations or subgroup analyses.
Tretinoin lotion 0.05% is a novel topical treatment for moderate to severe acne that leverages polymeric emulsion technology. The development rationale was to provide a tretinoin formulation with improved efficacy and tolerability, features that could be especially suited to black patients with acne.
In our post hoc analysis of black patients with acne, tretinoin lotion 0.05% generally was considered safe and well tolerated. The most commonly reported treatment-related AEs were of low incidence and included application-site reactions and skin-related events attributed to the known properties of tretinoin. Most noteworthy was the extremely low irritation potential of this novel tretinoin formulation. Treatment-related AEs generally were mild, and interestingly, the majority occurred in female patients. The incidence of the most common treatment-related AEs—application-site dryness (2.6%) and application-site pain (2.6%)—was lower than that reported in the white populations in the 2 studies (3.8% and 3.4%, respectively).(unpublished data, Ortho Dermatologics), though the differences were not significant (P=.625 and P=.799).
Approximately one-quarter of participants had mild to moderate erythema, scaling, itching, and stinging at baseline. All of these cutaneous symptoms improved with treatment. There were slight transient increases in scaling and stinging at week 4, with stinging more noticeable in the female population. There were no noticeable changes in mild to moderate burning during the study.
Postinflammatory hyperpigmentation is an important consideration in black patients with acne. It can arise from either acne-induced inflammation or injury. It can be of greater concern to the patient than the acne itself and often is the main reason black patients seek a dermatologist consultation. In a survey of adult female acne, nonwhite women experienced substantially more PIH than white women. In addition, clearance of PIH was most important for these nonwhite women (42% vs 8% for white women), whereas lesion clearance was the most important aspect for white women (58% vs 32% for nonwhite women).15 Erring on the side of increased tolerability is appropriate in black patients with acne, given that any irritant reactions can lead to pigmentary alterations—hyperpigmentation or hypopigmentation—that can cause considerable patient anxiety. The psychologic impact of PIH can be devastating, and an ideal acne treatment in these patients would be one that is effective against both PIH and acne. Tretinoin cream 0.1% monotherapy has been shown to be effective in reducing PIH.16 Postinflammatory hyperpigmentation lesions and normal skin were assessed by clinical and colorimetric evaluations and by analysis of biopsy specimens. Although facial PIH lesions in the 24 tretinoin-treated patients were significantly lighter after 40 weeks of treatment compared to vehicle in this study (P<.001), overall improvement was first noted after 4 weeks (P=.009). Normal skin also was minimally lightened by tretinoin; however, exuberant local skin reactions, including peeling, developed in 50% of patients. Mild to moderate PIH was present in the majority of tretinoin-treated patients at baseline in our post hoc analysis, severe in 3.2% of cases, and both more common and severe in females. Mean scores reduced over the 12-week study period, from 0.6 to 0.4 in male patients and 0.9 to 0.7 in female patients. Hypopigmentation was rare and mild at baseline and did not increase over the course of the study. A pilot study with a cream formulation of tazarotene in patients with acne from darker racial groups showed the retinoid to be effective in treating PIH following 18 weeks of once-daily application.17 Further longer-term studies on treating PIH with tretinoin lotion 0.05% are warranted given its tolerability profile.
CONCLUSION
This novel tretinoin lotion 0.05% formulation is a safe and well-tolerated topical treatment for moderate to severe comedonal and inflammatory acne in black patients. Tretinoin lotion 0.05% does not appear to induce PIH and may afford an effective, well-tolerated, dual-treatment option.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for medical writing support. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
Acne vulgaris (acne) is the most common dermatologic condition in black patients.1,2 However, among outpatient visits, racial disparities exist in both the likelihood of seeing a dermatologist and being treated.3 Black patients are less likely to visit a dermatologist or receive any acne medication. Acne in black skin is frequently associated with postinflammatory hyperpigmentation (PIH), an important consideration in treatment choice and maintenance.
There is a paucity of clinical studies that specifically evaluate acne treatment in this patient population. An 8-week, vehicle-controlled study with tretinoin cream 0.025% in 27 black patients with acne reported notable decreases in papules, pustules, and hyperpigmented macules in 83% of patients treated with tretinoin compared to only 13% receiving vehicle.4 However, irritation and inflammation were problematic. An open-label study of adapalene gel 0.1% in 65 black South Africans also demonstrated significant improvement in inflammatory and noninflammatory lesions and PIH (P<.01), with seemingly better tolerability.5,6 A meta-analysis of 5 randomized studies from the United States and Europe (N=655) compared the efficacy and safety of adapalene gel 0.1% in black (n=46) and white patients.7 There was no significant difference in percentage reduction in comedonal (44%) or total (42%) lesion counts. The percentage reduction in inflammatory lesion counts (53%) was significantly greater in black patients (P=.012). Tolerability also was better; black patients experienced significantly less erythema and scaling (P<.001 and P=.026, respectively), though erythema can be underestimated in darker skin tones because of the masking effects of melanin.5,7 Dryness was more common, though a smaller percentage of black patients reported moderate or severe dryness compared to white patients (7% vs 18%).7
Black patients also are less likely to receive combination therapy, and again clinical data are limited.3 A more recent subgroup analysis evaluated the safety and efficacy of adapalene 0.1%–benzoyl peroxide 2.5% gel in black patients with moderate acne from 3 studies (n=238 out of a total of 3855 patients).8 Similar results were obtained as in the overall study populations, with 64.3% and 48.5% reductions in inflammatory and noninflammatory lesion counts, respectively, at week 12. The most common treatment-related adverse event (AE) in both treatment groups was dry skin (11.3%).8
Extensive clinical data in a predominantly white population have shown that topical retinoids (eg, tretinoin, adapalene, tazarotene) are highly effective in treating acne, and they are recommended as the cornerstone of topical therapy.9 However, there is a common perception that they are primarily effective in comedonal acne10 and that their use is associated with notable cutaneous irritation.11,12 Several attempts have been made to alleviate the tolerability issue using novel delivery systems. A new lotion formulation of tretinoin recently was developed and leveraged polymeric emulsion technology with the aim to improve both efficacy and tolerability of tretinoin. Herein, we performed a post hoc analysis of 2 large phase 3 clinical studies13 in patients with moderate or severe acne treated with tretinoin lotion 0.05% to evaluate its safety and tolerability in a black population.
METHODS
Study Design
We conducted a post hoc analysis of 2 identical multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies13 in black patients with moderate or severe acne. Protocols received approval from the appropriate institutional review board for each center before patient enrollment, and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as well as in compliance with local regulatory requirements. All patients were informed of the study details and provided written consent before entering the studies.
Patients were enrolled with an evaluator global severity score (EGSS) of 3 (moderate) or 4 (severe). Participants were randomized (1:1) to receive tretinoin lotion 0.05% or vehicle applied to the face once daily for 12 weeks.
Study Population
Eligible patients for the post hoc analysis included male and female patients with black skin who were 9 years and older and presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 or fewer nodules. A washout period of up to 1 month was required for patients who previously used prescription and over-the-counter acne treatments, and a washout period of 6 months was required for systemic retinoids.
Safety Evaluation
Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a 4-point scale (0=none; 3=severe). Severity of hypopigmentation and hyperpigmentation also was assessed using this 4-point scale. The investigator assessed erythema and scaling at the time of each study visit. Reports of itching, burning, and stinging were solicited from participants and recorded as an average score of their symptoms during the period since the prior visit.
Adverse events were evaluated throughout and summarized by treatment group, severity, and relationship to study medication.
