User login
Should I suspect obstructive sleep apnea if a patient has hard-to-control hypertension?
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
Sleep disturbances in cancer patients: Underrecognized and undertreated
Many cancer patients don't sleep well, for a variety of reasons. It is an important problem: not only does poor sleep worsen quality of life, it may affect prognosis. Moreover, treatment is available.
Yet many physicians caring for cancer patients do not ask about sleep problems, underestimating their impact or focusing on more urgent problems. Also, patients may not want to bring up the topic because they consider poor sleep to be unavoidable and untreatable and because they fear that reporting it may shift the focus of their treatment from trying to cure the cancer to easing its symptoms.
This practical review will help health care professionals avoid the common barriers to diagnosis and treatment of poor sleep in cancer patients. Because there are few data on other sleep disorders such as sleep apnea and restless leg syndrome, we will focus on the most common one in cancer patients—insomnia—and its effects on other symptoms and quality of life.
MORE PATIENTS SURVIVE CANCER NOW
Today, more patients are surviving cancer, but cancer symptoms and the side effects of surgery, chemotherapy, and radiation therapy may persist for years.1,2 The most common complaints include cancer-related fatigue, leg restlessness, anxiety, insomnia, and excessive sleepiness.3
Sleep disturbances appear to contribute to the other problems and are relatively easier to quantify. Most studies of sleep disorders in cancer patients have looked specifically at insomnia,4 although a few have explored the prevalence of other sleep disorders, such as sleep-disordered breathing and limb movements during sleep.5
The International Classification of Sleep Disorders, 2nd edition,6 defines insomnia as difficulty going to sleep or staying asleep (the latter defined as waking up in the middle of the night, with wakeful episodes lasting more than 30 minutes), early-morning awakenings (waking 30 minutes or more before the intended time), or nonrestorative sleep, causing significant distress or impairment of day-time functioning.
INSOMNIA WORSENS QUALITY OF LIFE
Insomnia significantly worsens quality of life in cancer patients, and if it can be detected and effectively treated, quality of life is likely to improve. Studies in cancer patients have found that those with insomnia:
- Were less able to cope with stress and carry on their activities of daily living3
- Were much less able to function and reported more pain, less energy, and greater difficulty in dealing with emotional problems7
- Had poor quality of life, both physically and emotionally.3,8
PERHAPS MORE THAN HALF OF CANCER PATIENTS HAVE INSOMNIA
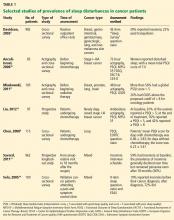
Depending on the methods used and populations studied, at least 30% and perhaps more than half of patients with cancer have insomnia (Table 1).3,4,8–14 It is one of the most commonly reported complaints in this group,15–17 and it occurs before, during, and after treatment of cancer.
Although the prevalence may differ in various cancers, it is still higher than in the general population. In a study of about 450 patients with cancer or depression and 300 healthy volunteers, 62% of the cancer patients reported moderate to severe sleep disturbance, compared with 52% of the depressed patients and 30% of the healthy volunteers.18
When Davidson et al3 surveyed nearly 1,000 cancer patients, one-third said they had insomnia. The problem was most prevalent in lung and breast cancer patients.
In a longitudinal study by Savard et al,13 the prevalence of insomnia declined over time but remained high even at the end of 18 months. It was more prevalent in patients with gynecologic and breast cancer than in those with prostate cancer.13,19
SLEEP PROBLEMS ARE UNDERREPORTED
Sleep problems in cancer patients often go unrecognized because patients do not report them. In a survey of 150 patients,20 44% reported having had sleep problems during the preceding month. However, only one-third of those with sleep problems told their health care providers. This highlights the need for physicians to address sleep complaints in cancer patients at every visit and, if needed, to refer them to a sleep specialist for further evaluation and management.
INSOMNIA IS OFTEN ASSOCIATED WITH OTHER PROBLEMS
Many things can interfere with sleep in cancer patients: the cancer itself (eg, pain due to tumor invasion), medical treatments (eg, narcotics, chemotherapy, neuroleptics, sympathomimetics, steroids, sedative hypnotics), psychosocial disturbances (eg, depression, anxiety, stress), and comorbid medical issues.
In this population, insomnia is often part of a cluster of symptoms that includes pain, fatigue, depression, and anxiety. These act synergistically, worsening quality of life.21–24
Cancer-related fatigue and insomnia
Cancer-related fatigue is a distressing, persistent, subjective sense of tiredness or exhaustion that is related to cancer or cancer treatment, that is not proportional to recent activity and that interferes with usual functioning.25 It has been reported by up to 90% of cancer patients in some studies.26–28
Cancer-related fatigue worsens quality of life and is one of the most distressing and persistent symptoms experienced before, during, and after cancer treatment.29,30 Furthermore, it can lead to sleep disturbances and daytime somnolence and further aggravate insomnia.31,32 The two conditions are often reported as part of a cluster of interrelated symptoms that include pain, depression, and loss of concentration and other cognitive functions, suggesting that they may share a common etiology.33–35
Åhsberg et al36 examined different aspects of perceived cancer-related fatigue in patients undergoing radiotherapy and found correlations between lack of energy, sleepiness, and cancer-related fatigue.
Current understanding of the possible link between cancer-related fatigue and insomnia suggests that interventions targeting the insomnia and daytime sleepiness could decrease the fatigue as well.31
Pain and insomnia in cancer patients
Pain is reported by 60% to 90% of patients with advanced cancer,37,38 its intensity usually varying with the extent of disease. Too often, it is inadequately controlled.39 Furthermore, it is thought to contribute to insomnia.40
In a study of more than 1,600 cancer patients, nearly 60% reported insomnia in addition to pain.41 The severity of pain directly correlated with the probability of insomnia.
Conversely, research suggests that sleep disturbances, primarily insomnia, can increase cancer patients’ sensitivity to pain.42 One hypothesis is that adequate sleep is needed to promote processes relevant to recovery from pain, both physiologic (ie, tissue repair) and psychological (ie, transient cessation of the perception of pain signals).43
Paradoxically, opioids can worsen insomnia
Cancer pain is often treated with opioids, which, paradoxically, can cause or worsen insomnia.
Although opioids induce sleep, they also depress respiration, and at night, they can cause or worsen sleep-disordered breathing (obstructive or central sleep apnea or ataxic breathing), leading to episodes of hypoxia, arousals, and fragmented sleep.44 Moreover, opioids can lead to daytime sedation. Further, psychostimulants such as methylphenidate, given to counteract opioid-induced sedation, can cause anxiety and insomnia. Thus, the interaction between cancer-related pain, insomnia, and pain management leads to a vicious cycle. Understanding this process, we can try to break the cycle and help patients with cancer sleep better.
However, how best to treat sleep-disordered breathing in patients taking opioids long-term is not well established.
In general, the primary intervention is to reduce the opioid dose. Practitioners should continually assess the need for these drugs and consider referral to a drug-behavior treatment center to help with discontinuation of opioid use when deemed medically appropriate.45 Other strategies include positive airway pressure ventilation including continuous positive airway pressure, bilevel pressure devices with backup rate, or adaptive servoventilators. In some cases oxygen supplementation may be required.
Sleep-disordered breathing, when recognized and diagnosed, should be managed in partnership with a sleep specialist.
Depression and insomnia in cancer patients
By some estimates, up to half of cancer patients suffer from depression at some point in their illness.28 And not without reason: these patients face uncertainty about their life, and this often results in depression or anxiety.46
Many cancer patients with depression also have insomnia.28 Indeed, patients with persistent insomnia are at greater risk of developing psychological disorders such as depression and anxiety.47
In a survey of cancer patients, insomnia symptoms were more often attributed to thoughts or concerns about health, family, friends, the cancer diagnosis, and finances than to the actual physical effects of cancer.48
CANCER TREATMENT AND INSOMNIA
Many cancer patients experience sleep disturbances even before starting treatment.49 Liu et al50 showed that, in 76 women about to undergo chemotherapy for breast cancer, those who already had sleep disturbances, fatigue, and depression had more problems, and more severe problems, during chemotherapy.
Radiation therapy and chemotherapy have been reported to cause or precipitate insomnia (Table 2).8,13
Hormonal therapy and biological therapy can also cause or worsen preexisting insomnia.51,52 For example, androgen deprivation therapy for prostate cancer and hormonal therapy for breast cancer are often associated with sleep problems.49,50 Possible mechanisms of insomnia include hot flashes, night sweats, and anxiety caused by such treatments. Biological agents such as interferons, interleukins, and tumor necrosis factor (TNF) alpha, which are often used to treat malignant melanoma, can affect the sleep-wake cycle, leading to insomnia.53
Corticosteroids sharply raise serum cortisol levels, which can lead to insomnia. Cancer patients receiving dexamethasone to prevent radiation-induced emesis experienced more insomnia than patients who did not receive dexamethasone.54
IMMUNOLOGIC BASIS OF INSOMNIA IN CANCER PATIENTS
Cancer cells produce inflammatory cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), and TNF alpha, and inflammation plays a role in tumor progression and possibly tumorigenesis.55
Specific cytokines also help regulate the sleep-wake cycle. Levels of IL-6 and TNF alpha peak during sleep, and daytime IL-6 levels are inversely related to the amount of nocturnal sleep.56 Vgontzas et al57 showed that although mean levels of 24-hour IL-6 and TNF alpha secretion were not significantly different in patients with insomnia vs healthy controls, chronic insomnia was associated with a shift in IL-6 and TNF alpha secretion from nighttime to daytime.57
Cancer and its treatment can affect secretion of the cytokines that play a role in the sleep-wake cycle. Thus, the sleep disturbances associated with cancer may also be related to the abnormalities in cytokine levels caused by either cancer or its treatment.
