User login
Duration of VTE Risk in Medically Ill Patients
Patients who are hospitalized for acute medical illness are at an increased risk of developing venous thromboembolism (VTE), which comprises deep‐vein thrombosis (DVT) and pulmonary embolism (PE).13 In a recent real‐world study of 158,325 US medical patients by Spyropoulos et al,4 4.0% of patients developed DVT, 1.5% developed PE, and 0.2% developed both DVT and PE. Furthermore, results from a population‐based case‐control study indicate that hospitalization for medical illness accounted for a proportion of VTE events similar to that of hospitalization for surgery (22% and 24%, respectively).5
Thromboprophylaxis reduces VTE incidence in at‐risk medical patients and is recommended according to evidence‐based guidelines from the American College of Chest Physicians (ACCP).1 The ACCP guidelines advocate that acutely ill medical patients admitted to the hospital with congestive heart failure (CHF) or severe lung disease/chronic obstructive pulmonary disease (COPD) or those who are confined to bed and have one or more additional risk factors (including active cancer, previous VTE, sepsis, acute neurologic disease, or inflammatory bowel disease) receive pharmacological prophylaxis with lowmolecular weight heparin (LMWH), low‐dose unfractionated heparin (UFH), or fondaparinux.1 Although guidelines provide recommendations for the duration of prophylaxis after major orthopedic surgery, such recommendations are unavailable for medical patients. In clinical trials of acutely ill medical patients, prophylaxis regimens found to be effective were provided for a duration of hospitalization of 6‐14 days.68 The mean length of hospital stay for medical illnesses is decreasing and is currently shorter than 6‐14 days.9, 10
In clinical practice, the duration of VTE risk during and after hospitalization is not well understood in medical patients, particularly in the context of shortening hospital stays. Such information could, however, provide insight into whether current thromboprophylaxis practices reflect real‐world need. To gain a greater understanding of the period during which patients are at risk of VTE, this retrospective, observational study assessed the incidence and time course of symptomatic VTE events during and after hospitalization in a large population of US medical patients.
METHODS
Data and Patient Selection
This study employed linked administrative claims data and hospital billing data contained in the Thomson Reuters MarketScan Inpatient Drug Link File. This combines longitudinal patient‐level inpatient and outpatient medical and pharmaceutical claims data from the MarketScan Commercial claims data from the MarketScan Commercial Claims and Encounters (Commercial) and Medicare Supplemental and Coordination of Benefits (Medicare Supplemental) databases, with hospital discharge records detailing services used and drugs administered during a hospitalization, which are included in the Hospital Drug Database. The linked data sources enable analysis of a patient's experience before, during, and after a hospitalization. The present study was not designed to obtain bleeding rates.
The study cohort comprised patients considered to be at‐risk for VTE as a result of a medical hospitalization occurring between January 1, 2005, and December 31, 2008. At‐risk medical hospitalizations were those for which the primary diagnosis was for cancer, CHF, severe lung disease/COPD, or infectious disease (see Supporting Information, Appendix I, for International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes used to identify patients with medical illnesses). Included patients were required to be at least 18 years of age at the time of admission and were required to be continuously enrolled in their insurance benefits for at least 12 months before admission (the baseline period) and for at least 180 days after the admission date (the evaluation period) to ensure that all administrative claims data during that period were captured. Patients who died in‐hospital from any cause were exempted from the continuous enrollment criterion, as long as they had been continuously enrolled prior to inpatient death. Patients transferred from or discharged to another acute‐care facility were excluded because of the possibility for incomplete inpatient data capture. For patients who had multiple medical hospitalizations between January 1, 2005, and December 31, 2008, the hospitalization around which the analysis focused was randomly selected from the set of potential medical hospitalizations.