Statistical Analysis
The safety analysis set comprised all randomized patients who were presumed to have used the study drug at least once and who provided at least 1 postbaseline evaluation. All AEs occurring during the studies were recorded and coded using the Medical Dictionary for Regulatory Activities version 18.0. Treatment group comparisons were made by tabulating the frequency of participants reporting 1 or more AEs during the study.
Cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and tolerability (itching, burning, and stinging) scores were presented by treatment group with descriptive statistics at baseline and weeks 4, 8, and 12. Frequencies and percentages for each outcome category were included in the statistics.
RESULTS
Baseline Characteristics
A total of 308 patients were included in the post hoc analysis. Overall, 257 (83.4%) patients completed the studies, including 138 (83.6%) patients receiving tretinoin lotion 0.05% and 119 (83.2%) receiving vehicle (Figure 1). Completion rates were similar in the female and male subgroups (83.3% and 83.8%, respectively). The most common reasons for study discontinuations were lost to follow-up (n=32; 10.4%) or participant request (n=13; 4.2%) and were similar irrespective of treatment or sex. There were no study discontinuations due to AEs.

Demographic data (Table) were similar across the 2 treatment arms. The mean age (standard deviation [SD]) of the participants was 22.1 (8.35) years (range, 9–58 years). Participants were predominantly female (209/308 [67.9%]) and tended to be a little older than the males (mean age, 23.6 vs 18.8 years).

There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).

Safety
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).

Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.
Hyperpigmentation and Hypopigmentation
There was a progressive improvement in baseline hyperpigmentation severity in participants treated with tretinoin lotion 0.05%; mean scores reduced from 0.8 at baseline to 0.6 by week 12 (Figure 3), with a similar improvement in both sexes (Figure 4). Moderate to severe hyperpigmentation was reported in 24 (17.3%) participants by week 12 compared to 41 (26.4%) at baseline; the majority (n=21) were female at week 12. Moderate to severe hyperpigmentation was reported in 24 (19.7%) participants treated with vehicle at week 12.


Hypopigmentation at baseline was rare and mild, and again most common in female patients. There was no increase in hypopigmentation over the course of the study.
COMMENT
Topical retinoids (eg, tretinoin, adapalene, tazarotene) are recommended as the cornerstone of topical acne treatment, with safety and efficacy well documented in large pivotal trials.14 However, data in black patients are lacking. Acne is the most common dermatologic condition in these patients, and yet investigation into this important population is limited to small study populations or subgroup analyses.
Tretinoin lotion 0.05% is a novel topical treatment for moderate to severe acne that leverages polymeric emulsion technology. The development rationale was to provide a tretinoin formulation with improved efficacy and tolerability, features that could be especially suited to black patients with acne.
In our post hoc analysis of black patients with acne, tretinoin lotion 0.05% generally was considered safe and well tolerated. The most commonly reported treatment-related AEs were of low incidence and included application-site reactions and skin-related events attributed to the known properties of tretinoin. Most noteworthy was the extremely low irritation potential of this novel tretinoin formulation. Treatment-related AEs generally were mild, and interestingly, the majority occurred in female patients. The incidence of the most common treatment-related AEs—application-site dryness (2.6%) and application-site pain (2.6%)—was lower than that reported in the white populations in the 2 studies (3.8% and 3.4%, respectively).(unpublished data, Ortho Dermatologics), though the differences were not significant (P=.625 and P=.799).
Approximately one-quarter of participants had mild to moderate erythema, scaling, itching, and stinging at baseline. All of these cutaneous symptoms improved with treatment. There were slight transient increases in scaling and stinging at week 4, with stinging more noticeable in the female population. There were no noticeable changes in mild to moderate burning during the study.
Postinflammatory hyperpigmentation is an important consideration in black patients with acne. It can arise from either acne-induced inflammation or injury. It can be of greater concern to the patient than the acne itself and often is the main reason black patients seek a dermatologist consultation. In a survey of adult female acne, nonwhite women experienced substantially more PIH than white women. In addition, clearance of PIH was most important for these nonwhite women (42% vs 8% for white women), whereas lesion clearance was the most important aspect for white women (58% vs 32% for nonwhite women).15 Erring on the side of increased tolerability is appropriate in black patients with acne, given that any irritant reactions can lead to pigmentary alterations—hyperpigmentation or hypopigmentation—that can cause considerable patient anxiety. The psychologic impact of PIH can be devastating, and an ideal acne treatment in these patients would be one that is effective against both PIH and acne. Tretinoin cream 0.1% monotherapy has been shown to be effective in reducing PIH.16 Postinflammatory hyperpigmentation lesions and normal skin were assessed by clinical and colorimetric evaluations and by analysis of biopsy specimens. Although facial PIH lesions in the 24 tretinoin-treated patients were significantly lighter after 40 weeks of treatment compared to vehicle in this study (P<.001), overall improvement was first noted after 4 weeks (P=.009). Normal skin also was minimally lightened by tretinoin; however, exuberant local skin reactions, including peeling, developed in 50% of patients. Mild to moderate PIH was present in the majority of tretinoin-treated patients at baseline in our post hoc analysis, severe in 3.2% of cases, and both more common and severe in females. Mean scores reduced over the 12-week study period, from 0.6 to 0.4 in male patients and 0.9 to 0.7 in female patients. Hypopigmentation was rare and mild at baseline and did not increase over the course of the study. A pilot study with a cream formulation of tazarotene in patients with acne from darker racial groups showed the retinoid to be effective in treating PIH following 18 weeks of once-daily application.17 Further longer-term studies on treating PIH with tretinoin lotion 0.05% are warranted given its tolerability profile.
CONCLUSION
This novel tretinoin lotion 0.05% formulation is a safe and well-tolerated topical treatment for moderate to severe comedonal and inflammatory acne in black patients. Tretinoin lotion 0.05% does not appear to induce PIH and may afford an effective, well-tolerated, dual-treatment option.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for medical writing support. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388,390.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(suppl 2):S98-S106.
- Rogers AT, Semenov YR, Kwatra SG, et al. Racial disparities in the management of acne: evidence from the National Ambulatory Medial Care Survey, 2005-2014. J Dermatolog Treat. 2018;29:287-289.
- Halder RM. The role of retinoids in the management of cutaneous conditions in blacks. J Am Acad Dermatol. 1998;39(suppl 2):S98-S103.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Jacyk WK, Mpofu P. Adapalene gel 0.1% for topical treatment of acne vulgaris in African patients. Cutis. 2001;68(suppl 4):48-54.
- Czernielewski J, Poncet M, Mizzi F. Efficacy and cutaneous safety of adapalene in black patients versus white patients with acne vulgaris. Cutis. 2002;70:243-248.
- Alexis AF, Johnson LA, Kerrouche N, et al. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170-174.
- Leyden JJ, Shalita A, Thiboutot D, et al. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216-224.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids?J Cutan Med Surg. 2015;19:530-538.
- Kircik LH. Evaluating tretinoin formulations in the treatment of acne. J Drugs Dermatol. 2014;13:466-470.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Leyden J, Stein-Gold l, Weiss J. Why topical retionoids are the mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017;7:293-304.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388,390.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(suppl 2):S98-S106.
- Rogers AT, Semenov YR, Kwatra SG, et al. Racial disparities in the management of acne: evidence from the National Ambulatory Medial Care Survey, 2005-2014. J Dermatolog Treat. 2018;29:287-289.
- Halder RM. The role of retinoids in the management of cutaneous conditions in blacks. J Am Acad Dermatol. 1998;39(suppl 2):S98-S103.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Jacyk WK, Mpofu P. Adapalene gel 0.1% for topical treatment of acne vulgaris in African patients. Cutis. 2001;68(suppl 4):48-54.
