User login
Indurated Thigh Plaque With Associated Lymphadenopathy
The Diagnosis: Rosai-Dorfman Disease
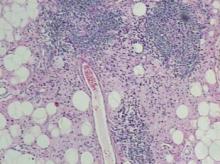
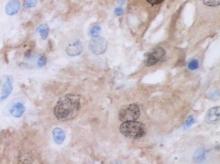
Punch biopsies of the mass from the right thigh were obtained. Hematoxylin and eosin staining revealed a reactive fibroinflammatory process of the dermis and subcutaneous tissue with fibrosis and prominent lymphoplasmacytic and histiocytic infiltrate with emperipolesis (Figure 1). The histiocytes stained positive for S-100 (Figure 2) and CD68. Markers for CD34, smooth muscle actin, and keratin were negative, as were acid-fast bacteria (Ziehl-Neelsen) and fungal (Gomori methenamine-silver) stains. The morphologic and immunohistochemical features of the biopsy specimens were consistent with Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy.
Rosai-Dorfman disease is a benign histiocytic proliferative disorder that usually involves the lymph nodes but may involve any organ system including the skin and soft tissue.1,2 Systemic RDD usually presents in children or young adults as massive cervical lymphadenopathy, often with fever, polyclonal hyperglobulinemia, mild anemia, elevated erythrocyte sedimentation rate, and occasionally neutropenia.2 Rosai-Dorfman disease was first described as a distinct clinicopathologic entity in 1969.3
Extranodal involvement occurs in approximately 40% of patients, with cutaneous disease in approximately 10% of cases. Other sites that may be affected include the eyelid and orbit, salivary glands, lungs, central nervous system, and bone.2 Purely cutaneous RDD is rare and has a variable skin distribution. Compared to systemic RDD, cutaneous disease affects older patients with a predilection for females and white individuals.4
Histologic examination of cutaneous RDD reveals a dense dermal infiltrate of foamy histiocytes with scattered lymphocytes, plasma cells, and neutrophils.2 Emperipolesis, consisting of intact lymphocytes and less commonly plasma cells in the cytoplasm of the histiocytes, is a characteristic feature of cutaneous RDD. Occasional findings include fibrosis, lymphoid aggregates, foamy histiocytes inside of dilated lymphatics, thick-walled venules with surrounding plasma cells, and multinucleate histiocytes.2 Immunohistochemistry is helpful in diagnosing RDD, as the histiocytes stain strongly positive for S-100, occasionally positive for CD68, and negative for CD1a.5
The pathogenesis of RDD remains unknown. Some cases of RDD have had positive serologies for human herpesvirus 6 and Epstein-Barr virus, though an infectious origin has not been proven.5 Clonality studies have shown the cellular infiltrate to be polyclonal, supporting a reactive rather than neoplastic process.6 The disease course of RDD varies from self-limited to protracted with remissions and exacerbations. Many cutaneous RDD lesions spontaneously heal and only require treatment when disseminated, destructive, or causing physical compromise.2 Treatment modalities for cutaneous RDD have exhibited varying degrees of success and include surgical excision; thalidomide; isotretinoin; radiotherapy; alkylating agents; and oral, topical, and intralesional steroids.2
The mass in our patient was surgically excised, though surgical margins remained positive. He was followed by the hematology-oncology service and later reported the development of 2 additional masses in the right leg with intermittent tenderness. Magnetic resonance imaging of the head was negative for intracranial abnormalities, though it did demonstrate prominent nasopharyngeal soft tissue and sinus disease. A complete skeletal radiograph survey and whole-body technetium bone scan did not show evidence of bone involvement. Chest computed tomography was negative for mass lesions. The patient deferred a bone marrow biopsy and was reportedly doing well at follow-up without further treatment.
1. Rubenstein MA, Farnsworth NN, Pielop JA, et al. Cutaneous Rosai-Dorfman disease. Dermatol Online J. 2006;12:8.
2. Goodman WT, Barrett TL. Histiocytoses. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. 2nd ed. Spain: Mosby Elsevier; 2008:1395-1410.
3. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
4. Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
5. Wartman DG, Perry A, Werchniak AE. Multiple nodules and plaques on the face and trunk. cutaneous Rosai Dorfman disease (RDD). Arch Dermatol. 2006;142:1501-1506.
6. Paulli M, Bergamaschi G, Tonon L. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Br J Haematol. 1995;91:415-418.
The Diagnosis: Rosai-Dorfman Disease
Punch biopsies of the mass from the right thigh were obtained. Hematoxylin and eosin staining revealed a reactive fibroinflammatory process of the dermis and subcutaneous tissue with fibrosis and prominent lymphoplasmacytic and histiocytic infiltrate with emperipolesis (Figure 1). The histiocytes stained positive for S-100 (Figure 2) and CD68. Markers for CD34, smooth muscle actin, and keratin were negative, as were acid-fast bacteria (Ziehl-Neelsen) and fungal (Gomori methenamine-silver) stains. The morphologic and immunohistochemical features of the biopsy specimens were consistent with Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy.
