Article

Quality and Quantity of the Elbow Arthroscopy Literature: A Systematic Review and Meta-Analysis
- Author:
- Erickson BJ
- Chalmers PN
- Cvetanovich GL
- Frank RM
- Romeo AA
- Harris JD
The purpose of this article is to perform a systematic review and meta-analysis of elbow arthroscopy literature to answer the following questions...
Article

The Effect of Humeral Inclination on Range of Motion in Reverse Total Shoulder Arthroplasty: A Systematic Review
- Author:
- Erickson BJ
- Harris JD
- Romeo AA
Reverse total shoulder arthroplasty (RTSA) is a treatment option for patients with rotator cuff tear arthropathy, pseudoparalysis, and a...
Article
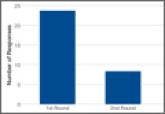
Orthopedic Practice Patterns Relating to Anterior Cruciate Ligament Reconstruction in Elite Athletes
- Author:
- Erickson BJ
- Harris JD
- Fillingham YA
We conducted an online survey of National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) team...
Article
Causes and Rates of Unplanned Readmissions After Elective Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis
- Author:
- Ramkumar PN
- Chu CT
- Harris JD
To address the lack of consensus on the leading reasons for readmissions after primary elective unilateral total joint arthroplasties (TJAs), we...
Article
Infrapatellar Branch of Saphenous Neurectomy for Painful Neuroma: A Case Report
- Author:
- Harris JD
- Fazalare JJ
- Griesser MJ
- Flanigan DC
We present the case of an 18-year-old woman who was healthy other than a history of multiple arthroscopic right knee surgeries culminating in...
Article
Intramuscular Synovial Cyst of the Shoulder
- Author:
- Griesser MJ
- Harris JD
- Jones GL
In this article, we report a case of intramuscular synovial cyst of the rotator cuff musculature with associated supraspinatus and infraspinatus...
Article
Synovial Chondromatosis of the Elbow Causing a Mechanical Block to Range of Motion: A Case Report and Review of the Literature
- Author:
- Griesser MJ
- Harris JD
- Likes RL
- Jones GL
We report a unique case of elbow synovial chondromatosis with sudden onset of severe loss of elbow extension and flexion range of motion caused by...