- Czernielewski J, Poncet M, Mizzi F. Efficacy and cutaneous safety of adapalene in black patients versus white patients with acne vulgaris. Cutis. 2002;70:243-248.
- Alexis AF, Johnson LA, Kerrouche N, et al. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170-174.
- Leyden JJ, Shalita A, Thiboutot D, et al. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216-224.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids?J Cutan Med Surg. 2015;19:530-538.
- Kircik LH. Evaluating tretinoin formulations in the treatment of acne. J Drugs Dermatol. 2014;13:466-470.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Leyden J, Stein-Gold l, Weiss J. Why topical retionoids are the mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017;7:293-304.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
Practice Points
- Acne vulgaris is the most common dermatologic disorder seen in black patients, though data on treatment effects is lacking.
- Postinflammatory hyperpigmentation (PIH) frequently coexists with acne, and retinoids are known to treat both.
- Tretinoin lotion 0.05% is effective in treating both inflammatory and noninflammatory lesions in black patients with acne and reducing PIH without the irritant contact dermatitis seen with other retinoid formulations.
Efinaconazole Solution 10% for Treatment of Toenail Onychomycosis in Latino Patients
Onychomycosis is a common progressive fungal infection of the nail bed, matrix, or plate leading to destruction and deformity of the toenails and fingernails.1,2 It represents up to 50% of all nail disorders1,3 with a notable increasing prevalence in the United States.4-6
Latinos represent the largest ethnic minority group in the United States,7 which is growing rapidly through immigration, particularly in the southern United States. Prevalence data are limited. An incidence of 9.3% secondary to dermatophytes was recorded in a dermatology clinic setting (N=2000).8 Onychomycosis was reported in 31.9% of a group of Latino immigrants in North Carolina (N=518), with higher prevalence in poultry workers, possibly due to the work environment.9
Efinaconazole solution 10% was shown to be well tolerated and more effective than a vehicle in a phase 2 study in Mexico.10 Two identical phase 3 studies of 1655 participants assessed the safety and efficacy of efinaconazole solution 10% in the treatment of onychomycosis.11 This post hoc analysis compares the data for Latino versus non-Latino populations.
Methods
We evaluated the results of 2 multicenter, randomized, double-blind, vehicle-controlled studies that included a total of 1655 participants with mild to moderate toenail onychomycosis (20%–50% clinical involvement). Participants were randomized to efinaconazole solu-tion 10% or vehicle once daily (3:1) for 48 weeks with a 4-week posttreatment follow-up period.11
Our post hoc analysis included 270 Latino patients, defined as an individual of Cuban, Mexican, Puerto Rican, or South or Central American origin or other Latino culture, regardless of race. In addition, data were compared to the 1380 non-Latino patients in the 2 studies. Patients who were randomized in error and never received treatment were excluded from the intention-to-treat analysis.
Efficacy Evaluation
The primary efficacy end point was complete cure rate (0% clinical involvement of target toenail, and both negative potassium hydroxide examination and fungal culture) at week 52. Secondary end points included mycologic cure, complete/almost complete cure (≤5% clinical involvement of target toenail, mycologic cure), and treatment success (≤10% clinical involvement of target toenail) at week 52.
Safety Evaluation
Safety assessments included monitoring and recording of adverse events (AEs) at every postbaseline study visit through week 52. All AEs were classified using the Medical Dictionary for Regulatory Activities (version 12.1). Treatment-emergent AEs (ie, events that began after the first application of study drug) that occurred during the study were summarized for each treatment group by the number of patients reporting each event, as well as by system organ class, preferred term, severity, seriousness, and relationship to the study drug.
Results
A total of 270 Latino participants with toenail onychomycosis (efinaconazole solution 10%, n=193; vehicle, n=77) were included in our study. The mean age of participants at baseline was 45.9 years. They were predominantly male (69.6%) and white Latinos (91.1%). The mean area of target toenail involvement was 36.6%, and the mean number of affected nontarget toenails was 2.5. Latino participants tended to be younger than non-Latino participants (45.9 vs 52.6 years), with a higher proportion of females (30.4% vs 21.3%). Disease severity was similar in both populations. Diabetes was reported in 7.0% and 6.7% of Latino and non-Latino participants, respectively, and mean weight was 83.6 and 86.6 kg, respectively.
Primary Efficacy End Points (Observed Case [OC])
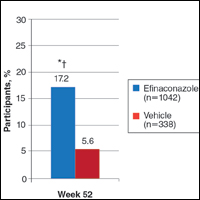
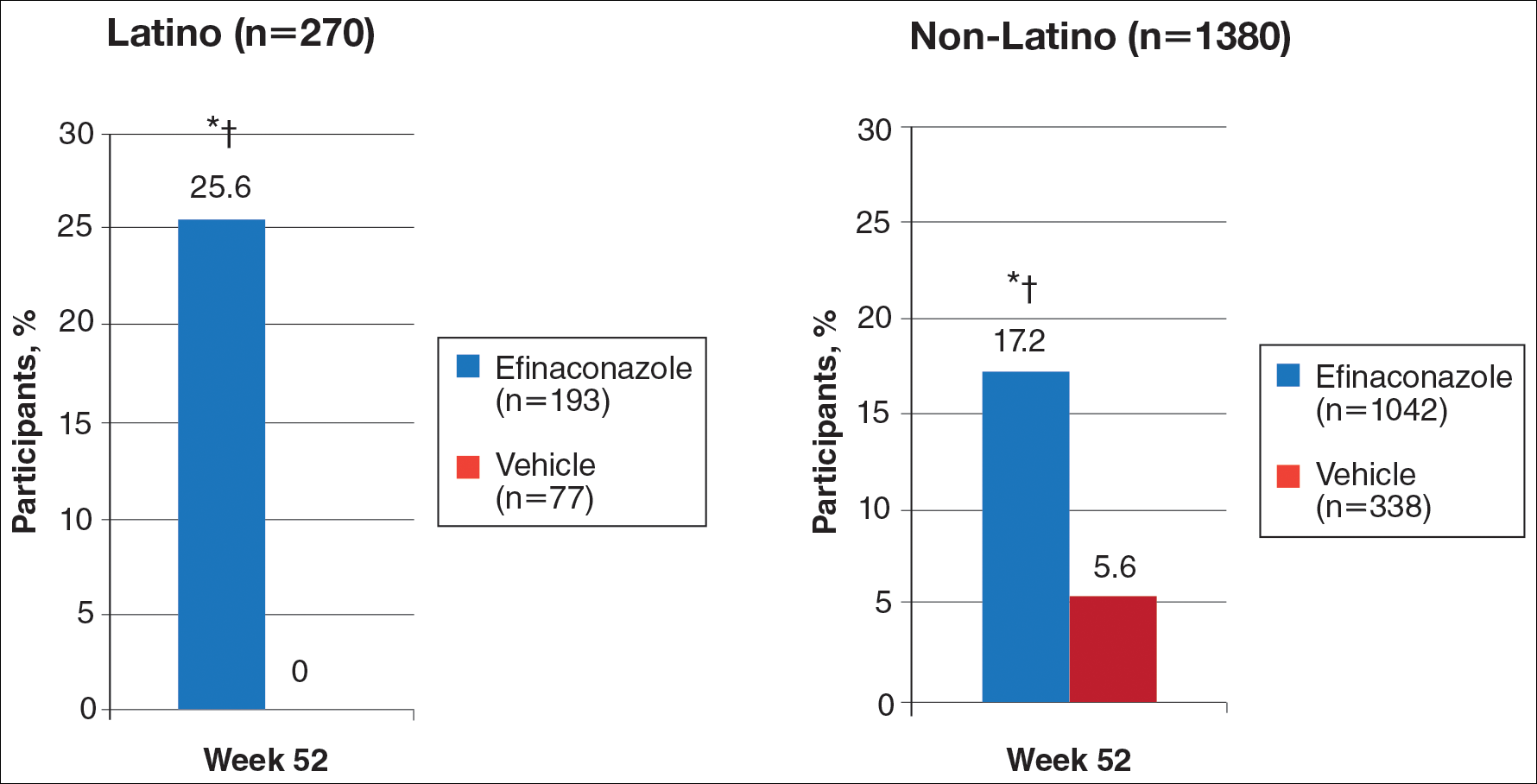
At week 52, 25.6% of Latino participants in the efinaconazole group achieved complete cure versus 0% in the vehicle group (P<.001)(Figure 1). The efficacy of efinaconazole was statistically superior in Latino participants versus non-Latino participants (17.2% [P=.012]). The net effect (calculated by active treatment minus vehicle) for Latino participants also was superior to non-Latino participants (25.6% vs 11.6%).
