Article

Multiple Glomangiomas in a Patient With a History of Metastatic Melanoma
- Author:
- Sepehr Hamidi, MD
- Gene H. Kim, MD
- Brittney K. DeClerck, MD
In patients with a recent history of malignancy, multiple glomangiomas may mimic cutaneous metastases. Therefore, multiple biopsies and histologic...
Article
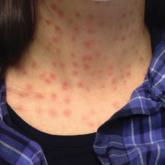
Rapid Development of Perifolliculitis Following Mesotherapy
- Author:
- Weihuang Vivian Ning, MD
- Sameer Bashey, MD
- Gene H. Kim, MD
Mesotherapy is a common procedure performed in both medical and nomedical settings for cosmetic rejuvenation. Complications can occur from...