Rosai-Dorfman disease is a benign histiocytic proliferative disorder that usually involves the lymph nodes but may involve any organ system including the skin and soft tissue.1,2 Systemic RDD usually presents in children or young adults as massive cervical lymphadenopathy, often with fever, polyclonal hyperglobulinemia, mild anemia, elevated erythrocyte sedimentation rate, and occasionally neutropenia.2 Rosai-Dorfman disease was first described as a distinct clinicopathologic entity in 1969.3
Extranodal involvement occurs in approximately 40% of patients, with cutaneous disease in approximately 10% of cases. Other sites that may be affected include the eyelid and orbit, salivary glands, lungs, central nervous system, and bone.2 Purely cutaneous RDD is rare and has a variable skin distribution. Compared to systemic RDD, cutaneous disease affects older patients with a predilection for females and white individuals.4
Histologic examination of cutaneous RDD reveals a dense dermal infiltrate of foamy histiocytes with scattered lymphocytes, plasma cells, and neutrophils.2 Emperipolesis, consisting of intact lymphocytes and less commonly plasma cells in the cytoplasm of the histiocytes, is a characteristic feature of cutaneous RDD. Occasional findings include fibrosis, lymphoid aggregates, foamy histiocytes inside of dilated lymphatics, thick-walled venules with surrounding plasma cells, and multinucleate histiocytes.2 Immunohistochemistry is helpful in diagnosing RDD, as the histiocytes stain strongly positive for S-100, occasionally positive for CD68, and negative for CD1a.5
The pathogenesis of RDD remains unknown. Some cases of RDD have had positive serologies for human herpesvirus 6 and Epstein-Barr virus, though an infectious origin has not been proven.5 Clonality studies have shown the cellular infiltrate to be polyclonal, supporting a reactive rather than neoplastic process.6 The disease course of RDD varies from self-limited to protracted with remissions and exacerbations. Many cutaneous RDD lesions spontaneously heal and only require treatment when disseminated, destructive, or causing physical compromise.2 Treatment modalities for cutaneous RDD have exhibited varying degrees of success and include surgical excision; thalidomide; isotretinoin; radiotherapy; alkylating agents; and oral, topical, and intralesional steroids.2
The mass in our patient was surgically excised, though surgical margins remained positive. He was followed by the hematology-oncology service and later reported the development of 2 additional masses in the right leg with intermittent tenderness. Magnetic resonance imaging of the head was negative for intracranial abnormalities, though it did demonstrate prominent nasopharyngeal soft tissue and sinus disease. A complete skeletal radiograph survey and whole-body technetium bone scan did not show evidence of bone involvement. Chest computed tomography was negative for mass lesions. The patient deferred a bone marrow biopsy and was reportedly doing well at follow-up without further treatment.
The Diagnosis: Rosai-Dorfman Disease
Punch biopsies of the mass from the right thigh were obtained. Hematoxylin and eosin staining revealed a reactive fibroinflammatory process of the dermis and subcutaneous tissue with fibrosis and prominent lymphoplasmacytic and histiocytic infiltrate with emperipolesis (Figure 1). The histiocytes stained positive for S-100 (Figure 2) and CD68. Markers for CD34, smooth muscle actin, and keratin were negative, as were acid-fast bacteria (Ziehl-Neelsen) and fungal (Gomori methenamine-silver) stains. The morphologic and immunohistochemical features of the biopsy specimens were consistent with Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy.