Prophylaxis
Pharmacological prophylaxis was identified via charge codes during hospitalization or via pharmacy claims after discharge for UFH, enoxaparin, dalteparin, warfarin, and fondaparinux. All dosages of a pharmacological agent were considered prophylactic only if there was no evidence of VTE during the admission, with the exception of warfarin (Supporting Information, Appendix II). Post‐discharge use of anticoagulation therapy was measured for up to 35 days after discharge from the hospital. Use of mechanical prophylaxis during hospitalization was identified via charge codes for graduated compression stockings and charge codes indicating use of intermittent pneumatic compression devices and/or venous foot pumps. The appropriateness of prophylaxis was not assessed.
Analysis
The risk of VTE was estimated across an evaluation period of 180 days by measuring VTE occurrence and person‐time exposure. Inpatient VTE occurrence was defined as any nonprimary diagnosis of DVT and/or PE during the at‐risk hospitalization. VTE after discharge was defined as an ICD‐9‐CM diagnosis code, whether primary or secondary, for DVT or PE in the evaluation period during an emergency room or inpatient admission, or on an outpatient claim with 1 or more of the following confirmatory events: an emergency room or inpatient admission for VTE within 2 days of the outpatient diagnosis; a prescription claim for enoxaparin, fondaparinux, or UFH within 15 days after diagnosis; or a prescription claim for warfarin within 15 days after diagnosis and no evidence of atrial fibrillation or atrial flutter in the 6 months before the outpatient diagnosis for DVT or PE. Person‐time exposure was measured as the number of days from the hospital admission date to the first occurrence of VTE, or censoring at a subsequent at‐risk hospitalization, death, or 180 days after admission.