Secondary Efficacy End Points (OC)
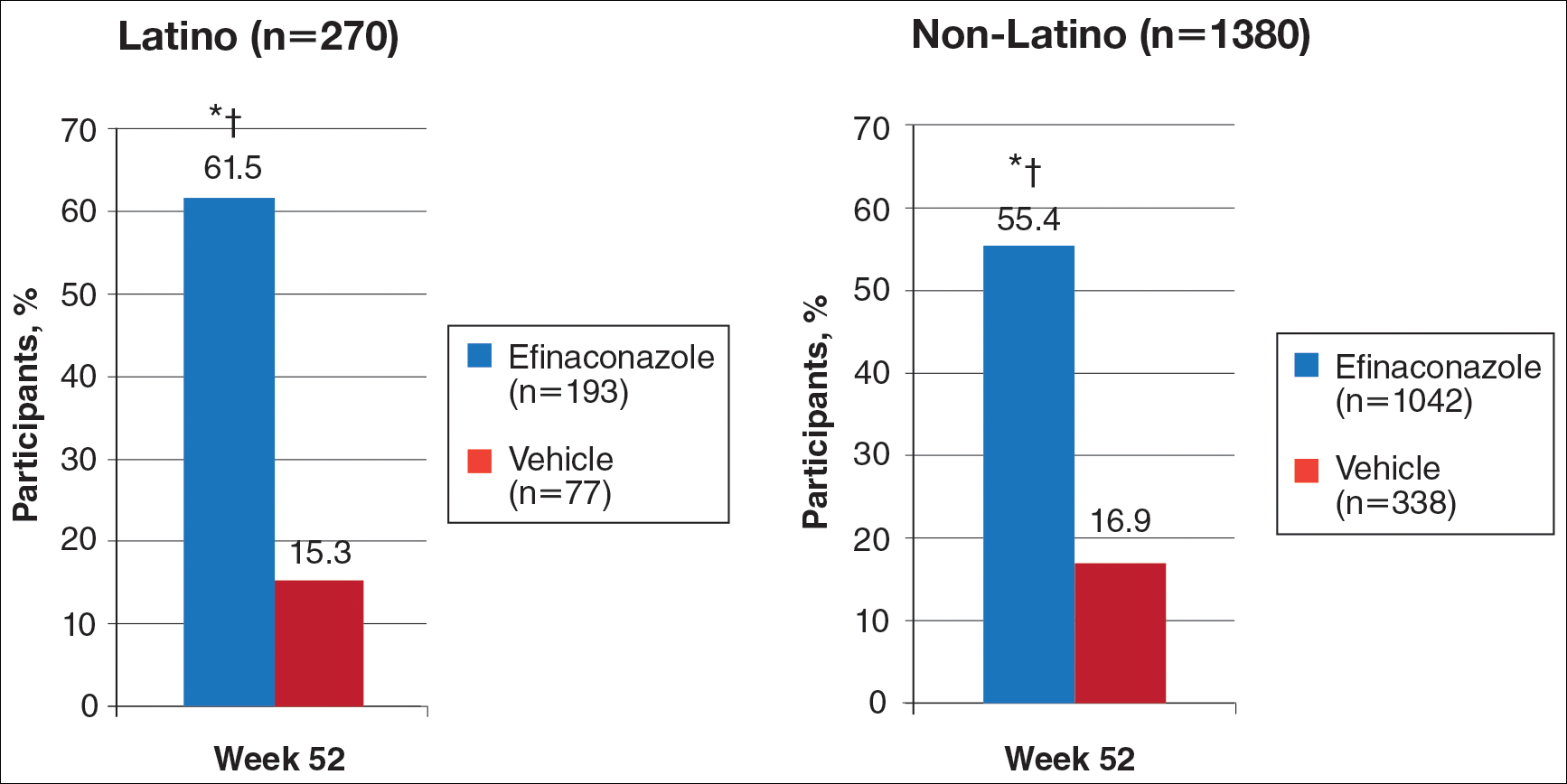
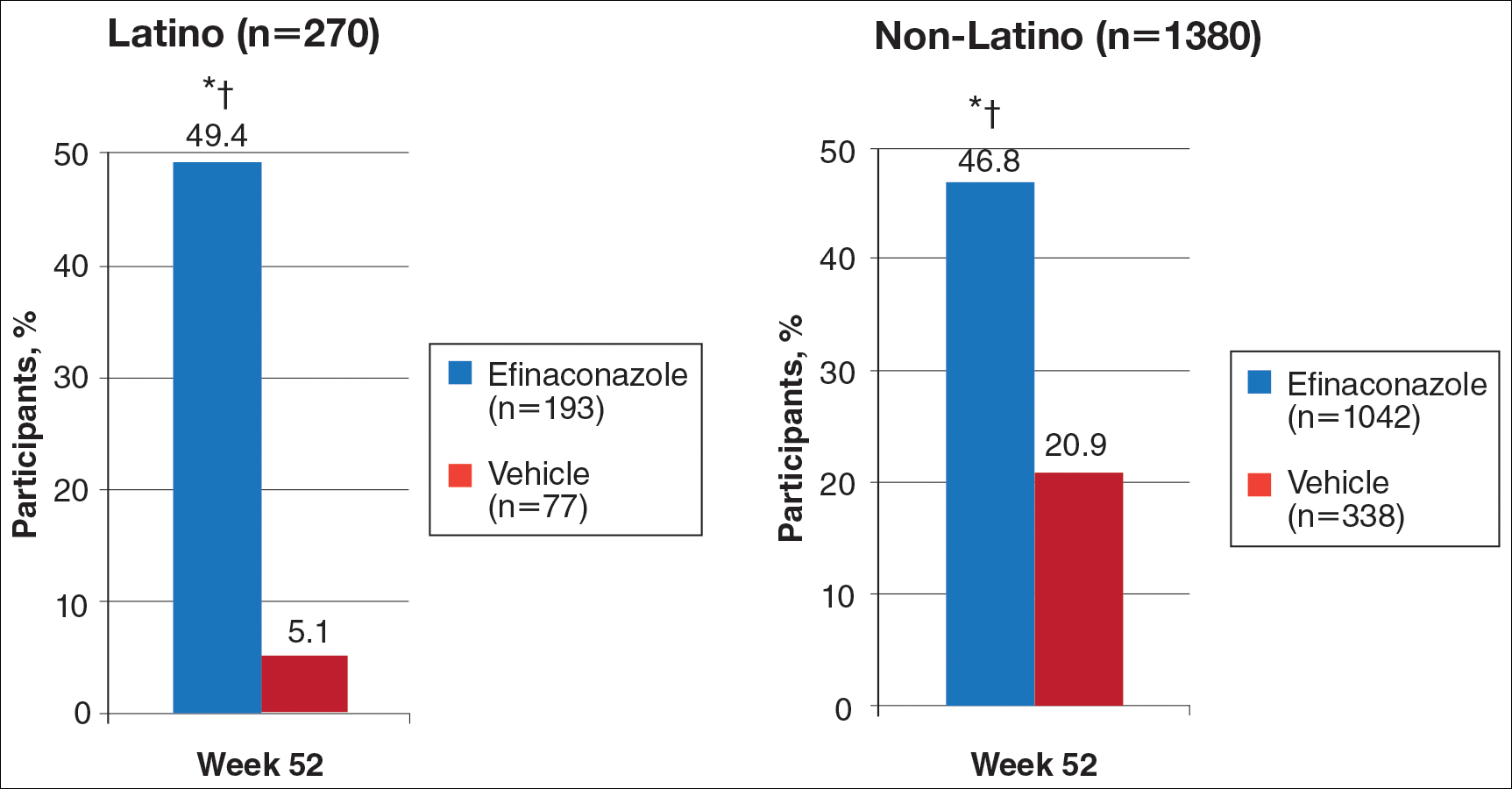
At week 52, 61.5% of Latino participants in the efina-conazole group achieved mycologic cure versus 15.3% in the vehicle group (P<.001)(Figure 2). The net effect for Latino participants was superior to non-Latino participants (46.2% vs 38.5%). More Latino participants in the efinaconazole group compared to vehicle group achieved complete/almost complete cure (32.7% vs 1.7%) or treatment success (49.4% vs 5.1%)(all P<.001)(Figure 3). Although there was no significant difference between the 2 groups for secondary efficacy end points, the net effect of efinaconazole was greater for all end points.