Rosai-Dorfman disease is a benign histiocytic proliferative disorder that usually involves the lymph nodes but may involve any organ system including the skin and soft tissue.1,2 Systemic RDD usually presents in children or young adults as massive cervical lymphadenopathy, often with fever, polyclonal hyperglobulinemia, mild anemia, elevated erythrocyte sedimentation rate, and occasionally neutropenia.2 Rosai-Dorfman disease was first described as a distinct clinicopathologic entity in 1969.3
Extranodal involvement occurs in approximately 40% of patients, with cutaneous disease in approximately 10% of cases. Other sites that may be affected include the eyelid and orbit, salivary glands, lungs, central nervous system, and bone.2 Purely cutaneous RDD is rare and has a variable skin distribution. Compared to systemic RDD, cutaneous disease affects older patients with a predilection for females and white individuals.4
Histologic examination of cutaneous RDD reveals a dense dermal infiltrate of foamy histiocytes with scattered lymphocytes, plasma cells, and neutrophils.2 Emperipolesis, consisting of intact lymphocytes and less commonly plasma cells in the cytoplasm of the histiocytes, is a characteristic feature of cutaneous RDD. Occasional findings include fibrosis, lymphoid aggregates, foamy histiocytes inside of dilated lymphatics, thick-walled venules with surrounding plasma cells, and multinucleate histiocytes.2 Immunohistochemistry is helpful in diagnosing RDD, as the histiocytes stain strongly positive for S-100, occasionally positive for CD68, and negative for CD1a.5
The pathogenesis of RDD remains unknown. Some cases of RDD have had positive serologies for human herpesvirus 6 and Epstein-Barr virus, though an infectious origin has not been proven.5 Clonality studies have shown the cellular infiltrate to be polyclonal, supporting a reactive rather than neoplastic process.6 The disease course of RDD varies from self-limited to protracted with remissions and exacerbations. Many cutaneous RDD lesions spontaneously heal and only require treatment when disseminated, destructive, or causing physical compromise.2 Treatment modalities for cutaneous RDD have exhibited varying degrees of success and include surgical excision; thalidomide; isotretinoin; radiotherapy; alkylating agents; and oral, topical, and intralesional steroids.2
The mass in our patient was surgically excised, though surgical margins remained positive. He was followed by the hematology-oncology service and later reported the development of 2 additional masses in the right leg with intermittent tenderness. Magnetic resonance imaging of the head was negative for intracranial abnormalities, though it did demonstrate prominent nasopharyngeal soft tissue and sinus disease. A complete skeletal radiograph survey and whole-body technetium bone scan did not show evidence of bone involvement. Chest computed tomography was negative for mass lesions. The patient deferred a bone marrow biopsy and was reportedly doing well at follow-up without further treatment.
1. Rubenstein MA, Farnsworth NN, Pielop JA, et al. Cutaneous Rosai-Dorfman disease. Dermatol Online J. 2006;12:8.
2. Goodman WT, Barrett TL. Histiocytoses. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. 2nd ed. Spain: Mosby Elsevier; 2008:1395-1410.
3. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
4. Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
5. Wartman DG, Perry A, Werchniak AE. Multiple nodules and plaques on the face and trunk. cutaneous Rosai Dorfman disease (RDD). Arch Dermatol. 2006;142:1501-1506.
6. Paulli M, Bergamaschi G, Tonon L. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Br J Haematol. 1995;91:415-418.
1. Rubenstein MA, Farnsworth NN, Pielop JA, et al. Cutaneous Rosai-Dorfman disease. Dermatol Online J. 2006;12:8.
2. Goodman WT, Barrett TL. Histiocytoses. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. 2nd ed. Spain: Mosby Elsevier; 2008:1395-1410.
3. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
4. Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
5. Wartman DG, Perry A, Werchniak AE. Multiple nodules and plaques on the face and trunk. cutaneous Rosai Dorfman disease (RDD). Arch Dermatol. 2006;142:1501-1506.
6. Paulli M, Bergamaschi G, Tonon L. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Br J Haematol. 1995;91:415-418.
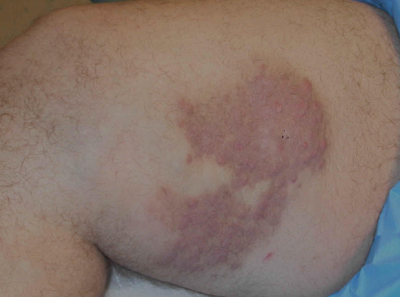
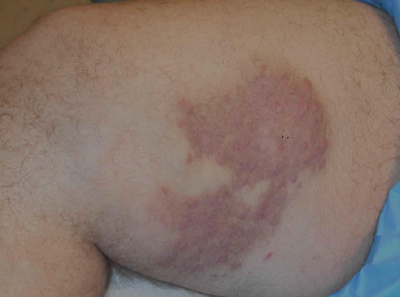
A 38-year-old white man with a history of lower extremity varicose veins was referred for evaluation of a plaque on the medial aspect of the right thigh and right inguinal lymphadenopathy. The patient reported having the lesion for approximately 1 year; over time it had become progressively more indurated. He denied pain, discomfort, and pruritus at the site, as well as any systemic symptoms. The patient had no known allergies, was not taking any medications, smoked 1 pack of tobacco daily for 20 years, drank alcohol socially, and was not sexually active. His family history was noncontributory. Prior to the dermatology consultation, a computed tomography scan of the right leg, abdomen, and pelvis was obtained and demonstrated a subcutaneous mass in the distal third of the medial aspect of the right thigh as well as pericaval and right inguinal lymphadenopathy. Physical examination revealed a violaceous, indurated, nodular plaque on the medial aspect of the right thigh measuring approximately 10×5 cm. Multiple small, nontender, mobile lymph nodes were palpated on the right side of the inguinal region. The remainder of the physical examination was unremarkable.