Cumulative risk of VTE over the 180‐day evaluation period was calculated by the Kaplan‐Meier product limit method of survival analysis and displayed for deciles of cumulative risk at 180 days after the hospital admission date. The risk of VTE at each point of time during the evaluation period (the hazard function) was first calculated on a daily basis and then smoothed via LOESS regression, a locally weighted regression procedure.
RESULTS
Patient Demographics
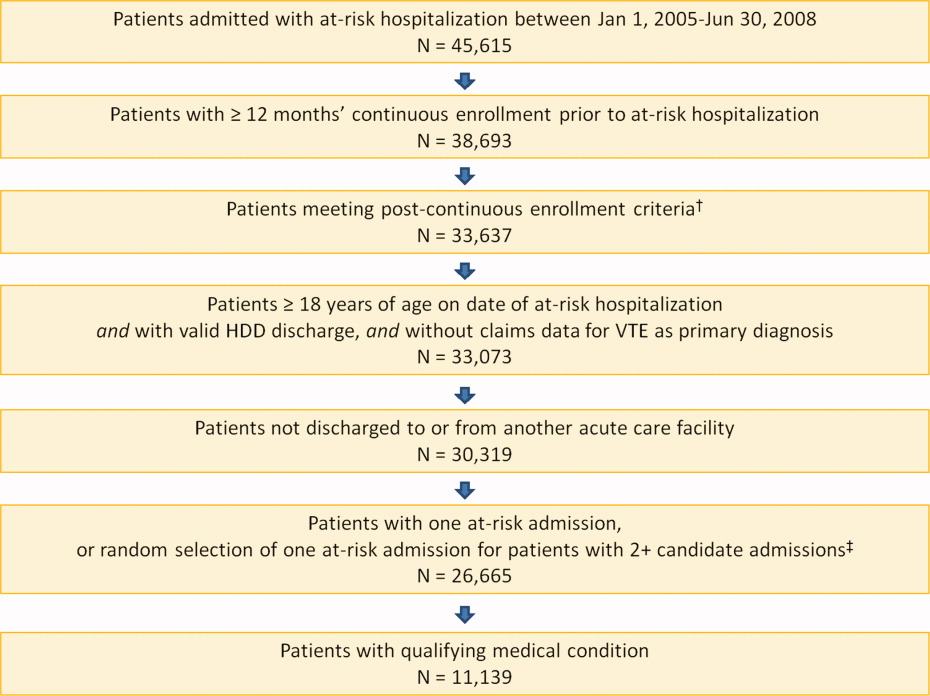
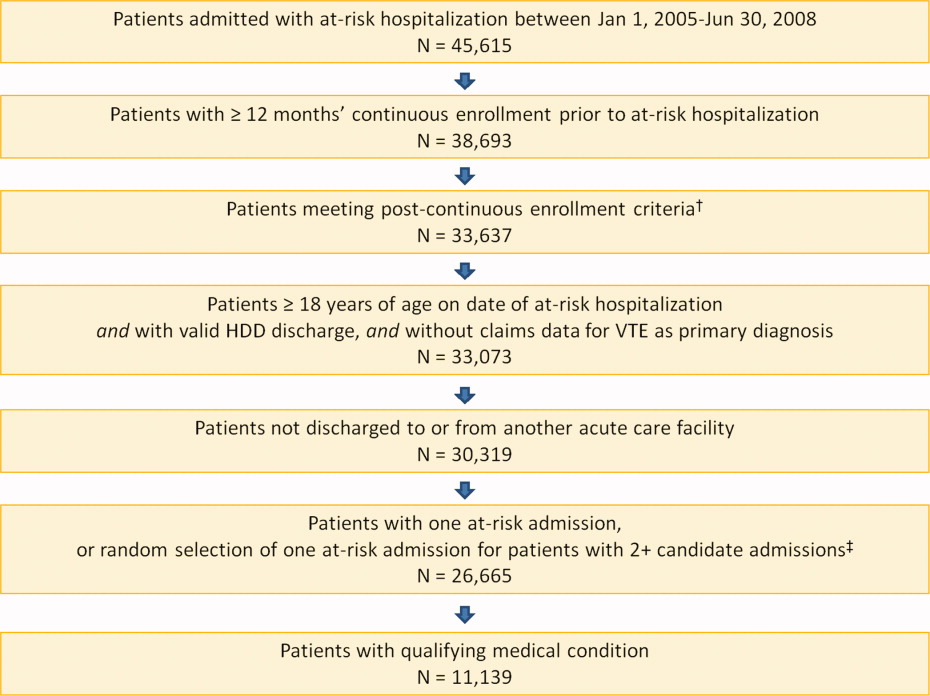
A total of 11,139 medical patients were included in the analysis (Figure 1), with a mean standard deviation (SD) age of 67.6 13.9 years, and 51.6% were women (Table 1). Of the reasons for admission to the hospital, 51.5% of patients were admitted for severe lung disease/COPD, 20.1% were admitted for cancer, 15.3% were admitted for CHF, and 13.1% were admitted for severe infectious disease. Most patients were treated in an urban hospital (87.5%), in a hospital without teaching status (87.9%), and in the South Census region (74.1%). The majority of patients were treated in medium‐sized to large care facilities. Risk factors for VTE during the baseline period included hospitalization for a medical condition with a high risk for VTE (75.6%), a prior at‐risk hospitalization (18.6%), cancer therapy (10.0% of all medical patients combined and 18.5% of cancer patients), trauma (9.2%), and previous VTE (4.3%).