Mills et al58 found that inflammatory markers such as vascular endothelial growth factor and soluble intercellular adhesion molecule-1 were significantly elevated during chemotherapy in breast cancer patients, and the elevated vascular endothelial growth factor levels were associated with poorer sleep during treatment.
Further research is warranted to establish causality, to help us understand the mechanisms of insomnia and other cancer symptoms, and to develop new treatments for these complaints.
POOR SLEEP AND CANCER RISK AND OUTCOMES
Sleep disturbances have negative health consequences in cancer. Their impact ranges from plausible carcinogenesis to affecting the course of the disease and cancer survival.
Poor sleep and risk of cancer
Epidemiologic studies have examined a possible link between circadian rhythm disruption and breast cancer risk, using both direct measures such as melatonin levels and indirect measures such as sleep duration and shift work. (Melatonin production is related to sleep duration, and night-shift work leads to disruption of sleep pattern and quality of sleep, thus lowering melatonin levels.59)
The findings were mixed. Breast cancer risk was significantly and inversely associated with urinary melatonin levels (6-sulfatoxymelatonin) in the Nurses’ Health Study II,60 but not in the Guernsey III study in the United Kingdom.61 Breast cancer risk was significantly lower with longer sleep duration in Finnish women62 and in Chinese women in Singapore,63 but not in American women.64,65 Results of three cohort studies66–68 and two case-control studies69,70 suggested a higher breast cancer risk in women who work evening or overnight shifts. Shorter sleep duration was associated with a higher risk of colorectal adenomas.71
These studies make a strong case for an association of cancer with circadian rhythm disruption and shorter sleep duration, possibly from an effect on melatonin levels. However, one should be cautious in interpreting epidemiologic studies: although they show sleep disturbances to be associated with cancer risk, they do not establish causality.
Insomnia and cancer outcomes
Evidence is growing that sleep disturbances may affect compliance with treatment, immune function, and outcomes—including survival—in cancer patients.23,24
In patients newly diagnosed with various types of cancer, Degner and Sloan72 showed that those who suffered from insomnia, nausea, poor appetite, and pain had a lower survival rate at 5 years, independent of the cancer stage. However, no separate analyses were performed to examine the specific influence of insomnia on cancer survival.
Thompson and Li73 analyzed data from 101 breast cancer patients with available Oncotype DX recurrence scores (a proprietary genetic test performed on tumor tissue that predicts the likelihood of recurrence). The scores were strongly correlated with average hours of sleep per night before breast cancer diagnosis, with fewer hours of sleep associated with a higher (worse) score.
Since these studies were retrospective and merely suggest associations, prospective studies, using more standardized questionnaires and objective measures, are needed to establish causality and to further our understanding of the mechanisms involved.
HELPING CANCER PATIENTS SLEEP BETTER
Insomnia is generally diagnosed with a thorough history that includes sleep, medical issues, substance use, and psychiatric issues. The sleep history should include specific insomniarelated complaints, presleep conditions and habits, sleep-wake habits, other sleep-related symptoms, and daytime consequences. To obtain the information, one can use questionnaires, sleep logs, psychological screening tests, and bed-partner interviews.74
Managing insomnia involves both pharmacologic and nonpharmacologic treatment. It is also important to treat the associated disorders such as depression and anxiety disorders that often accompany insomnia. Long-term management of cancer patients should not be limited to surveillance of cancer but should also involve aggressive treatment of clusters of symptoms such as insomnia, cancer-related fatigue, and pain to yield better long-term quality of life.75–77
Nonpharmacologic treatment: Cognitive-behavioral therapy
Nonpharmacologic interventions use psychological and behavioral therapies. The American Academy of Sleep Medicine guidelines recommend cognitive behavioral therapy for all patients with insomnia, either alone or in combination with hypnotic medications.
Cognitive-behavioral therapy for insomnia includes various components that help the patient learn coping skills and ways to prevent or mitigate the severity of future episodes (Table 3). Various randomized controlled trials found it to be effective for treating insomnia in the general population.77–79
Several studies found that cognitive-behavioral therapy for insomnia was effective in cancer patients, not only improving sleep quality but also decreasing psychological distress, resulting in better overall quality of life.80,81
Savard et al81 conducted a randomized controlled trial of cognitive-behavioral therapy for insomnia in 57 patients with breast cancer, examining subjective and objective sleep measures, psychological functioning, quality of life, and immunologic responses. They found significant improvements in sleep efficiency, mood, quality of life, depression, anxiety, and need for sleep medications. Improvements in subjective sleep measures persisted on 12-month follow-up.
Berger et al,82 in another randomized controlled trial, assessed behavioral therapy using stimulus control, modified sleep restriction, relaxation therapy, and sleep hygiene in breast cancer patients receiving adjuvant chemotherapy. Behavioral therapy improved sleep quality over time, as measured by the Pittsburgh Sleep Quality Index.
Espie et al83 evaluated the effect of cognitive-behavioral therapy on prostate, colorectal, gynecologic, and breast cancer patients, with similar results.83
Cognitive-behavioral therapy is at least as effective as drug therapy for insomnia in the general population. In the limited studies done in cancer patients, it has been shown to be effective irrespective of the type of cancer and is associated with better long-term outcomes. It diminishes the distress associated with early insomnia, can reduce anxiety, and can promote sleep.
A National Institutes of Health conference on insomnia concluded that cognitivebehavioral therapy is at least as effective as medications for brief treatment of chronic insomnia and that its beneficial effects, in contrast to those produced by medications, may last beyond the termination of treatment.84
It is important to think about numerous factors when considering options such as cognitive-behavioral therapy, as patients with cancer have different complications that may affect sleep quality, such as cancer-related fatigue, cancer-related depression, psychological reactions to the disease, side effects of treatment, and cancer-related pain. These need to be addressed as well.
If cognitive-behavioral therapy is not available, self-help interventions (eg, written material, videos, television and Internet resources) can be used. These have several advantages over professionally administered interventions, including greater accessibility, less burden for the patient, and lower cost. Research is under way evaluating this approach in cancer patients.85
Drug therapy
The focus of therapy should be to treat underlying disorders that may be causing or contributing to insomnia. However, a substantial number of patients may need to be assessed for pharmacotherapy for insomnia.
Sleep problems in the general population are commonly treated with drugs, and most of the recommendations in cancer patients are based on experience in the general population. However, sleep medications should be used cautiously in cancer patients, since to our knowledge there have been no studies of these agents in patients with cancer.
Side effects also need to be considered. For example, sleep medications can profoundly worsen cancer-related fatigue.
Hypnotics are often prescribed for cancer patients.86,87 A study in five major oncology centers showed that about half of the 1,500 patients were prescribed at least one psychotropic drug.86 In this study, hypnotics were the most frequently prescribed drugs, accounting for 48% of total prescriptions, and 44% of the psychotropic prescriptions were written for sleep.
Benzodiazepine receptor agonists such as zaleplon, zolpidem, and eszopiclone can be used for problems with falling asleep and staying asleep.88,89 They are better tolerated than older, long-acting benzodiazepines,90 which can cause alterations in sleep-cycle architecture or rebound insomnia. The earlier agents can also cause adverse effects such as tolerance, drowsiness, and cognitive impairment.
A National Institutes of Health conference stated that benzodiazepine receptor agonists are efficacious in the short-term management of insomnia and that their adverse effects are much less frequent and severe than those of the benzodiazepines or other sedating drugs.84 It also stated that all antidepressants, antihistamines (H1 receptor antagonists), and anti-psychotics have potentially significant adverse effects that raise concerns about their risk-to-benefit ratio and their suitability as treatment for chronic insomnia.
Benzodiazepines are commonly prescribed for insomnia. They increase sleep efficiency, decrease arousals, and increase stage 2 sleep.
Melatonin receptor agonists have been approved by the US Food and Drug Administration for treating insomnia. A recent meta-analysis of eight studies in healthy patients showed improvements in subjective and objective sleep outcomes with the use of ramelteon.91 The dosages primarily used were 4 to 32 mg. However, most of the studies used a dosage of 4 to 8 mg.
Antidepressants. Some of the antidepressants are also used for insomnia, but they can cause daytime fatigue.
Mirtazapine was shown to be effective for insomnia and coexistent mood disorder in cancer patients, but larger trials are needed.92
A recent clinical trial with secondary data analyses evaluated the effect of paroxetine on insomnia, depression, and fatigue in patients with cancer. Paroxetine significantly reduced insomnia in both depressed and nondepressed patients after 2 to 3 weeks of treatment.93
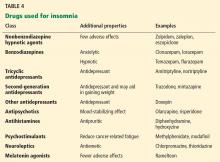
Table 4 summarizes classes of drugs used for insomnia and their additional therapeutic properties.
- Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol 2006; 16:197–205.
- Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology 2006; 15:306–320.
- Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med 2002; 54:1309–1321.
- Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 2001; 19:895–908.
- Payne RJ, Hier MP, Kost KM, et al. High prevalence of obstructive sleep apnea among patients with head and neck cancer. J Otolaryngol 2005; 34:304–311.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Second Edition (ICSD-2); 2005.
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage 2002; 24:471–480.
- Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer 2008; 62:391–400.
- Liu L, Ancoli-Israel S. Sleep disturbances in cancer. Psychiatr Ann 2008; 38:627–634.
- Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 2006; 14:201–209.
- Miaskowski C, Lee K, Dunn L, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs 2011; 34:255–268.
- Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep 2012; 35:237–245.
- Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol 2011; 29:3580–3586.
- Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care 2005; 3:23–31.
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med 2007; 20:434–443.
- Schroevers MJ, Ranchor AV, Sanderman R. The role of age at the onset of cancer in relation to survivors’ long-term adjustment: a controlled comparison over an eight-year period. Psychooncology 2004; 13:740–752.
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008; 112(suppl 11):2577–2592.
- Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 2003; 25:307–318.
- Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol 2009; 27:5233–5239.
- Engstrom CA, Strohl RA, Rose L, Lewandowski L, Stefanek ME. Sleep alterations in cancer patients. Cancer Nurs 1999; 22:143–148.
- Hoffman A, Given BA, von Eye A, Given CW, Gift AG. A study on the relationship between fatigue, pain, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2006; 33:404.
- Hoffman AJ, Given BA, von Eye A, Gift AG, Given CW. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2007; 34:785–792.
- Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of disturbance in women with breast cancer: an exploratory study. J Psychosom Res 2003; 54:85–91.
- Shapiro SL, Lopez AM, Schwartz GE, et al. Quality of life and breast cancer: relationship to psychosocial variables. J Clin Psychol 2001; 57:501–519.
- Mock V, Atkinson A, Barsevick A, et al; National Comprehensive Cancer Network. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 2000; 14:151–161.
- Cella D, Davis K, Breitbart W, Curt G;Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 2001; 19:3385–3391.
- Sateia MJ, Lang BJ. Sleep and cancer: recent developments. Curr Oncol Rep 2008; 10:309–318.
- Ahluwalia M. Fatigue, pain, and depression among older adults with cancer: still underrecognized and undertreated. Geriatrics and Aging 2008; 11:495–501.
- Enderlin CA, Coleman EA, Cole C, Richards KC, Hutchins LF, Sherman AC. Sleep across chemotherapy treatment: a growing concern for women older than 50 with breast cancer. Oncol Nurs Forum 2010; 37:461–A3.
- Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum 1994; 21:23–36.
- Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw 2008; 6:3–13.
- Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 2003; 25:307–318.
- Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarsh 2004; 36:197–206.
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. J Natl Cancer Inst Monogr 2004;76–78.
- Paice JA. Assessment of symptom clusters in people with cancer. J Natl Cancer Inst Monogr 2004;98–102.
- Åhsberg E, Fürst CJ. Dimensions of fatigue during radiotherapy—an application of the Swedish Occupational Fatigue Inventory (SOFI) on cancer patients. Acta Oncol 2001; 40:37–43.
- Foley KM. The treatment of cancer pain. N Engl J Med 1985; 313:84–95.
- Twycross RG, Fairfield S. Pain in far-advanced cancer. Pain 1982; 14:303–310.
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994; 330:592–596.
- Fleming L, Gillespie S, Espie CA. The development and impact of insomnia on cancer survivors: Psychooncology 2010; 19:991–996.
- Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage 1994; 9:372–382.
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004; 8:119–132.
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr 1999; 20:244–252.
- Yue HJ, Guilleminault C. Opioid medication and sleep-disordered breathing. Med Clin North Am 2010; 94:435–446.
- Teichtahl H, Wang D. Sleep-disordered breathing with chronic opioid use. Expert Opin Drug Saf 2007; 6:641–649.
- Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 2001; 10:245–255.
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 1997; 42:209–212.
- Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer 1999; 79:1479–1486.
- Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs 1999; 22:185–194.
- Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology 2009; 18:187–194.
- Savard J, Hervouet S, Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology 2013; 22:1381–1388.
- Fenlon DR, Corner JL, Haviland J. Menopausal hot flushes after breast cancer. Eur J Cancer Care (Engl) 2009; 18:140–148.
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008; 26:971–982.
- Kirkbride P, Bezjak A, Pater J, et al. Dexamethasone for the prophylaxis of radiation-induced emesis: a National Cancer Institute of Canada Clinical Trials Group phase III study. J Clin Oncol 2000; 18:1960–1966.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867.
- Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 2002; 31:15–36.
- Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 2002; 51:887–892.
- Mills PJ, Parker B, Jones V, et al. The effects of standard anthracycline-based chemotherapy on soluble ICAM-1 and vascular endothelial growth factor levels in breast cancer. Clin Cancer Res 2004; 10:4998–5003.
- Reiter RJ, Tan DX, Korkmaz A, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog 2007; 13:303–328.
- Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 2005; 97:1084–1087.
- Travis RC, Allen DS, Fentiman IS, Key TJ. Melatonin and breast cancer: a prospective study. J Natl Cancer Inst 2004; 96:475–482.
- Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res 2005; 65:9595–9600.
- Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis 2008; 29:1244–1248.
- McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res 2006; 15:241–249.
- Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res 2006; 66:5521–5525.
- Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control 2006; 17:39–44.
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology 2006; 17:108–111.
- Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst 2001; 93:1563–1568.
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 2001; 93:1557–1562.
- Hansen J. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst 2001; 93:1513–1515.
- Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011; 117:841–847.
- Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage 1995; 10:423–431.
- Thompson CL, Li L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res Treat 2012; 134:1291–1295.
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008; 4:487–504.
- Fan HG, Houédé-Tchen N, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 2005; 23:8025–8032.
- Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs 2001; 17:241–248.
- Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer 2008; 16:399–405.
- National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 2005; 28:1049–1057.
- Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005; 25:559–592.
- Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003; 71:189–200.
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol 2005; 23:6083–6096.
- Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology 2009; 18:634–646.
- Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol 2008; 26:4651–4658.
- National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 2005; 28:1049–1057.
- Savard J, Villa J, Simard S, Ivers H, Morin CM. Feasibility of a self-help treatment for insomnia comorbid with cancer. Psychooncology 2011; 20:1013–1019.
- Derogatis LR, Feldstein M, Morrow G, et al. A survey of psychotropic drug prescriptions in an oncology population. Cancer 1979; 44:1919–1929.
- Stiefel FC, Kornblith AB, Holland JC. Changes in the prescription patterns of psychotropic drugs for cancer patients during a 10-year period. Cancer 1990; 65:1048–1053.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008; 100:1155–1166.
- Minton O, Stone P, Richardson A, Sharpe M, Hotopf M. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2008;CD006704.
- Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003; 26:793–799.
- Liu J, Wang LN. Ramelteon in the treatment of chronic insomnia: systematic review and meta-analysis. Int J Clin Pract 2012; 66:867–873.
- Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Turhan L, Alkis N. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Support Care Cancer 2008; 16:1291–1298.
- Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012; 13:1184–1190.
Many cancer patients don't sleep well, for a variety of reasons. It is an important problem: not only does poor sleep worsen quality of life, it may affect prognosis. Moreover, treatment is available.
Yet many physicians caring for cancer patients do not ask about sleep problems, underestimating their impact or focusing on more urgent problems. Also, patients may not want to bring up the topic because they consider poor sleep to be unavoidable and untreatable and because they fear that reporting it may shift the focus of their treatment from trying to cure the cancer to easing its symptoms.
This practical review will help health care professionals avoid the common barriers to diagnosis and treatment of poor sleep in cancer patients. Because there are few data on other sleep disorders such as sleep apnea and restless leg syndrome, we will focus on the most common one in cancer patients—insomnia—and its effects on other symptoms and quality of life.
MORE PATIENTS SURVIVE CANCER NOW
Today, more patients are surviving cancer, but cancer symptoms and the side effects of surgery, chemotherapy, and radiation therapy may persist for years.1,2 The most common complaints include cancer-related fatigue, leg restlessness, anxiety, insomnia, and excessive sleepiness.3
Sleep disturbances appear to contribute to the other problems and are relatively easier to quantify. Most studies of sleep disorders in cancer patients have looked specifically at insomnia,4 although a few have explored the prevalence of other sleep disorders, such as sleep-disordered breathing and limb movements during sleep.5
The International Classification of Sleep Disorders, 2nd edition,6 defines insomnia as difficulty going to sleep or staying asleep (the latter defined as waking up in the middle of the night, with wakeful episodes lasting more than 30 minutes), early-morning awakenings (waking 30 minutes or more before the intended time), or nonrestorative sleep, causing significant distress or impairment of day-time functioning.
INSOMNIA WORSENS QUALITY OF LIFE
Insomnia significantly worsens quality of life in cancer patients, and if it can be detected and effectively treated, quality of life is likely to improve. Studies in cancer patients have found that those with insomnia:
- Were less able to cope with stress and carry on their activities of daily living3
- Were much less able to function and reported more pain, less energy, and greater difficulty in dealing with emotional problems7
- Had poor quality of life, both physically and emotionally.3,8
PERHAPS MORE THAN HALF OF CANCER PATIENTS HAVE INSOMNIA
Depending on the methods used and populations studied, at least 30% and perhaps more than half of patients with cancer have insomnia (Table 1).3,4,8–14 It is one of the most commonly reported complaints in this group,15–17 and it occurs before, during, and after treatment of cancer.
Although the prevalence may differ in various cancers, it is still higher than in the general population. In a study of about 450 patients with cancer or depression and 300 healthy volunteers, 62% of the cancer patients reported moderate to severe sleep disturbance, compared with 52% of the depressed patients and 30% of the healthy volunteers.18
When Davidson et al3 surveyed nearly 1,000 cancer patients, one-third said they had insomnia. The problem was most prevalent in lung and breast cancer patients.