Safety
Adverse event rates were higher in the efinaconazole group than the vehicle group (65.3% vs 54.4%) and were similar in both populations; they were generally mild (61.8% vs 54.5%) or moderate (35.3% vs 45.5%) in severity, not related to study medication (96.8% vs 98.0%), and resolved without sequelae. Only 3 Latino participants (1.6%) discontinued efinaconazole treatment compared to 29 (2.8%) in the non-Latino population.
Comment
With the continued growth of the Latino population in the United States and likely higher prevalence of onychomycosis,9 this post hoc analysis provides important insights into treatment of onychomycosis in this patient population.
Efinaconazole solution 10% was significantly more effective than vehicle in the Latino population (P<.001) and also appeared significantly more effective than the non-Latino population across the 2 phase 3 studies (P=.012). Interestingly, complete cure rates (25.6%) were identical to those reported in the phase 2 study of Mexican patients treated with efinaconazole for 36 weeks.10 Specific data with other topical therapies, such as tavaborole, in Latino patients are not available. One phase 3 study of tavaborole for onychomycosis included 89 Mexican patients (15% of the total study population), but complete cure rates for the overall active treatment group were higher in a second phase 3 study (6.5% vs 9.1%) that did not include participants outside the United States or Canada.12
It is not clear why phase 3 efficacy results with efinaconazole appear better in the Latino population. There are a number of predisposing factors for onychomycosis that are important treatment considerations in Latinos. Obesity is an important factor in the development of onychomycosis,13 with more than 42% of Latino adults in the United States reportedly obese compared to 32.6% of non-Latino adults.14 Obese patients reportedly have shown a poorer response to efinaconazole treatment15; however, in our analysis, the mean weight of the 2 subpopulations was similar at baseline. Diabetes also is associated with an increased risk for onychomycosis16,17 and may be a more important issue in Latinos perhaps due to differences in health care access, social and cultural factors, and/or genetics, as well as the greater incidence of obesity. Prior reports suggest the efficacy of efinaconazole is not substantially influenced by the presence of diabetes,18 and in our 2 subpopulations, baseline incidence of coexisting diabetes was similar. These factors are unlikely to account for the better treatment success seen in our analysis. Efinaconazole has been reported to be more effective in females,19 though the reasons are less clear. The higher proportion of female Latinos (30.4% vs 21.3%) in our study may have had an impact on the results reported, though this baseline characteristic cannot be considered in isolation.
When considering the net effect (active minus vehicle), the apparent benefits of efinaconazole in Latino patients with onychomycosis were more marked. Vehicle complete cure rates at week 52 were 0% compared with 5.6% of non-Latino participants. Vehicle cure rates in randomized controlled trials of toenail onychomycosis are relatively low and appear to be independent of the study characteristics.20 Vehicle cure rates of 2 topical treatments—efinaconazole and tavaborole—reported in their 2 respective phase 3 studies were 3.3% and 5.5% for efinaconzole11 and 0.5% and 1.5% for tavaborole.12 It has been suggested that the higher results seen with the efinaconazole vehicle relate to the formulation, though there is no reason to expect it to perform differently in a Latino population. It also has been suggested that baseline disease severity might impact vehicle treatment outcome.20 In our analysis, the percentage affected nail at baseline was higher in the Latino participants treated with vehicle (38.9% vs 36.2%).
Although the overall level of AEs was similar in Latino versus non-Latino participants treated with efinaconazole, events were generally milder in the Latino subpopulation and fewer participants discontinued because of AEs.
Our study had a number of limitations. A study period of 52 weeks may be too brief to evaluate clinical cure in onychomycosis, as continued improvement could occur with either longer treatment or follow-up. Also, the pivotal studies were not set up to specifically study Latino participants; the demographics and study disposition may not be representative of the general Latino population.
Conclusion
Once-daily treatment with efinaconazole solution 10% may provide a useful topical option in the treatment of Latino patients with toenail onychomycosis.
Acknowledgment
The authors would like to thank Brian Bulley, MSc (Konic Limited, West Sussex, United Kingdom), for medical writing support. Valeant Pharmaceuticals North America LLC funded Konic Limited’s activities pertaining to this manuscript. Dr. Cook-Bolden did not receive funding or any form of compensation for authorship of this publication.
- Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
- Crissey JT. Common dermatophyte infections. a simple diagnostic test and current management. Postgrad Med. 1998;103:191-192, 197-200, 205.
- Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
- Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Census 2010: 50 million Latinos. Hispanics account for more than half of nation’s growth in past decade. Pew Hispanic Center website. http://pewhispanic.org/files/reports/140.pdf. Published March 24, 2011. Accessed November 22, 2016.
- Sanchez MR. Cutaneous diseases in Latinos. Dermatol Clin. 2002;21:689-697.
- Pichardo-Geisinger R, Mun˜oz-Ali D, Arcury TA, et al. Dermatologist-diagnosed skin diseases among immigrant Latino poultry processors and other manual workers in North Carolina, USA. Int J Dermatol. 2013;52:1342-1348.
- Tschen EH, Bucko AD, Oizumi N, et al. Efinaconazole solution in the treatment of toenail onychomycosis: a phase 2, multicenter, randomized, double-blind study. J Drugs Dermatol. 2013;12:186-192.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Chan MK, Chong LY. A prospective epidemiology survey of foot disease in Hong Kong. J Am Podiatr Med Assoc. 2002;92:450-456.
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of Obesity Among Adults: United States, 2011-2012. Hyattsville, MD: National Center for Health Statistics, 2013. NCHS data brief, no. 131.
- Elewski BE, Tosti A. Risk factors and comorbidities for onychomycosis: implications for treatment with topical therapy. J Clin Aesthet Dermatol. 2015;8:38-42.