| Characteristic | Medical Patients (N = 11,139) |
|---|---|
| |
| Gender | |
| Men | 5389 (48.4) |
| Women | 5750 (51.6) |
| Reason for hospitalization | |
| Cancer | 2243 (20.1) |
| CHF | 1705 (15.3) |
| Severe lung disease/COPD | 5736 (51.5) |
| Severe infectious disease | 1455 (13.1) |
| Age group, years | |
| 1834 | 230 (2.1) |
| 3544 | 442 (4.0) |
| 4554 | 1188 (10.7) |
| 5564 | 2644 (23.7) |
| 6574 | 2657 (23.9) |
| 7584 | 2969 (26.7) |
| 85 years | 1009 (9.1) |
| Median age SD, years | 67.6 13.9 |
| Primary payer* | |
| Medicare | 6819 (61.2) |
| Commercial | 4320 (38.8) |
| Geographical area | |
| Northeast | 122 (1.1) |
| North Central | 2649 (23.8) |
| South | 8258 (74.1) |
| West | 110 (1.0) |
| Urban location | 9743 (87.5) |
| Teaching hospital | 1345 (12.1) |
| Licensed bed size | |
| 1199 | 1621 (14.6) |
| 200299 | 2869 (25.8) |
| 300499 | 4005 (36.0) |
| 500 | 2644 (23.7) |
VTE Prophylaxis
Patients stayed in hospital for a mean SD duration of 5.3 5.3 days, varying from 4.6 3.9 days in patients with CHF to 6.7 6.5 days in patients with infectious disease, during which 46.7% of patients received pharmacological VTE prophylaxis. Inpatient pharmacological prophylaxis rates ranged from 64.1% in patients with CHF to 30.7% in patients with cancer (Table 2). Overall, the most commonly used form of inpatient pharmacological prophylaxis was enoxaparin (26.8% of all patients), followed by UFH (13.5% of all patients). Mechanical prophylaxis was received by 12.2% of all patients. Mean SD VTE prophylaxis duration during hospitalization was 5.0 4.7 days, varying from 4.2 4.0 days in patients with cancer to 6.2 5.5 days in patients with infectious disease.
| n (%) | Infectious Disease (n = 1455) | CHF (n = 1705) | Severe Lung Disease/COPD (n = 5736) | Cancer (n = 2243) | Any Medical (N = 11,139) |
|---|---|---|---|---|---|
| |||||
| Pharmacological prophylaxis during hospitalization* | 599 (41.2) | 1093 (64.1) | 2820 (49.2) | 688 (30.7) | 5200 (46.7) |
| Enoxaparin | 362 (24.9) | 466 (27.3) | 1877 (32.7) | 282 (12.6) | 2987 (26.8) |
| UFH | 191 (13.1) | 400 (23.5) | 527 (9.2) | 383 (17.1) | 1501 (13.5) |
| Warfarin | 135 (9.3) | 498 (29.2) | 622 (10.8) | 95 (4.2) | 1350 (12.1) |
| Dalteparin | 16 (1.1) | 21 (1.2) | 109 (1.9) | 16 (0.7) | 162 (1.5) |
| Fondaparinux | 5 (0.3) | 4 (0.2) | 22 (0.4) | 2 (0.1) | 33 (0.3) |
| Mechanical prophylaxis in hospital | 148 (10.2) | 65 (3.8) | 343 (6.0) | 803 (35.8) | 1359 (12.2) |
| Anticoagulation within 35 days after discharge | 104 (7.1) | 315 (18.5) | 397 (6.9) | 166 (7.4) | 982 (8.8) |
| Enoxaparin | 15 (1.0) | 14 (0.8) | 32 (0.6) | 25 (1.1) | 86 (0.8) |
| UFH | 17 (1.2) | 10 (0.6) | 23 (0.4) | 35 (1.6) | 85 (0.8) |
| Warfarin | 79 (5.4) | 302 (17.7) | 357 (6.2) | 116 (5.2) | 854 (7.7) |
| Dalteparin | 0 | 0 | 2 (<0.1) | 1 (<0.1) | 3 (<0.1) |
| Fondaparinux | 1 (0.1) | 0 | 0 | 2 (0.1) | 3 (<0.1) |
| Antiplatelet therapy within 35 days after discharge | 72 (4.9) | 217 (12.7) | 351 (6.1) | 53 (2.4) | 693 (6.2) |
In the 35 days after discharge, 8.8% of patients received anticoagulation therapy, most commonly warfarin (7.7%). The rate of outpatient prophylaxis was highest in patients hospitalized for CHF (18.5%) compared with other medical conditions (7%).