In a longitudinal study by Savard et al,13 the prevalence of insomnia declined over time but remained high even at the end of 18 months. It was more prevalent in patients with gynecologic and breast cancer than in those with prostate cancer.13,19
SLEEP PROBLEMS ARE UNDERREPORTED
Sleep problems in cancer patients often go unrecognized because patients do not report them. In a survey of 150 patients,20 44% reported having had sleep problems during the preceding month. However, only one-third of those with sleep problems told their health care providers. This highlights the need for physicians to address sleep complaints in cancer patients at every visit and, if needed, to refer them to a sleep specialist for further evaluation and management.
INSOMNIA IS OFTEN ASSOCIATED WITH OTHER PROBLEMS
Many things can interfere with sleep in cancer patients: the cancer itself (eg, pain due to tumor invasion), medical treatments (eg, narcotics, chemotherapy, neuroleptics, sympathomimetics, steroids, sedative hypnotics), psychosocial disturbances (eg, depression, anxiety, stress), and comorbid medical issues.
In this population, insomnia is often part of a cluster of symptoms that includes pain, fatigue, depression, and anxiety. These act synergistically, worsening quality of life.21–24
Cancer-related fatigue and insomnia
Cancer-related fatigue is a distressing, persistent, subjective sense of tiredness or exhaustion that is related to cancer or cancer treatment, that is not proportional to recent activity and that interferes with usual functioning.25 It has been reported by up to 90% of cancer patients in some studies.26–28
Cancer-related fatigue worsens quality of life and is one of the most distressing and persistent symptoms experienced before, during, and after cancer treatment.29,30 Furthermore, it can lead to sleep disturbances and daytime somnolence and further aggravate insomnia.31,32 The two conditions are often reported as part of a cluster of interrelated symptoms that include pain, depression, and loss of concentration and other cognitive functions, suggesting that they may share a common etiology.33–35
Åhsberg et al36 examined different aspects of perceived cancer-related fatigue in patients undergoing radiotherapy and found correlations between lack of energy, sleepiness, and cancer-related fatigue.
Current understanding of the possible link between cancer-related fatigue and insomnia suggests that interventions targeting the insomnia and daytime sleepiness could decrease the fatigue as well.31
Pain and insomnia in cancer patients
Pain is reported by 60% to 90% of patients with advanced cancer,37,38 its intensity usually varying with the extent of disease. Too often, it is inadequately controlled.39 Furthermore, it is thought to contribute to insomnia.40
In a study of more than 1,600 cancer patients, nearly 60% reported insomnia in addition to pain.41 The severity of pain directly correlated with the probability of insomnia.
Conversely, research suggests that sleep disturbances, primarily insomnia, can increase cancer patients’ sensitivity to pain.42 One hypothesis is that adequate sleep is needed to promote processes relevant to recovery from pain, both physiologic (ie, tissue repair) and psychological (ie, transient cessation of the perception of pain signals).43
Paradoxically, opioids can worsen insomnia
Cancer pain is often treated with opioids, which, paradoxically, can cause or worsen insomnia.
Although opioids induce sleep, they also depress respiration, and at night, they can cause or worsen sleep-disordered breathing (obstructive or central sleep apnea or ataxic breathing), leading to episodes of hypoxia, arousals, and fragmented sleep.44 Moreover, opioids can lead to daytime sedation. Further, psychostimulants such as methylphenidate, given to counteract opioid-induced sedation, can cause anxiety and insomnia. Thus, the interaction between cancer-related pain, insomnia, and pain management leads to a vicious cycle. Understanding this process, we can try to break the cycle and help patients with cancer sleep better.
However, how best to treat sleep-disordered breathing in patients taking opioids long-term is not well established.
In general, the primary intervention is to reduce the opioid dose. Practitioners should continually assess the need for these drugs and consider referral to a drug-behavior treatment center to help with discontinuation of opioid use when deemed medically appropriate.45 Other strategies include positive airway pressure ventilation including continuous positive airway pressure, bilevel pressure devices with backup rate, or adaptive servoventilators. In some cases oxygen supplementation may be required.
Sleep-disordered breathing, when recognized and diagnosed, should be managed in partnership with a sleep specialist.
Depression and insomnia in cancer patients
By some estimates, up to half of cancer patients suffer from depression at some point in their illness.28 And not without reason: these patients face uncertainty about their life, and this often results in depression or anxiety.46
Many cancer patients with depression also have insomnia.28 Indeed, patients with persistent insomnia are at greater risk of developing psychological disorders such as depression and anxiety.47
In a survey of cancer patients, insomnia symptoms were more often attributed to thoughts or concerns about health, family, friends, the cancer diagnosis, and finances than to the actual physical effects of cancer.48
CANCER TREATMENT AND INSOMNIA
Many cancer patients experience sleep disturbances even before starting treatment.49 Liu et al50 showed that, in 76 women about to undergo chemotherapy for breast cancer, those who already had sleep disturbances, fatigue, and depression had more problems, and more severe problems, during chemotherapy.
Radiation therapy and chemotherapy have been reported to cause or precipitate insomnia (Table 2).8,13
Hormonal therapy and biological therapy can also cause or worsen preexisting insomnia.51,52 For example, androgen deprivation therapy for prostate cancer and hormonal therapy for breast cancer are often associated with sleep problems.49,50 Possible mechanisms of insomnia include hot flashes, night sweats, and anxiety caused by such treatments. Biological agents such as interferons, interleukins, and tumor necrosis factor (TNF) alpha, which are often used to treat malignant melanoma, can affect the sleep-wake cycle, leading to insomnia.53
Corticosteroids sharply raise serum cortisol levels, which can lead to insomnia. Cancer patients receiving dexamethasone to prevent radiation-induced emesis experienced more insomnia than patients who did not receive dexamethasone.54
IMMUNOLOGIC BASIS OF INSOMNIA IN CANCER PATIENTS
Cancer cells produce inflammatory cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), and TNF alpha, and inflammation plays a role in tumor progression and possibly tumorigenesis.55
Specific cytokines also help regulate the sleep-wake cycle. Levels of IL-6 and TNF alpha peak during sleep, and daytime IL-6 levels are inversely related to the amount of nocturnal sleep.56 Vgontzas et al57 showed that although mean levels of 24-hour IL-6 and TNF alpha secretion were not significantly different in patients with insomnia vs healthy controls, chronic insomnia was associated with a shift in IL-6 and TNF alpha secretion from nighttime to daytime.57
Cancer and its treatment can affect secretion of the cytokines that play a role in the sleep-wake cycle. Thus, the sleep disturbances associated with cancer may also be related to the abnormalities in cytokine levels caused by either cancer or its treatment.
Mills et al58 found that inflammatory markers such as vascular endothelial growth factor and soluble intercellular adhesion molecule-1 were significantly elevated during chemotherapy in breast cancer patients, and the elevated vascular endothelial growth factor levels were associated with poorer sleep during treatment.
Further research is warranted to establish causality, to help us understand the mechanisms of insomnia and other cancer symptoms, and to develop new treatments for these complaints.
POOR SLEEP AND CANCER RISK AND OUTCOMES
Sleep disturbances have negative health consequences in cancer. Their impact ranges from plausible carcinogenesis to affecting the course of the disease and cancer survival.
Poor sleep and risk of cancer
Epidemiologic studies have examined a possible link between circadian rhythm disruption and breast cancer risk, using both direct measures such as melatonin levels and indirect measures such as sleep duration and shift work. (Melatonin production is related to sleep duration, and night-shift work leads to disruption of sleep pattern and quality of sleep, thus lowering melatonin levels.59)
The findings were mixed. Breast cancer risk was significantly and inversely associated with urinary melatonin levels (6-sulfatoxymelatonin) in the Nurses’ Health Study II,60 but not in the Guernsey III study in the United Kingdom.61 Breast cancer risk was significantly lower with longer sleep duration in Finnish women62 and in Chinese women in Singapore,63 but not in American women.64,65 Results of three cohort studies66–68 and two case-control studies69,70 suggested a higher breast cancer risk in women who work evening or overnight shifts. Shorter sleep duration was associated with a higher risk of colorectal adenomas.71
These studies make a strong case for an association of cancer with circadian rhythm disruption and shorter sleep duration, possibly from an effect on melatonin levels. However, one should be cautious in interpreting epidemiologic studies: although they show sleep disturbances to be associated with cancer risk, they do not establish causality.
Insomnia and cancer outcomes
Evidence is growing that sleep disturbances may affect compliance with treatment, immune function, and outcomes—including survival—in cancer patients.23,24
In patients newly diagnosed with various types of cancer, Degner and Sloan72 showed that those who suffered from insomnia, nausea, poor appetite, and pain had a lower survival rate at 5 years, independent of the cancer stage. However, no separate analyses were performed to examine the specific influence of insomnia on cancer survival.
Thompson and Li73 analyzed data from 101 breast cancer patients with available Oncotype DX recurrence scores (a proprietary genetic test performed on tumor tissue that predicts the likelihood of recurrence). The scores were strongly correlated with average hours of sleep per night before breast cancer diagnosis, with fewer hours of sleep associated with a higher (worse) score.
Since these studies were retrospective and merely suggest associations, prospective studies, using more standardized questionnaires and objective measures, are needed to establish causality and to further our understanding of the mechanisms involved.