- Tosti A, Hay R, Arenas-Guzmán R. Patients at risk of onychomycosis–risk factor identification and active prevention. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):13-16.
- Sigurgeirsson B, Steingrímsson O. Risk factors associated with onychomycosis. J Eur Acad Dermatol Venereol. 2004;18:48-51.
- Vlahovic TC, Joseph WS. Efinaconazole topical, 10% for the treatment of toenail onychomycosis in patients with diabetes. J Drugs Dermatol. 2014;13:1186-1190.
- Rosen T. Evaluation of gender as a clinically relevant outcome variable in the treatment of onychomycosis with efinaconazole topical solution 10%. Cutis. 2015;96:197-201.
- Gupta AK, Paquet M. Placebo cure rates in the treatment of onychomycosis. J Am Podiatr Med Assoc. 2014;104:277-282.
Onychomycosis is a common progressive fungal infection of the nail bed, matrix, or plate leading to destruction and deformity of the toenails and fingernails.1,2 It represents up to 50% of all nail disorders1,3 with a notable increasing prevalence in the United States.4-6
Latinos represent the largest ethnic minority group in the United States,7 which is growing rapidly through immigration, particularly in the southern United States. Prevalence data are limited. An incidence of 9.3% secondary to dermatophytes was recorded in a dermatology clinic setting (N=2000).8 Onychomycosis was reported in 31.9% of a group of Latino immigrants in North Carolina (N=518), with higher prevalence in poultry workers, possibly due to the work environment.9
Efinaconazole solution 10% was shown to be well tolerated and more effective than a vehicle in a phase 2 study in Mexico.10 Two identical phase 3 studies of 1655 participants assessed the safety and efficacy of efinaconazole solution 10% in the treatment of onychomycosis.11 This post hoc analysis compares the data for Latino versus non-Latino populations.
Methods
We evaluated the results of 2 multicenter, randomized, double-blind, vehicle-controlled studies that included a total of 1655 participants with mild to moderate toenail onychomycosis (20%–50% clinical involvement). Participants were randomized to efinaconazole solu-tion 10% or vehicle once daily (3:1) for 48 weeks with a 4-week posttreatment follow-up period.11
Our post hoc analysis included 270 Latino patients, defined as an individual of Cuban, Mexican, Puerto Rican, or South or Central American origin or other Latino culture, regardless of race. In addition, data were compared to the 1380 non-Latino patients in the 2 studies. Patients who were randomized in error and never received treatment were excluded from the intention-to-treat analysis.
Efficacy Evaluation
The primary efficacy end point was complete cure rate (0% clinical involvement of target toenail, and both negative potassium hydroxide examination and fungal culture) at week 52. Secondary end points included mycologic cure, complete/almost complete cure (≤5% clinical involvement of target toenail, mycologic cure), and treatment success (≤10% clinical involvement of target toenail) at week 52.
Safety Evaluation
Safety assessments included monitoring and recording of adverse events (AEs) at every postbaseline study visit through week 52. All AEs were classified using the Medical Dictionary for Regulatory Activities (version 12.1). Treatment-emergent AEs (ie, events that began after the first application of study drug) that occurred during the study were summarized for each treatment group by the number of patients reporting each event, as well as by system organ class, preferred term, severity, seriousness, and relationship to the study drug.
Results
A total of 270 Latino participants with toenail onychomycosis (efinaconazole solution 10%, n=193; vehicle, n=77) were included in our study. The mean age of participants at baseline was 45.9 years. They were predominantly male (69.6%) and white Latinos (91.1%). The mean area of target toenail involvement was 36.6%, and the mean number of affected nontarget toenails was 2.5. Latino participants tended to be younger than non-Latino participants (45.9 vs 52.6 years), with a higher proportion of females (30.4% vs 21.3%). Disease severity was similar in both populations. Diabetes was reported in 7.0% and 6.7% of Latino and non-Latino participants, respectively, and mean weight was 83.6 and 86.6 kg, respectively.
Primary Efficacy End Points (Observed Case [OC])
At week 52, 25.6% of Latino participants in the efinaconazole group achieved complete cure versus 0% in the vehicle group (P<.001)(Figure 1). The efficacy of efinaconazole was statistically superior in Latino participants versus non-Latino participants (17.2% [P=.012]). The net effect (calculated by active treatment minus vehicle) for Latino participants also was superior to non-Latino participants (25.6% vs 11.6%).

Secondary Efficacy End Points (OC)
At week 52, 61.5% of Latino participants in the efina-conazole group achieved mycologic cure versus 15.3% in the vehicle group (P<.001)(Figure 2). The net effect for Latino participants was superior to non-Latino participants (46.2% vs 38.5%). More Latino participants in the efinaconazole group compared to vehicle group achieved complete/almost complete cure (32.7% vs 1.7%) or treatment success (49.4% vs 5.1%)(all P<.001)(Figure 3). Although there was no significant difference between the 2 groups for secondary efficacy end points, the net effect of efinaconazole was greater for all end points.


Safety
Adverse event rates were higher in the efinaconazole group than the vehicle group (65.3% vs 54.4%) and were similar in both populations; they were generally mild (61.8% vs 54.5%) or moderate (35.3% vs 45.5%) in severity, not related to study medication (96.8% vs 98.0%), and resolved without sequelae. Only 3 Latino participants (1.6%) discontinued efinaconazole treatment compared to 29 (2.8%) in the non-Latino population.
Comment
With the continued growth of the Latino population in the United States and likely higher prevalence of onychomycosis,9 this post hoc analysis provides important insights into treatment of onychomycosis in this patient population.
Efinaconazole solution 10% was significantly more effective than vehicle in the Latino population (P<.001) and also appeared significantly more effective than the non-Latino population across the 2 phase 3 studies (P=.012). Interestingly, complete cure rates (25.6%) were identical to those reported in the phase 2 study of Mexican patients treated with efinaconazole for 36 weeks.10 Specific data with other topical therapies, such as tavaborole, in Latino patients are not available. One phase 3 study of tavaborole for onychomycosis included 89 Mexican patients (15% of the total study population), but complete cure rates for the overall active treatment group were higher in a second phase 3 study (6.5% vs 9.1%) that did not include participants outside the United States or Canada.12
It is not clear why phase 3 efficacy results with efinaconazole appear better in the Latino population. There are a number of predisposing factors for onychomycosis that are important treatment considerations in Latinos. Obesity is an important factor in the development of onychomycosis,13 with more than 42% of Latino adults in the United States reportedly obese compared to 32.6% of non-Latino adults.14 Obese patients reportedly have shown a poorer response to efinaconazole treatment15; however, in our analysis, the mean weight of the 2 subpopulations was similar at baseline. Diabetes also is associated with an increased risk for onychomycosis16,17 and may be a more important issue in Latinos perhaps due to differences in health care access, social and cultural factors, and/or genetics, as well as the greater incidence of obesity. Prior reports suggest the efficacy of efinaconazole is not substantially influenced by the presence of diabetes,18 and in our 2 subpopulations, baseline incidence of coexisting diabetes was similar. These factors are unlikely to account for the better treatment success seen in our analysis. Efinaconazole has been reported to be more effective in females,19 though the reasons are less clear. The higher proportion of female Latinos (30.4% vs 21.3%) in our study may have had an impact on the results reported, though this baseline characteristic cannot be considered in isolation.