Time Course of VTE Risk and Hazard Function
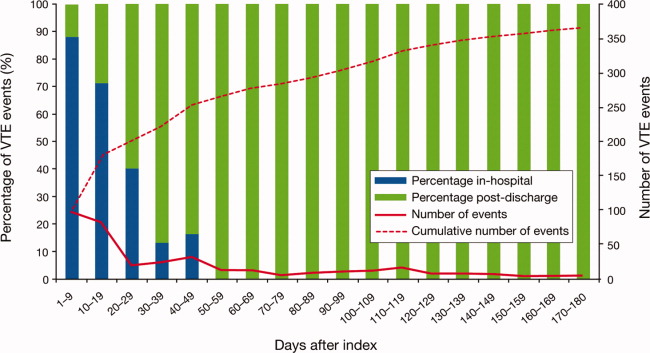
Overall, there were 366 symptomatic VTE events, representing a VTE rate of 3.3%. These events comprised 241 DVT‐only events, 98 PE‐only events, and 27 events with evidence of both DVT and PE. In total, 43.4% of events occurred during hospitalization (Figure 2). The VTE rate was 5.7% in patients with cancer (30.5% of events occurring in hospital), 4.3% with infectious disease (61.9% in hospital), 3.1% with CHF (54.7% in hospital), and 2.1% with severe lung disease/COPD (42.6% in hospital). The highest number of VTE events, 97 events (62 DVT only, 26 PE only, and 9 events both DVT and PE), occurred in the first 9 days after the hospital admission date, of which 87.6% were during hospitalization. During days 10‐19, there were 82 VTE events (50 DVT only, 24 PE only, and 8 both DVT and PE), 70.7% of which occurred in the hospital. Over the following 10‐day periods, VTE incidence gradually declined (Figure 2) and fluctuated at a background level of 4‐8 events during each 10‐day interval from 120 to 180 days.
The cumulative probability of VTE among all patients was 0.035 (Figure 3A). Half of the VTE risk had accumulated by day 23, and 75% had accumulated by day 71. By day 30, the proportion of cumulative risk was 52.6% overall, and ranged from 41.9% with cancer to 72.9% with infectious disease (Figure 3).