HELPING CANCER PATIENTS SLEEP BETTER
Insomnia is generally diagnosed with a thorough history that includes sleep, medical issues, substance use, and psychiatric issues. The sleep history should include specific insomniarelated complaints, presleep conditions and habits, sleep-wake habits, other sleep-related symptoms, and daytime consequences. To obtain the information, one can use questionnaires, sleep logs, psychological screening tests, and bed-partner interviews.74
Managing insomnia involves both pharmacologic and nonpharmacologic treatment. It is also important to treat the associated disorders such as depression and anxiety disorders that often accompany insomnia. Long-term management of cancer patients should not be limited to surveillance of cancer but should also involve aggressive treatment of clusters of symptoms such as insomnia, cancer-related fatigue, and pain to yield better long-term quality of life.75–77
Nonpharmacologic treatment: Cognitive-behavioral therapy
Nonpharmacologic interventions use psychological and behavioral therapies. The American Academy of Sleep Medicine guidelines recommend cognitive behavioral therapy for all patients with insomnia, either alone or in combination with hypnotic medications.
Cognitive-behavioral therapy for insomnia includes various components that help the patient learn coping skills and ways to prevent or mitigate the severity of future episodes (Table 3). Various randomized controlled trials found it to be effective for treating insomnia in the general population.77–79
Several studies found that cognitive-behavioral therapy for insomnia was effective in cancer patients, not only improving sleep quality but also decreasing psychological distress, resulting in better overall quality of life.80,81
Savard et al81 conducted a randomized controlled trial of cognitive-behavioral therapy for insomnia in 57 patients with breast cancer, examining subjective and objective sleep measures, psychological functioning, quality of life, and immunologic responses. They found significant improvements in sleep efficiency, mood, quality of life, depression, anxiety, and need for sleep medications. Improvements in subjective sleep measures persisted on 12-month follow-up.
Berger et al,82 in another randomized controlled trial, assessed behavioral therapy using stimulus control, modified sleep restriction, relaxation therapy, and sleep hygiene in breast cancer patients receiving adjuvant chemotherapy. Behavioral therapy improved sleep quality over time, as measured by the Pittsburgh Sleep Quality Index.
Espie et al83 evaluated the effect of cognitive-behavioral therapy on prostate, colorectal, gynecologic, and breast cancer patients, with similar results.83
Cognitive-behavioral therapy is at least as effective as drug therapy for insomnia in the general population. In the limited studies done in cancer patients, it has been shown to be effective irrespective of the type of cancer and is associated with better long-term outcomes. It diminishes the distress associated with early insomnia, can reduce anxiety, and can promote sleep.
A National Institutes of Health conference on insomnia concluded that cognitivebehavioral therapy is at least as effective as medications for brief treatment of chronic insomnia and that its beneficial effects, in contrast to those produced by medications, may last beyond the termination of treatment.84
It is important to think about numerous factors when considering options such as cognitive-behavioral therapy, as patients with cancer have different complications that may affect sleep quality, such as cancer-related fatigue, cancer-related depression, psychological reactions to the disease, side effects of treatment, and cancer-related pain. These need to be addressed as well.
If cognitive-behavioral therapy is not available, self-help interventions (eg, written material, videos, television and Internet resources) can be used. These have several advantages over professionally administered interventions, including greater accessibility, less burden for the patient, and lower cost. Research is under way evaluating this approach in cancer patients.85
Drug therapy
The focus of therapy should be to treat underlying disorders that may be causing or contributing to insomnia. However, a substantial number of patients may need to be assessed for pharmacotherapy for insomnia.
Sleep problems in the general population are commonly treated with drugs, and most of the recommendations in cancer patients are based on experience in the general population. However, sleep medications should be used cautiously in cancer patients, since to our knowledge there have been no studies of these agents in patients with cancer.
Side effects also need to be considered. For example, sleep medications can profoundly worsen cancer-related fatigue.
Hypnotics are often prescribed for cancer patients.86,87 A study in five major oncology centers showed that about half of the 1,500 patients were prescribed at least one psychotropic drug.86 In this study, hypnotics were the most frequently prescribed drugs, accounting for 48% of total prescriptions, and 44% of the psychotropic prescriptions were written for sleep.
Benzodiazepine receptor agonists such as zaleplon, zolpidem, and eszopiclone can be used for problems with falling asleep and staying asleep.88,89 They are better tolerated than older, long-acting benzodiazepines,90 which can cause alterations in sleep-cycle architecture or rebound insomnia. The earlier agents can also cause adverse effects such as tolerance, drowsiness, and cognitive impairment.
A National Institutes of Health conference stated that benzodiazepine receptor agonists are efficacious in the short-term management of insomnia and that their adverse effects are much less frequent and severe than those of the benzodiazepines or other sedating drugs.84 It also stated that all antidepressants, antihistamines (H1 receptor antagonists), and anti-psychotics have potentially significant adverse effects that raise concerns about their risk-to-benefit ratio and their suitability as treatment for chronic insomnia.
Benzodiazepines are commonly prescribed for insomnia. They increase sleep efficiency, decrease arousals, and increase stage 2 sleep.
Melatonin receptor agonists have been approved by the US Food and Drug Administration for treating insomnia. A recent meta-analysis of eight studies in healthy patients showed improvements in subjective and objective sleep outcomes with the use of ramelteon.91 The dosages primarily used were 4 to 32 mg. However, most of the studies used a dosage of 4 to 8 mg.
Antidepressants. Some of the antidepressants are also used for insomnia, but they can cause daytime fatigue.
Mirtazapine was shown to be effective for insomnia and coexistent mood disorder in cancer patients, but larger trials are needed.92
A recent clinical trial with secondary data analyses evaluated the effect of paroxetine on insomnia, depression, and fatigue in patients with cancer. Paroxetine significantly reduced insomnia in both depressed and nondepressed patients after 2 to 3 weeks of treatment.93
Table 4 summarizes classes of drugs used for insomnia and their additional therapeutic properties.
Many cancer patients don't sleep well, for a variety of reasons. It is an important problem: not only does poor sleep worsen quality of life, it may affect prognosis. Moreover, treatment is available.
Yet many physicians caring for cancer patients do not ask about sleep problems, underestimating their impact or focusing on more urgent problems. Also, patients may not want to bring up the topic because they consider poor sleep to be unavoidable and untreatable and because they fear that reporting it may shift the focus of their treatment from trying to cure the cancer to easing its symptoms.
This practical review will help health care professionals avoid the common barriers to diagnosis and treatment of poor sleep in cancer patients. Because there are few data on other sleep disorders such as sleep apnea and restless leg syndrome, we will focus on the most common one in cancer patients—insomnia—and its effects on other symptoms and quality of life.
MORE PATIENTS SURVIVE CANCER NOW
Today, more patients are surviving cancer, but cancer symptoms and the side effects of surgery, chemotherapy, and radiation therapy may persist for years.1,2 The most common complaints include cancer-related fatigue, leg restlessness, anxiety, insomnia, and excessive sleepiness.3
Sleep disturbances appear to contribute to the other problems and are relatively easier to quantify. Most studies of sleep disorders in cancer patients have looked specifically at insomnia,4 although a few have explored the prevalence of other sleep disorders, such as sleep-disordered breathing and limb movements during sleep.5
The International Classification of Sleep Disorders, 2nd edition,6 defines insomnia as difficulty going to sleep or staying asleep (the latter defined as waking up in the middle of the night, with wakeful episodes lasting more than 30 minutes), early-morning awakenings (waking 30 minutes or more before the intended time), or nonrestorative sleep, causing significant distress or impairment of day-time functioning.
INSOMNIA WORSENS QUALITY OF LIFE
Insomnia significantly worsens quality of life in cancer patients, and if it can be detected and effectively treated, quality of life is likely to improve. Studies in cancer patients have found that those with insomnia:
- Were less able to cope with stress and carry on their activities of daily living3
- Were much less able to function and reported more pain, less energy, and greater difficulty in dealing with emotional problems7
- Had poor quality of life, both physically and emotionally.3,8
PERHAPS MORE THAN HALF OF CANCER PATIENTS HAVE INSOMNIA
Depending on the methods used and populations studied, at least 30% and perhaps more than half of patients with cancer have insomnia (Table 1).3,4,8–14 It is one of the most commonly reported complaints in this group,15–17 and it occurs before, during, and after treatment of cancer.
Although the prevalence may differ in various cancers, it is still higher than in the general population. In a study of about 450 patients with cancer or depression and 300 healthy volunteers, 62% of the cancer patients reported moderate to severe sleep disturbance, compared with 52% of the depressed patients and 30% of the healthy volunteers.18
When Davidson et al3 surveyed nearly 1,000 cancer patients, one-third said they had insomnia. The problem was most prevalent in lung and breast cancer patients.
In a longitudinal study by Savard et al,13 the prevalence of insomnia declined over time but remained high even at the end of 18 months. It was more prevalent in patients with gynecologic and breast cancer than in those with prostate cancer.13,19
SLEEP PROBLEMS ARE UNDERREPORTED
Sleep problems in cancer patients often go unrecognized because patients do not report them. In a survey of 150 patients,20 44% reported having had sleep problems during the preceding month. However, only one-third of those with sleep problems told their health care providers. This highlights the need for physicians to address sleep complaints in cancer patients at every visit and, if needed, to refer them to a sleep specialist for further evaluation and management.
INSOMNIA IS OFTEN ASSOCIATED WITH OTHER PROBLEMS
Many things can interfere with sleep in cancer patients: the cancer itself (eg, pain due to tumor invasion), medical treatments (eg, narcotics, chemotherapy, neuroleptics, sympathomimetics, steroids, sedative hypnotics), psychosocial disturbances (eg, depression, anxiety, stress), and comorbid medical issues.