When considering the net effect (active minus vehicle), the apparent benefits of efinaconazole in Latino patients with onychomycosis were more marked. Vehicle complete cure rates at week 52 were 0% compared with 5.6% of non-Latino participants. Vehicle cure rates in randomized controlled trials of toenail onychomycosis are relatively low and appear to be independent of the study characteristics.20 Vehicle cure rates of 2 topical treatments—efinaconazole and tavaborole—reported in their 2 respective phase 3 studies were 3.3% and 5.5% for efinaconzole11 and 0.5% and 1.5% for tavaborole.12 It has been suggested that the higher results seen with the efinaconazole vehicle relate to the formulation, though there is no reason to expect it to perform differently in a Latino population. It also has been suggested that baseline disease severity might impact vehicle treatment outcome.20 In our analysis, the percentage affected nail at baseline was higher in the Latino participants treated with vehicle (38.9% vs 36.2%).
Although the overall level of AEs was similar in Latino versus non-Latino participants treated with efinaconazole, events were generally milder in the Latino subpopulation and fewer participants discontinued because of AEs.
Our study had a number of limitations. A study period of 52 weeks may be too brief to evaluate clinical cure in onychomycosis, as continued improvement could occur with either longer treatment or follow-up. Also, the pivotal studies were not set up to specifically study Latino participants; the demographics and study disposition may not be representative of the general Latino population.
Conclusion
Once-daily treatment with efinaconazole solution 10% may provide a useful topical option in the treatment of Latino patients with toenail onychomycosis.
Acknowledgment
The authors would like to thank Brian Bulley, MSc (Konic Limited, West Sussex, United Kingdom), for medical writing support. Valeant Pharmaceuticals North America LLC funded Konic Limited’s activities pertaining to this manuscript. Dr. Cook-Bolden did not receive funding or any form of compensation for authorship of this publication.
Onychomycosis is a common progressive fungal infection of the nail bed, matrix, or plate leading to destruction and deformity of the toenails and fingernails.1,2 It represents up to 50% of all nail disorders1,3 with a notable increasing prevalence in the United States.4-6
Latinos represent the largest ethnic minority group in the United States,7 which is growing rapidly through immigration, particularly in the southern United States. Prevalence data are limited. An incidence of 9.3% secondary to dermatophytes was recorded in a dermatology clinic setting (N=2000).8 Onychomycosis was reported in 31.9% of a group of Latino immigrants in North Carolina (N=518), with higher prevalence in poultry workers, possibly due to the work environment.9
Efinaconazole solution 10% was shown to be well tolerated and more effective than a vehicle in a phase 2 study in Mexico.10 Two identical phase 3 studies of 1655 participants assessed the safety and efficacy of efinaconazole solution 10% in the treatment of onychomycosis.11 This post hoc analysis compares the data for Latino versus non-Latino populations.
Methods
We evaluated the results of 2 multicenter, randomized, double-blind, vehicle-controlled studies that included a total of 1655 participants with mild to moderate toenail onychomycosis (20%–50% clinical involvement). Participants were randomized to efinaconazole solu-tion 10% or vehicle once daily (3:1) for 48 weeks with a 4-week posttreatment follow-up period.11
Our post hoc analysis included 270 Latino patients, defined as an individual of Cuban, Mexican, Puerto Rican, or South or Central American origin or other Latino culture, regardless of race. In addition, data were compared to the 1380 non-Latino patients in the 2 studies. Patients who were randomized in error and never received treatment were excluded from the intention-to-treat analysis.
Efficacy Evaluation
The primary efficacy end point was complete cure rate (0% clinical involvement of target toenail, and both negative potassium hydroxide examination and fungal culture) at week 52. Secondary end points included mycologic cure, complete/almost complete cure (≤5% clinical involvement of target toenail, mycologic cure), and treatment success (≤10% clinical involvement of target toenail) at week 52.
Safety Evaluation
Safety assessments included monitoring and recording of adverse events (AEs) at every postbaseline study visit through week 52. All AEs were classified using the Medical Dictionary for Regulatory Activities (version 12.1). Treatment-emergent AEs (ie, events that began after the first application of study drug) that occurred during the study were summarized for each treatment group by the number of patients reporting each event, as well as by system organ class, preferred term, severity, seriousness, and relationship to the study drug.
Results
A total of 270 Latino participants with toenail onychomycosis (efinaconazole solution 10%, n=193; vehicle, n=77) were included in our study. The mean age of participants at baseline was 45.9 years. They were predominantly male (69.6%) and white Latinos (91.1%). The mean area of target toenail involvement was 36.6%, and the mean number of affected nontarget toenails was 2.5. Latino participants tended to be younger than non-Latino participants (45.9 vs 52.6 years), with a higher proportion of females (30.4% vs 21.3%). Disease severity was similar in both populations. Diabetes was reported in 7.0% and 6.7% of Latino and non-Latino participants, respectively, and mean weight was 83.6 and 86.6 kg, respectively.
Primary Efficacy End Points (Observed Case [OC])
At week 52, 25.6% of Latino participants in the efinaconazole group achieved complete cure versus 0% in the vehicle group (P<.001)(Figure 1). The efficacy of efinaconazole was statistically superior in Latino participants versus non-Latino participants (17.2% [P=.012]). The net effect (calculated by active treatment minus vehicle) for Latino participants also was superior to non-Latino participants (25.6% vs 11.6%).

Secondary Efficacy End Points (OC)
At week 52, 61.5% of Latino participants in the efina-conazole group achieved mycologic cure versus 15.3% in the vehicle group (P<.001)(Figure 2). The net effect for Latino participants was superior to non-Latino participants (46.2% vs 38.5%). More Latino participants in the efinaconazole group compared to vehicle group achieved complete/almost complete cure (32.7% vs 1.7%) or treatment success (49.4% vs 5.1%)(all P<.001)(Figure 3). Although there was no significant difference between the 2 groups for secondary efficacy end points, the net effect of efinaconazole was greater for all end points.


Safety
Adverse event rates were higher in the efinaconazole group than the vehicle group (65.3% vs 54.4%) and were similar in both populations; they were generally mild (61.8% vs 54.5%) or moderate (35.3% vs 45.5%) in severity, not related to study medication (96.8% vs 98.0%), and resolved without sequelae. Only 3 Latino participants (1.6%) discontinued efinaconazole treatment compared to 29 (2.8%) in the non-Latino population.
Comment
With the continued growth of the Latino population in the United States and likely higher prevalence of onychomycosis,9 this post hoc analysis provides important insights into treatment of onychomycosis in this patient population.
Efinaconazole solution 10% was significantly more effective than vehicle in the Latino population (P<.001) and also appeared significantly more effective than the non-Latino population across the 2 phase 3 studies (P=.012). Interestingly, complete cure rates (25.6%) were identical to those reported in the phase 2 study of Mexican patients treated with efinaconazole for 36 weeks.10 Specific data with other topical therapies, such as tavaborole, in Latino patients are not available. One phase 3 study of tavaborole for onychomycosis included 89 Mexican patients (15% of the total study population), but complete cure rates for the overall active treatment group were higher in a second phase 3 study (6.5% vs 9.1%) that did not include participants outside the United States or Canada.12
It is not clear why phase 3 efficacy results with efinaconazole appear better in the Latino population. There are a number of predisposing factors for onychomycosis that are important treatment considerations in Latinos. Obesity is an important factor in the development of onychomycosis,13 with more than 42% of Latino adults in the United States reportedly obese compared to 32.6% of non-Latino adults.14 Obese patients reportedly have shown a poorer response to efinaconazole treatment15; however, in our analysis, the mean weight of the 2 subpopulations was similar at baseline. Diabetes also is associated with an increased risk for onychomycosis16,17 and may be a more important issue in Latinos perhaps due to differences in health care access, social and cultural factors, and/or genetics, as well as the greater incidence of obesity. Prior reports suggest the efficacy of efinaconazole is not substantially influenced by the presence of diabetes,18 and in our 2 subpopulations, baseline incidence of coexisting diabetes was similar. These factors are unlikely to account for the better treatment success seen in our analysis. Efinaconazole has been reported to be more effective in females,19 though the reasons are less clear. The higher proportion of female Latinos (30.4% vs 21.3%) in our study may have had an impact on the results reported, though this baseline characteristic cannot be considered in isolation.