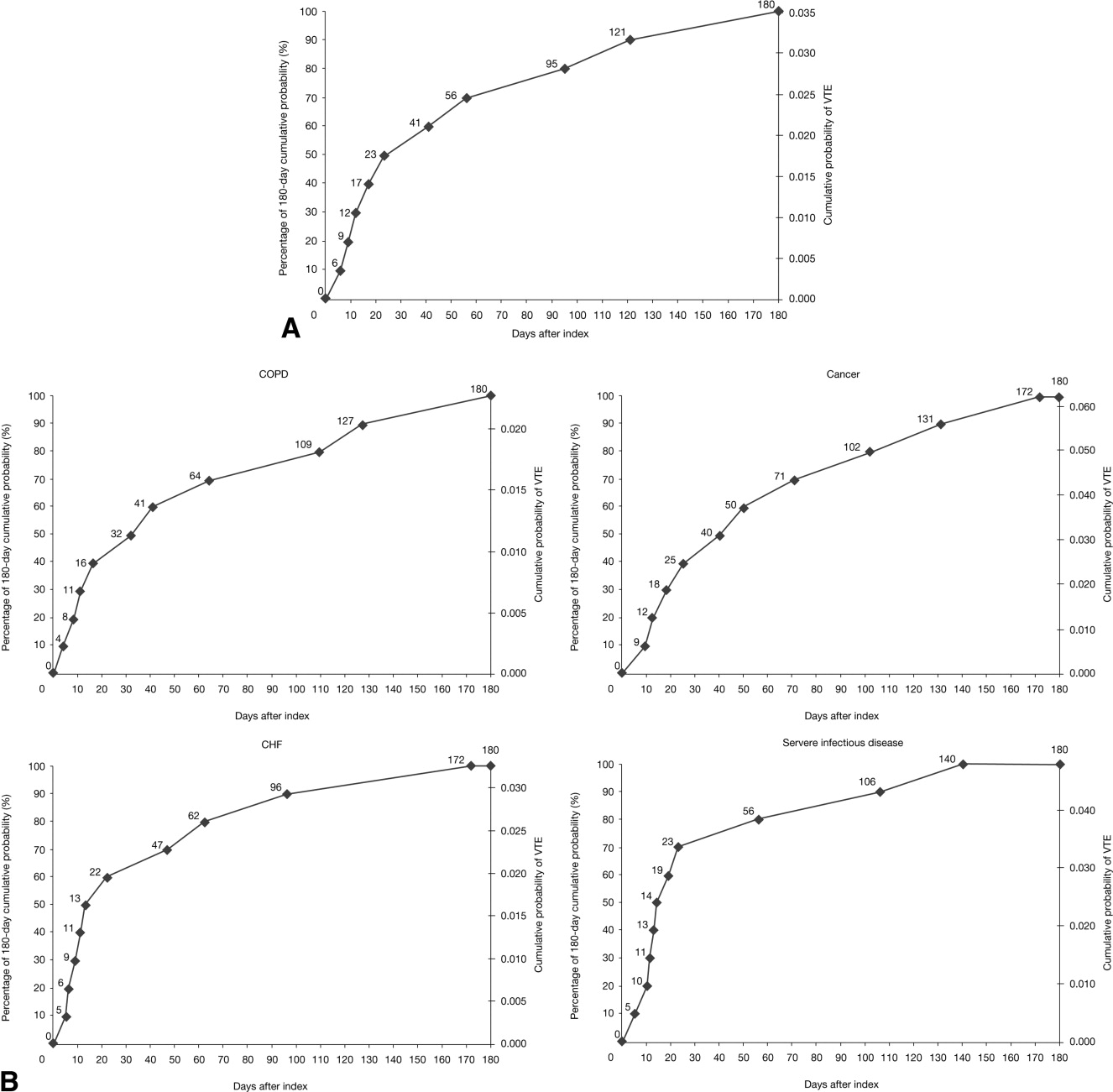
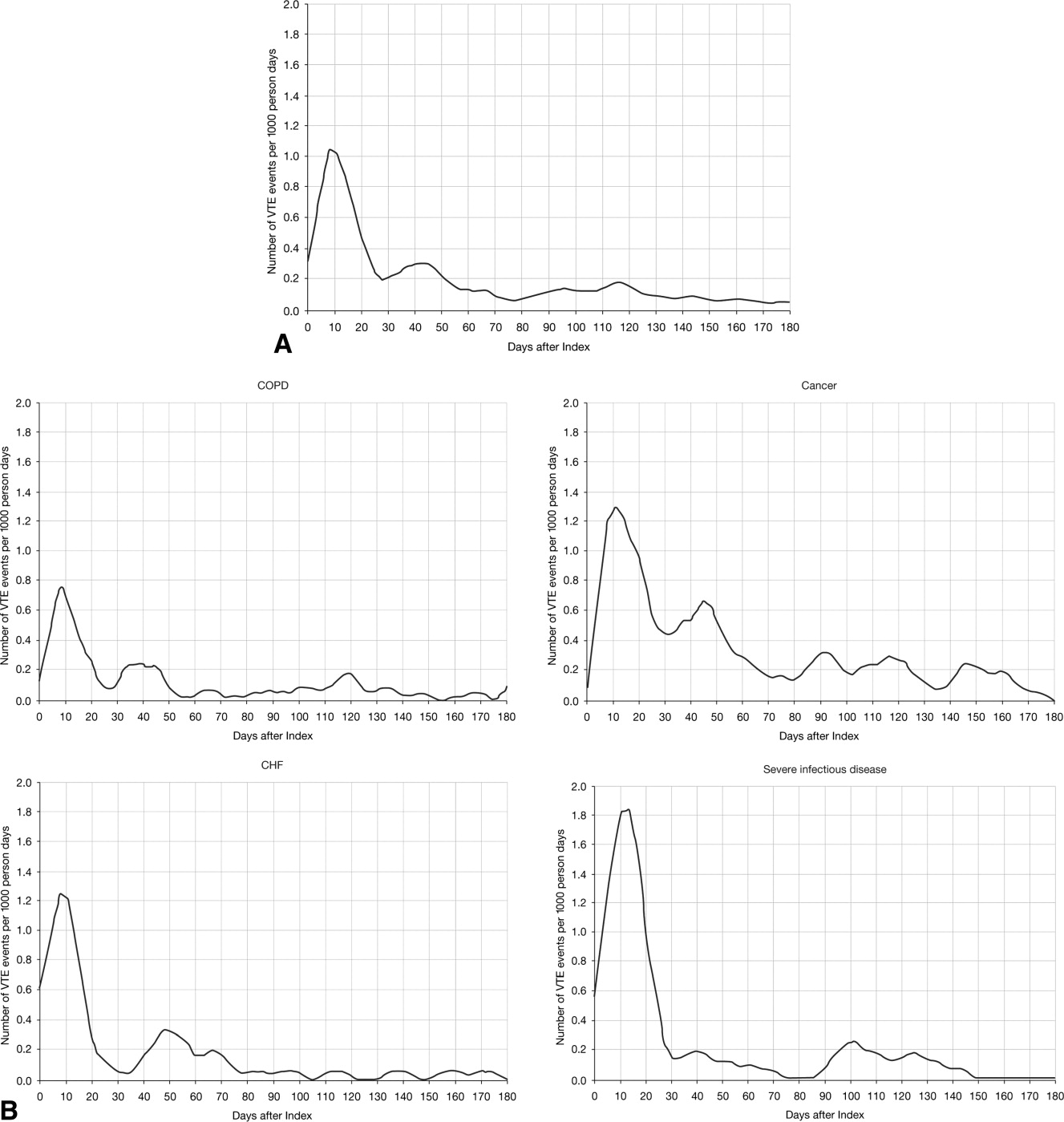
The VTE hazard peaked at approximately 1.05 VTE events per 1000 person‐days on day 8 after the hospital admission date overall (Figure 4A). The cumulative hazard at the peak day was 18.2% of the total VTE hazard over the 180‐day evaluation period. The hazard peak ranged from day 7 in patients with severe lung disease/COPD to day 12 in patients with infectious disease (Figure 4B). The cumulative hazard at the peak day was 39.7% for patients with infectious disease, 29.2% for patients with CHF, and approximately 19% for cancer or severe lung disease/COPD. After the peak risk day, the VTE hazard function decreased until the curve reached an inflection point, at day 28, when the cumulative risk was 51.8% (Figure 4A). After the inflection point, the VTE hazard increased to 0.3 VTE events per 1000 person‐days at approximately day 40‐45 and then decreased to <0.2 events per 1000 person‐days. The timing of the inflection varied by approximately 1 week across the medical illnesses (ranging from day 25 for severe lung disease/COPD to day 33 for CHF), with the cumulative risk at the inflection point ranging from 41.9% with cancer to 72.9% with infectious disease.
DISCUSSION
The results from this large, real‐world study provide new insights into the duration of risk of symptomatic VTE in medical patients and demonstrate that the number of VTE events was highest during days 0‐19, with the peak of VTE hazard at day 8. Half of the total 180‐day cumulative risk had been incurred by day 23 after hospital admission, and the period of greatest increased risk extended up to at least 30 days. Importantly, more than half of VTE events occurred after discharge (56.6%). A particularly high proportion of VTE events (69.5%) had occurred after discharge in patients with cancer. Although it was assumed that most VTE events that could be reasonably attributed to an at‐risk hospitalization would occur within 90 days as shown previously,4, 11 the 180‐day evaluation period was used to examine whether there was a prolonged period of continually diminished VTE risk from 90 to 180 days. Thus, events occurring within the later portions of the evaluation period may or may not have been attributable to the index hospitalization, potentially reflecting a background rate of VTE as noted above. Although these events are included in our estimate of the 180‐day cumulative risk of VTE, interpretation of the study results excluding such events is possible by examining the cumulative risk that had been incurred at each time point during the evaluation period.