In this population, insomnia is often part of a cluster of symptoms that includes pain, fatigue, depression, and anxiety. These act synergistically, worsening quality of life.21–24
Cancer-related fatigue and insomnia
Cancer-related fatigue is a distressing, persistent, subjective sense of tiredness or exhaustion that is related to cancer or cancer treatment, that is not proportional to recent activity and that interferes with usual functioning.25 It has been reported by up to 90% of cancer patients in some studies.26–28
Cancer-related fatigue worsens quality of life and is one of the most distressing and persistent symptoms experienced before, during, and after cancer treatment.29,30 Furthermore, it can lead to sleep disturbances and daytime somnolence and further aggravate insomnia.31,32 The two conditions are often reported as part of a cluster of interrelated symptoms that include pain, depression, and loss of concentration and other cognitive functions, suggesting that they may share a common etiology.33–35
Åhsberg et al36 examined different aspects of perceived cancer-related fatigue in patients undergoing radiotherapy and found correlations between lack of energy, sleepiness, and cancer-related fatigue.
Current understanding of the possible link between cancer-related fatigue and insomnia suggests that interventions targeting the insomnia and daytime sleepiness could decrease the fatigue as well.31
Pain and insomnia in cancer patients
Pain is reported by 60% to 90% of patients with advanced cancer,37,38 its intensity usually varying with the extent of disease. Too often, it is inadequately controlled.39 Furthermore, it is thought to contribute to insomnia.40
In a study of more than 1,600 cancer patients, nearly 60% reported insomnia in addition to pain.41 The severity of pain directly correlated with the probability of insomnia.
Conversely, research suggests that sleep disturbances, primarily insomnia, can increase cancer patients’ sensitivity to pain.42 One hypothesis is that adequate sleep is needed to promote processes relevant to recovery from pain, both physiologic (ie, tissue repair) and psychological (ie, transient cessation of the perception of pain signals).43
Paradoxically, opioids can worsen insomnia
Cancer pain is often treated with opioids, which, paradoxically, can cause or worsen insomnia.
Although opioids induce sleep, they also depress respiration, and at night, they can cause or worsen sleep-disordered breathing (obstructive or central sleep apnea or ataxic breathing), leading to episodes of hypoxia, arousals, and fragmented sleep.44 Moreover, opioids can lead to daytime sedation. Further, psychostimulants such as methylphenidate, given to counteract opioid-induced sedation, can cause anxiety and insomnia. Thus, the interaction between cancer-related pain, insomnia, and pain management leads to a vicious cycle. Understanding this process, we can try to break the cycle and help patients with cancer sleep better.
However, how best to treat sleep-disordered breathing in patients taking opioids long-term is not well established.
In general, the primary intervention is to reduce the opioid dose. Practitioners should continually assess the need for these drugs and consider referral to a drug-behavior treatment center to help with discontinuation of opioid use when deemed medically appropriate.45 Other strategies include positive airway pressure ventilation including continuous positive airway pressure, bilevel pressure devices with backup rate, or adaptive servoventilators. In some cases oxygen supplementation may be required.
Sleep-disordered breathing, when recognized and diagnosed, should be managed in partnership with a sleep specialist.
Depression and insomnia in cancer patients
By some estimates, up to half of cancer patients suffer from depression at some point in their illness.28 And not without reason: these patients face uncertainty about their life, and this often results in depression or anxiety.46
Many cancer patients with depression also have insomnia.28 Indeed, patients with persistent insomnia are at greater risk of developing psychological disorders such as depression and anxiety.47
In a survey of cancer patients, insomnia symptoms were more often attributed to thoughts or concerns about health, family, friends, the cancer diagnosis, and finances than to the actual physical effects of cancer.48
CANCER TREATMENT AND INSOMNIA
Many cancer patients experience sleep disturbances even before starting treatment.49 Liu et al50 showed that, in 76 women about to undergo chemotherapy for breast cancer, those who already had sleep disturbances, fatigue, and depression had more problems, and more severe problems, during chemotherapy.
Radiation therapy and chemotherapy have been reported to cause or precipitate insomnia (Table 2).8,13
Hormonal therapy and biological therapy can also cause or worsen preexisting insomnia.51,52 For example, androgen deprivation therapy for prostate cancer and hormonal therapy for breast cancer are often associated with sleep problems.49,50 Possible mechanisms of insomnia include hot flashes, night sweats, and anxiety caused by such treatments. Biological agents such as interferons, interleukins, and tumor necrosis factor (TNF) alpha, which are often used to treat malignant melanoma, can affect the sleep-wake cycle, leading to insomnia.53
Corticosteroids sharply raise serum cortisol levels, which can lead to insomnia. Cancer patients receiving dexamethasone to prevent radiation-induced emesis experienced more insomnia than patients who did not receive dexamethasone.54
IMMUNOLOGIC BASIS OF INSOMNIA IN CANCER PATIENTS
Cancer cells produce inflammatory cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), and TNF alpha, and inflammation plays a role in tumor progression and possibly tumorigenesis.55
Specific cytokines also help regulate the sleep-wake cycle. Levels of IL-6 and TNF alpha peak during sleep, and daytime IL-6 levels are inversely related to the amount of nocturnal sleep.56 Vgontzas et al57 showed that although mean levels of 24-hour IL-6 and TNF alpha secretion were not significantly different in patients with insomnia vs healthy controls, chronic insomnia was associated with a shift in IL-6 and TNF alpha secretion from nighttime to daytime.57
Cancer and its treatment can affect secretion of the cytokines that play a role in the sleep-wake cycle. Thus, the sleep disturbances associated with cancer may also be related to the abnormalities in cytokine levels caused by either cancer or its treatment.
Mills et al58 found that inflammatory markers such as vascular endothelial growth factor and soluble intercellular adhesion molecule-1 were significantly elevated during chemotherapy in breast cancer patients, and the elevated vascular endothelial growth factor levels were associated with poorer sleep during treatment.
Further research is warranted to establish causality, to help us understand the mechanisms of insomnia and other cancer symptoms, and to develop new treatments for these complaints.
POOR SLEEP AND CANCER RISK AND OUTCOMES
Sleep disturbances have negative health consequences in cancer. Their impact ranges from plausible carcinogenesis to affecting the course of the disease and cancer survival.
Poor sleep and risk of cancer
Epidemiologic studies have examined a possible link between circadian rhythm disruption and breast cancer risk, using both direct measures such as melatonin levels and indirect measures such as sleep duration and shift work. (Melatonin production is related to sleep duration, and night-shift work leads to disruption of sleep pattern and quality of sleep, thus lowering melatonin levels.59)
The findings were mixed. Breast cancer risk was significantly and inversely associated with urinary melatonin levels (6-sulfatoxymelatonin) in the Nurses’ Health Study II,60 but not in the Guernsey III study in the United Kingdom.61 Breast cancer risk was significantly lower with longer sleep duration in Finnish women62 and in Chinese women in Singapore,63 but not in American women.64,65 Results of three cohort studies66–68 and two case-control studies69,70 suggested a higher breast cancer risk in women who work evening or overnight shifts. Shorter sleep duration was associated with a higher risk of colorectal adenomas.71
These studies make a strong case for an association of cancer with circadian rhythm disruption and shorter sleep duration, possibly from an effect on melatonin levels. However, one should be cautious in interpreting epidemiologic studies: although they show sleep disturbances to be associated with cancer risk, they do not establish causality.
Insomnia and cancer outcomes
Evidence is growing that sleep disturbances may affect compliance with treatment, immune function, and outcomes—including survival—in cancer patients.23,24
In patients newly diagnosed with various types of cancer, Degner and Sloan72 showed that those who suffered from insomnia, nausea, poor appetite, and pain had a lower survival rate at 5 years, independent of the cancer stage. However, no separate analyses were performed to examine the specific influence of insomnia on cancer survival.
Thompson and Li73 analyzed data from 101 breast cancer patients with available Oncotype DX recurrence scores (a proprietary genetic test performed on tumor tissue that predicts the likelihood of recurrence). The scores were strongly correlated with average hours of sleep per night before breast cancer diagnosis, with fewer hours of sleep associated with a higher (worse) score.
Since these studies were retrospective and merely suggest associations, prospective studies, using more standardized questionnaires and objective measures, are needed to establish causality and to further our understanding of the mechanisms involved.
HELPING CANCER PATIENTS SLEEP BETTER
Insomnia is generally diagnosed with a thorough history that includes sleep, medical issues, substance use, and psychiatric issues. The sleep history should include specific insomniarelated complaints, presleep conditions and habits, sleep-wake habits, other sleep-related symptoms, and daytime consequences. To obtain the information, one can use questionnaires, sleep logs, psychological screening tests, and bed-partner interviews.74
Managing insomnia involves both pharmacologic and nonpharmacologic treatment. It is also important to treat the associated disorders such as depression and anxiety disorders that often accompany insomnia. Long-term management of cancer patients should not be limited to surveillance of cancer but should also involve aggressive treatment of clusters of symptoms such as insomnia, cancer-related fatigue, and pain to yield better long-term quality of life.75–77
Nonpharmacologic treatment: Cognitive-behavioral therapy
Nonpharmacologic interventions use psychological and behavioral therapies. The American Academy of Sleep Medicine guidelines recommend cognitive behavioral therapy for all patients with insomnia, either alone or in combination with hypnotic medications.
Cognitive-behavioral therapy for insomnia includes various components that help the patient learn coping skills and ways to prevent or mitigate the severity of future episodes (Table 3). Various randomized controlled trials found it to be effective for treating insomnia in the general population.77–79
Several studies found that cognitive-behavioral therapy for insomnia was effective in cancer patients, not only improving sleep quality but also decreasing psychological distress, resulting in better overall quality of life.80,81
Savard et al81 conducted a randomized controlled trial of cognitive-behavioral therapy for insomnia in 57 patients with breast cancer, examining subjective and objective sleep measures, psychological functioning, quality of life, and immunologic responses. They found significant improvements in sleep efficiency, mood, quality of life, depression, anxiety, and need for sleep medications. Improvements in subjective sleep measures persisted on 12-month follow-up.