When considering the net effect (active minus vehicle), the apparent benefits of efinaconazole in Latino patients with onychomycosis were more marked. Vehicle complete cure rates at week 52 were 0% compared with 5.6% of non-Latino participants. Vehicle cure rates in randomized controlled trials of toenail onychomycosis are relatively low and appear to be independent of the study characteristics.20 Vehicle cure rates of 2 topical treatments—efinaconazole and tavaborole—reported in their 2 respective phase 3 studies were 3.3% and 5.5% for efinaconzole11 and 0.5% and 1.5% for tavaborole.12 It has been suggested that the higher results seen with the efinaconazole vehicle relate to the formulation, though there is no reason to expect it to perform differently in a Latino population. It also has been suggested that baseline disease severity might impact vehicle treatment outcome.20 In our analysis, the percentage affected nail at baseline was higher in the Latino participants treated with vehicle (38.9% vs 36.2%).
Although the overall level of AEs was similar in Latino versus non-Latino participants treated with efinaconazole, events were generally milder in the Latino subpopulation and fewer participants discontinued because of AEs.
Our study had a number of limitations. A study period of 52 weeks may be too brief to evaluate clinical cure in onychomycosis, as continued improvement could occur with either longer treatment or follow-up. Also, the pivotal studies were not set up to specifically study Latino participants; the demographics and study disposition may not be representative of the general Latino population.
Conclusion
Once-daily treatment with efinaconazole solution 10% may provide a useful topical option in the treatment of Latino patients with toenail onychomycosis.
Acknowledgment
The authors would like to thank Brian Bulley, MSc (Konic Limited, West Sussex, United Kingdom), for medical writing support. Valeant Pharmaceuticals North America LLC funded Konic Limited’s activities pertaining to this manuscript. Dr. Cook-Bolden did not receive funding or any form of compensation for authorship of this publication.
- Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
- Crissey JT. Common dermatophyte infections. a simple diagnostic test and current management. Postgrad Med. 1998;103:191-192, 197-200, 205.
- Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
- Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Census 2010: 50 million Latinos. Hispanics account for more than half of nation’s growth in past decade. Pew Hispanic Center website. http://pewhispanic.org/files/reports/140.pdf. Published March 24, 2011. Accessed November 22, 2016.
- Sanchez MR. Cutaneous diseases in Latinos. Dermatol Clin. 2002;21:689-697.
- Pichardo-Geisinger R, Mun˜oz-Ali D, Arcury TA, et al. Dermatologist-diagnosed skin diseases among immigrant Latino poultry processors and other manual workers in North Carolina, USA. Int J Dermatol. 2013;52:1342-1348.
- Tschen EH, Bucko AD, Oizumi N, et al. Efinaconazole solution in the treatment of toenail onychomycosis: a phase 2, multicenter, randomized, double-blind study. J Drugs Dermatol. 2013;12:186-192.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Chan MK, Chong LY. A prospective epidemiology survey of foot disease in Hong Kong. J Am Podiatr Med Assoc. 2002;92:450-456.
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of Obesity Among Adults: United States, 2011-2012. Hyattsville, MD: National Center for Health Statistics, 2013. NCHS data brief, no. 131.
- Elewski BE, Tosti A. Risk factors and comorbidities for onychomycosis: implications for treatment with topical therapy. J Clin Aesthet Dermatol. 2015;8:38-42.
- Tosti A, Hay R, Arenas-Guzmán R. Patients at risk of onychomycosis–risk factor identification and active prevention. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):13-16.
- Sigurgeirsson B, Steingrímsson O. Risk factors associated with onychomycosis. J Eur Acad Dermatol Venereol. 2004;18:48-51.
- Vlahovic TC, Joseph WS. Efinaconazole topical, 10% for the treatment of toenail onychomycosis in patients with diabetes. J Drugs Dermatol. 2014;13:1186-1190.
- Rosen T. Evaluation of gender as a clinically relevant outcome variable in the treatment of onychomycosis with efinaconazole topical solution 10%. Cutis. 2015;96:197-201.
- Gupta AK, Paquet M. Placebo cure rates in the treatment of onychomycosis. J Am Podiatr Med Assoc. 2014;104:277-282.
- Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
- Crissey JT. Common dermatophyte infections. a simple diagnostic test and current management. Postgrad Med. 1998;103:191-192, 197-200, 205.
- Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
- Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Census 2010: 50 million Latinos. Hispanics account for more than half of nation’s growth in past decade. Pew Hispanic Center website. http://pewhispanic.org/files/reports/140.pdf. Published March 24, 2011. Accessed November 22, 2016.
- Sanchez MR. Cutaneous diseases in Latinos. Dermatol Clin. 2002;21:689-697.
- Pichardo-Geisinger R, Mun˜oz-Ali D, Arcury TA, et al. Dermatologist-diagnosed skin diseases among immigrant Latino poultry processors and other manual workers in North Carolina, USA. Int J Dermatol. 2013;52:1342-1348.
- Tschen EH, Bucko AD, Oizumi N, et al. Efinaconazole solution in the treatment of toenail onychomycosis: a phase 2, multicenter, randomized, double-blind study. J Drugs Dermatol. 2013;12:186-192.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Chan MK, Chong LY. A prospective epidemiology survey of foot disease in Hong Kong. J Am Podiatr Med Assoc. 2002;92:450-456.
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of Obesity Among Adults: United States, 2011-2012. Hyattsville, MD: National Center for Health Statistics, 2013. NCHS data brief, no. 131.
- Elewski BE, Tosti A. Risk factors and comorbidities for onychomycosis: implications for treatment with topical therapy. J Clin Aesthet Dermatol. 2015;8:38-42.
- Tosti A, Hay R, Arenas-Guzmán R. Patients at risk of onychomycosis–risk factor identification and active prevention. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):13-16.
- Sigurgeirsson B, Steingrímsson O. Risk factors associated with onychomycosis. J Eur Acad Dermatol Venereol. 2004;18:48-51.
- Vlahovic TC, Joseph WS. Efinaconazole topical, 10% for the treatment of toenail onychomycosis in patients with diabetes. J Drugs Dermatol. 2014;13:1186-1190.
- Rosen T. Evaluation of gender as a clinically relevant outcome variable in the treatment of onychomycosis with efinaconazole topical solution 10%. Cutis. 2015;96:197-201.
- Gupta AK, Paquet M. Placebo cure rates in the treatment of onychomycosis. J Am Podiatr Med Assoc. 2014;104:277-282.
Practice Points
- Onychomycosis is a common disease of importance in the increasing Latino population of the United States, especially due to predisposing factors such as obesity and diabetes mellitus. Specific data on the treatment of this patient population are lacking.
- Two large phase 3 studies with topical efinaconazole treatment included a notable number of Latino patients.
- Complete cure rates with efinaconazole in Latino participants were notably greater than those observed in the non-Latino population, and treatment was well tolerated in both groups.
- Treatment of onychomycosis is important to possibly prevent a more serious infectious disease involving the lower extremities, especially in those with comorbidities such as obesity, diabetes, and peripheral vascular disease.