Berger et al,82 in another randomized controlled trial, assessed behavioral therapy using stimulus control, modified sleep restriction, relaxation therapy, and sleep hygiene in breast cancer patients receiving adjuvant chemotherapy. Behavioral therapy improved sleep quality over time, as measured by the Pittsburgh Sleep Quality Index.
Espie et al83 evaluated the effect of cognitive-behavioral therapy on prostate, colorectal, gynecologic, and breast cancer patients, with similar results.83
Cognitive-behavioral therapy is at least as effective as drug therapy for insomnia in the general population. In the limited studies done in cancer patients, it has been shown to be effective irrespective of the type of cancer and is associated with better long-term outcomes. It diminishes the distress associated with early insomnia, can reduce anxiety, and can promote sleep.
A National Institutes of Health conference on insomnia concluded that cognitivebehavioral therapy is at least as effective as medications for brief treatment of chronic insomnia and that its beneficial effects, in contrast to those produced by medications, may last beyond the termination of treatment.84
It is important to think about numerous factors when considering options such as cognitive-behavioral therapy, as patients with cancer have different complications that may affect sleep quality, such as cancer-related fatigue, cancer-related depression, psychological reactions to the disease, side effects of treatment, and cancer-related pain. These need to be addressed as well.
If cognitive-behavioral therapy is not available, self-help interventions (eg, written material, videos, television and Internet resources) can be used. These have several advantages over professionally administered interventions, including greater accessibility, less burden for the patient, and lower cost. Research is under way evaluating this approach in cancer patients.85
Drug therapy
The focus of therapy should be to treat underlying disorders that may be causing or contributing to insomnia. However, a substantial number of patients may need to be assessed for pharmacotherapy for insomnia.
Sleep problems in the general population are commonly treated with drugs, and most of the recommendations in cancer patients are based on experience in the general population. However, sleep medications should be used cautiously in cancer patients, since to our knowledge there have been no studies of these agents in patients with cancer.
Side effects also need to be considered. For example, sleep medications can profoundly worsen cancer-related fatigue.
Hypnotics are often prescribed for cancer patients.86,87 A study in five major oncology centers showed that about half of the 1,500 patients were prescribed at least one psychotropic drug.86 In this study, hypnotics were the most frequently prescribed drugs, accounting for 48% of total prescriptions, and 44% of the psychotropic prescriptions were written for sleep.
Benzodiazepine receptor agonists such as zaleplon, zolpidem, and eszopiclone can be used for problems with falling asleep and staying asleep.88,89 They are better tolerated than older, long-acting benzodiazepines,90 which can cause alterations in sleep-cycle architecture or rebound insomnia. The earlier agents can also cause adverse effects such as tolerance, drowsiness, and cognitive impairment.
A National Institutes of Health conference stated that benzodiazepine receptor agonists are efficacious in the short-term management of insomnia and that their adverse effects are much less frequent and severe than those of the benzodiazepines or other sedating drugs.84 It also stated that all antidepressants, antihistamines (H1 receptor antagonists), and anti-psychotics have potentially significant adverse effects that raise concerns about their risk-to-benefit ratio and their suitability as treatment for chronic insomnia.
Benzodiazepines are commonly prescribed for insomnia. They increase sleep efficiency, decrease arousals, and increase stage 2 sleep.
Melatonin receptor agonists have been approved by the US Food and Drug Administration for treating insomnia. A recent meta-analysis of eight studies in healthy patients showed improvements in subjective and objective sleep outcomes with the use of ramelteon.91 The dosages primarily used were 4 to 32 mg. However, most of the studies used a dosage of 4 to 8 mg.
Antidepressants. Some of the antidepressants are also used for insomnia, but they can cause daytime fatigue.
Mirtazapine was shown to be effective for insomnia and coexistent mood disorder in cancer patients, but larger trials are needed.92
A recent clinical trial with secondary data analyses evaluated the effect of paroxetine on insomnia, depression, and fatigue in patients with cancer. Paroxetine significantly reduced insomnia in both depressed and nondepressed patients after 2 to 3 weeks of treatment.93
Table 4 summarizes classes of drugs used for insomnia and their additional therapeutic properties.
- Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol 2006; 16:197–205.
- Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology 2006; 15:306–320.
- Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med 2002; 54:1309–1321.
- Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 2001; 19:895–908.
- Payne RJ, Hier MP, Kost KM, et al. High prevalence of obstructive sleep apnea among patients with head and neck cancer. J Otolaryngol 2005; 34:304–311.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Second Edition (ICSD-2); 2005.
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage 2002; 24:471–480.
- Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer 2008; 62:391–400.
- Liu L, Ancoli-Israel S. Sleep disturbances in cancer. Psychiatr Ann 2008; 38:627–634.
- Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 2006; 14:201–209.
- Miaskowski C, Lee K, Dunn L, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs 2011; 34:255–268.
- Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep 2012; 35:237–245.
- Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol 2011; 29:3580–3586.
- Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care 2005; 3:23–31.
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med 2007; 20:434–443.
- Schroevers MJ, Ranchor AV, Sanderman R. The role of age at the onset of cancer in relation to survivors’ long-term adjustment: a controlled comparison over an eight-year period. Psychooncology 2004; 13:740–752.
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008; 112(suppl 11):2577–2592.
- Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 2003; 25:307–318.
- Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol 2009; 27:5233–5239.
- Engstrom CA, Strohl RA, Rose L, Lewandowski L, Stefanek ME. Sleep alterations in cancer patients. Cancer Nurs 1999; 22:143–148.
- Hoffman A, Given BA, von Eye A, Given CW, Gift AG. A study on the relationship between fatigue, pain, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2006; 33:404.
- Hoffman AJ, Given BA, von Eye A, Gift AG, Given CW. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2007; 34:785–792.
- Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of disturbance in women with breast cancer: an exploratory study. J Psychosom Res 2003; 54:85–91.
- Shapiro SL, Lopez AM, Schwartz GE, et al. Quality of life and breast cancer: relationship to psychosocial variables. J Clin Psychol 2001; 57:501–519.
- Mock V, Atkinson A, Barsevick A, et al; National Comprehensive Cancer Network. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 2000; 14:151–161.
- Cella D, Davis K, Breitbart W, Curt G;Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 2001; 19:3385–3391.
- Sateia MJ, Lang BJ. Sleep and cancer: recent developments. Curr Oncol Rep 2008; 10:309–318.
- Ahluwalia M. Fatigue, pain, and depression among older adults with cancer: still underrecognized and undertreated. Geriatrics and Aging 2008; 11:495–501.
- Enderlin CA, Coleman EA, Cole C, Richards KC, Hutchins LF, Sherman AC. Sleep across chemotherapy treatment: a growing concern for women older than 50 with breast cancer. Oncol Nurs Forum 2010; 37:461–A3.
- Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum 1994; 21:23–36.
- Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw 2008; 6:3–13.
- Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 2003; 25:307–318.
- Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarsh 2004; 36:197–206.
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. J Natl Cancer Inst Monogr 2004;76–78.
- Paice JA. Assessment of symptom clusters in people with cancer. J Natl Cancer Inst Monogr 2004;98–102.
- Åhsberg E, Fürst CJ. Dimensions of fatigue during radiotherapy—an application of the Swedish Occupational Fatigue Inventory (SOFI) on cancer patients. Acta Oncol 2001; 40:37–43.
- Foley KM. The treatment of cancer pain. N Engl J Med 1985; 313:84–95.
- Twycross RG, Fairfield S. Pain in far-advanced cancer. Pain 1982; 14:303–310.
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994; 330:592–596.
- Fleming L, Gillespie S, Espie CA. The development and impact of insomnia on cancer survivors: Psychooncology 2010; 19:991–996.
- Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage 1994; 9:372–382.
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004; 8:119–132.
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr 1999; 20:244–252.
- Yue HJ, Guilleminault C. Opioid medication and sleep-disordered breathing. Med Clin North Am 2010; 94:435–446.
- Teichtahl H, Wang D. Sleep-disordered breathing with chronic opioid use. Expert Opin Drug Saf 2007; 6:641–649.
- Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 2001; 10:245–255.
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 1997; 42:209–212.
- Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer 1999; 79:1479–1486.
- Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs 1999; 22:185–194.
- Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology 2009; 18:187–194.
- Savard J, Hervouet S, Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology 2013; 22:1381–1388.
- Fenlon DR, Corner JL, Haviland J. Menopausal hot flushes after breast cancer. Eur J Cancer Care (Engl) 2009; 18:140–148.
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008; 26:971–982.
- Kirkbride P, Bezjak A, Pater J, et al. Dexamethasone for the prophylaxis of radiation-induced emesis: a National Cancer Institute of Canada Clinical Trials Group phase III study. J Clin Oncol 2000; 18:1960–1966.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867.
- Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 2002; 31:15–36.
- Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 2002; 51:887–892.
- Mills PJ, Parker B, Jones V, et al. The effects of standard anthracycline-based chemotherapy on soluble ICAM-1 and vascular endothelial growth factor levels in breast cancer. Clin Cancer Res 2004; 10:4998–5003.
- Reiter RJ, Tan DX, Korkmaz A, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog 2007; 13:303–328.
- Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 2005; 97:1084–1087.
- Travis RC, Allen DS, Fentiman IS, Key TJ. Melatonin and breast cancer: a prospective study. J Natl Cancer Inst 2004; 96:475–482.
- Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res 2005; 65:9595–9600.
- Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis 2008; 29:1244–1248.
- McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res 2006; 15:241–249.
- Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res 2006; 66:5521–5525.
- Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control 2006; 17:39–44.
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology 2006; 17:108–111.
- Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst 2001; 93:1563–1568.
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 2001; 93:1557–1562.
- Hansen J. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst 2001; 93:1513–1515.
- Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011; 117:841–847.
- Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage 1995; 10:423–431.
- Thompson CL, Li L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res Treat 2012; 134:1291–1295.
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008; 4:487–504.
- Fan HG, Houédé-Tchen N, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 2005; 23:8025–8032.
- Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs 2001; 17:241–248.
- Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer 2008; 16:399–405.
- National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 2005; 28:1049–1057.
- Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005; 25:559–592.
- Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003; 71:189–200.
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol 2005; 23:6083–6096.
- Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology 2009; 18:634–646.
- Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol 2008; 26:4651–4658.
- National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 2005; 28:1049–1057.
- Savard J, Villa J, Simard S, Ivers H, Morin CM. Feasibility of a self-help treatment for insomnia comorbid with cancer. Psychooncology 2011; 20:1013–1019.
- Derogatis LR, Feldstein M, Morrow G, et al. A survey of psychotropic drug prescriptions in an oncology population. Cancer 1979; 44:1919–1929.
- Stiefel FC, Kornblith AB, Holland JC. Changes in the prescription patterns of psychotropic drugs for cancer patients during a 10-year period. Cancer 1990; 65:1048–1053.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008; 100:1155–1166.
- Minton O, Stone P, Richardson A, Sharpe M, Hotopf M. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2008;CD006704.
- Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003; 26:793–799.
- Liu J, Wang LN. Ramelteon in the treatment of chronic insomnia: systematic review and meta-analysis. Int J Clin Pract 2012; 66:867–873.
- Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Turhan L, Alkis N. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Support Care Cancer 2008; 16:1291–1298.
- Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012; 13:1184–1190.
- Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol 2006; 16:197–205.
- Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology 2006; 15:306–320.
- Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med 2002; 54:1309–1321.
- Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 2001; 19:895–908.
- Payne RJ, Hier MP, Kost KM, et al. High prevalence of obstructive sleep apnea among patients with head and neck cancer. J Otolaryngol 2005; 34:304–311.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Second Edition (ICSD-2); 2005.
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage 2002; 24:471–480.
- Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer 2008; 62:391–400.
- Liu L, Ancoli-Israel S. Sleep disturbances in cancer. Psychiatr Ann 2008; 38:627–634.
- Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 2006; 14:201–209.
- Miaskowski C, Lee K, Dunn L, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs 2011; 34:255–268.
- Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep 2012; 35:237–245.
- Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol 2011; 29:3580–3586.
- Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care 2005; 3:23–31.
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med 2007; 20:434–443.
- Schroevers MJ, Ranchor AV, Sanderman R. The role of age at the onset of cancer in relation to survivors’ long-term adjustment: a controlled comparison over an eight-year period. Psychooncology 2004; 13:740–752.
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008; 112(suppl 11):2577–2592.
- Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 2003; 25:307–318.
- Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol 2009; 27:5233–5239.
- Engstrom CA, Strohl RA, Rose L, Lewandowski L, Stefanek ME. Sleep alterations in cancer patients. Cancer Nurs 1999; 22:143–148.
- Hoffman A, Given BA, von Eye A, Given CW, Gift AG. A study on the relationship between fatigue, pain, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2006; 33:404.
- Hoffman AJ, Given BA, von Eye A, Gift AG, Given CW. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2007; 34:785–792.
- Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of disturbance in women with breast cancer: an exploratory study. J Psychosom Res 2003; 54:85–91.
- Shapiro SL, Lopez AM, Schwartz GE, et al. Quality of life and breast cancer: relationship to psychosocial variables. J Clin Psychol 2001; 57:501–519.
- Mock V, Atkinson A, Barsevick A, et al; National Comprehensive Cancer Network. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 2000; 14:151–161.
- Cella D, Davis K, Breitbart W, Curt G;Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 2001; 19:3385–3391.
- Sateia MJ, Lang BJ. Sleep and cancer: recent developments. Curr Oncol Rep 2008; 10:309–318.
- Ahluwalia M. Fatigue, pain, and depression among older adults with cancer: still underrecognized and undertreated. Geriatrics and Aging 2008; 11:495–501.
- Enderlin CA, Coleman EA, Cole C, Richards KC, Hutchins LF, Sherman AC. Sleep across chemotherapy treatment: a growing concern for women older than 50 with breast cancer. Oncol Nurs Forum 2010; 37:461–A3.
- Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum 1994; 21:23–36.
- Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw 2008; 6:3–13.
- Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 2003; 25:307–318.
- Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarsh 2004; 36:197–206.
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. J Natl Cancer Inst Monogr 2004;76–78.
- Paice JA. Assessment of symptom clusters in people with cancer. J Natl Cancer Inst Monogr 2004;98–102.
- Åhsberg E, Fürst CJ. Dimensions of fatigue during radiotherapy—an application of the Swedish Occupational Fatigue Inventory (SOFI) on cancer patients. Acta Oncol 2001; 40:37–43.
- Foley KM. The treatment of cancer pain. N Engl J Med 1985; 313:84–95.
- Twycross RG, Fairfield S. Pain in far-advanced cancer. Pain 1982; 14:303–310.
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994; 330:592–596.
- Fleming L, Gillespie S, Espie CA. The development and impact of insomnia on cancer survivors: Psychooncology 2010; 19:991–996.
- Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage 1994; 9:372–382.
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004; 8:119–132.
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr 1999; 20:244–252.
- Yue HJ, Guilleminault C. Opioid medication and sleep-disordered breathing. Med Clin North Am 2010; 94:435–446.
- Teichtahl H, Wang D. Sleep-disordered breathing with chronic opioid use. Expert Opin Drug Saf 2007; 6:641–649.
- Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 2001; 10:245–255.
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 1997; 42:209–212.
- Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer 1999; 79:1479–1486.
- Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs 1999; 22:185–194.
- Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology 2009; 18:187–194.
- Savard J, Hervouet S, Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology 2013; 22:1381–1388.
- Fenlon DR, Corner JL, Haviland J. Menopausal hot flushes after breast cancer. Eur J Cancer Care (Engl) 2009; 18:140–148.
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008; 26:971–982.
- Kirkbride P, Bezjak A, Pater J, et al. Dexamethasone for the prophylaxis of radiation-induced emesis: a National Cancer Institute of Canada Clinical Trials Group phase III study. J Clin Oncol 2000; 18:1960–1966.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867.
- Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 2002; 31:15–36.
- Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 2002; 51:887–892.
- Mills PJ, Parker B, Jones V, et al. The effects of standard anthracycline-based chemotherapy on soluble ICAM-1 and vascular endothelial growth factor levels in breast cancer. Clin Cancer Res 2004; 10:4998–5003.
- Reiter RJ, Tan DX, Korkmaz A, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog 2007; 13:303–328.
- Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 2005; 97:1084–1087.
- Travis RC, Allen DS, Fentiman IS, Key TJ. Melatonin and breast cancer: a prospective study. J Natl Cancer Inst 2004; 96:475–482.
- Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res 2005; 65:9595–9600.
- Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis 2008; 29:1244–1248.
- McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res 2006; 15:241–249.
- Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res 2006; 66:5521–5525.
- Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control 2006; 17:39–44.
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology 2006; 17:108–111.
- Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst 2001; 93:1563–1568.
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 2001; 93:1557–1562.
- Hansen J. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst 2001; 93:1513–1515.
- Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011; 117:841–847.
- Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage 1995; 10:423–431.
- Thompson CL, Li L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res Treat 2012; 134:1291–1295.
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008; 4:487–504.
- Fan HG, Houédé-Tchen N, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 2005; 23:8025–8032.
- Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs 2001; 17:241–248.
- Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer 2008; 16:399–405.
- National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 2005; 28:1049–1057.
- Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005; 25:559–592.
- Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003; 71:189–200.
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol 2005; 23:6083–6096.
- Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology 2009; 18:634–646.
- Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol 2008; 26:4651–4658.
- National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 2005; 28:1049–1057.
- Savard J, Villa J, Simard S, Ivers H, Morin CM. Feasibility of a self-help treatment for insomnia comorbid with cancer. Psychooncology 2011; 20:1013–1019.
- Derogatis LR, Feldstein M, Morrow G, et al. A survey of psychotropic drug prescriptions in an oncology population. Cancer 1979; 44:1919–1929.
- Stiefel FC, Kornblith AB, Holland JC. Changes in the prescription patterns of psychotropic drugs for cancer patients during a 10-year period. Cancer 1990; 65:1048–1053.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008; 100:1155–1166.
- Minton O, Stone P, Richardson A, Sharpe M, Hotopf M. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2008;CD006704.
- Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003; 26:793–799.
- Liu J, Wang LN. Ramelteon in the treatment of chronic insomnia: systematic review and meta-analysis. Int J Clin Pract 2012; 66:867–873.
- Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Turhan L, Alkis N. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Support Care Cancer 2008; 16:1291–1298.
- Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012; 13:1184–1190.
KEY POINTS
- Sleep disturbances, primarily insomnia, profoundly affect all aspects of quality of life.
- Insomnia can be caused or worsened by a number of other conditions, such as pain, fatigue, depression, and anxiety, and these in turn can be worsened by insomnia.
- Cognitive-behavioral therapy is the treatment of choice for chronic insomnia. Underlying problems should be addressed.
- Drugs are often prescribed to help cancer patients sleep but should be used with caution, as there is limited information from clinical trials in this population.