Few other studies have assessed the duration of VTE risk in hospitalized medical patients. In a study by Spyropoulos et al,4 the median time to a DVT and/or PE event was 74 days, ranging from 62 days in severe infectious disease to 126 days in CHF. In another observational study that included patients who had recently been hospitalized but had not undergone surgery, 66.9% of patients who experienced DVT and/or PE events were diagnosed with DVT and/or PE within the first month after hospital discharge; 19.9% between months 1 and 2, and 13.2% between months 2 and 3.12
Fewer than half of the patients in the present study received thromboprophylaxis, which is consistent with other studies demonstrating the low prophylaxis rates in medical inpatients.9, 1315 In a recently published US study of discharge records that included 22,455 medical inpatients, prophylaxis rates were 59.4% in patients with CHF, 52.3% with cancer, 45.8% with severe lung disease/COPD, and 40.4% with infectious disease.14 Fewer than 10% of patients in the present study received prophylaxis after discharge, a result that is consistent with other studies.4, 9
The effect of extended prophylaxis in acutely ill medical patients with the LMWH enoxaparin beyond 6‐14 days has been investigated in the EXCLAIM study.16 This trial included approximately 5800 acutely ill medical patients at significant risk of developing VTE due to a recent reduction in mobility. Patients in the extended prophylaxis group had a lower risk of VTE (2.5% vs 4% for placebo; absolute risk reduction 1.5% [95.8% confidence interval 2.54% to 0.52%]), but had increased major bleeding events (0.8% vs 0.3% for placebo; absolute risk difference favoring placebo, 0.51% [95% confidence interval, 0.12% to 0.89%]). The patient populations with most benefit from an additional 28 days prophylaxis with enoxaparin, in addition to the usual short‐term prophylaxis of 10 days, were patients with restricted mobility (level 1; total bed rest/sedentary), elderly patients (age >75 years), and women. A limitation of the EXCLAIM trial is that estimates of efficacy and safety are difficult to interpret: after an interim analysis of adjudicated efficacy and safety outcomes, amendments were made to the original study protocol by changing eligibility criteria for patients with level 2 immobility (level 1 with bathroom privileges).16
The optimal duration of prophylaxis for medical patients has not been determined; prophylaxis is generally administered to at‐risk medical patients for the duration of hospitalization. In the current study, mean length of stay was 5.3 5.3 days overall. As hospital stays shorten, many medical patients who are prescribed inpatient prophylaxis alone are unlikely to receive the standard 6‐14 days of prophylaxis shown to be effective in clinical trials.68 Furthermore, the extended period of VTE risk in the present study and the finding that 56.6% of events occurred after discharge also suggest that current practices for inpatient prophylaxis alone may need to be evaluated.
This study reports real‐world data from a large, well‐defined population and obtains the incidence of symptomatic VTE events. Even though certain demographic data deviate from the national averagefor example, 74.1% of patients were treated in the South Census region, whereas this region is served by 37.6% of US hospitals17; 87.5% of hospitals had an urban location (compared with 60.1% of US hospitals18), and 85.4% of hospitals had a licensed bed size of at least 200 beds (compared with 28.2% of US hospitals, with the average US hospital having fewer than 100 beds19)these data may be beneficial in guiding policy and health care strategies for gaining understanding of the duration of risk for VTE.
Limitations of the study include characterization of the VTE risk period through examination of the cumulative risk and hazard of VTE across time, as the actual VTE risk period cannot be determined with exact precision. We used ICD‐9‐CM diagnosis coding to identify VTE. Since many cases of PE are asymptomatic and detected at autopsy,20 our approach may have missed such cases, as they would not have been recorded within the database. Furthermore, validation studies suggest that suboptimal specificity exists for ICD‐9‐CM diagnosis codes used to identify VTE.21 In an attempt to improve the specificity of our VTE identification algorithm, we required that post‐discharge VTE was recorded either during an emergency room or subsequent inpatient admission (which would be indicative of acute care for VTE) or on an outpatient claim with subsequent evidence of treatment for VTE. The true sensitivity and specificity of the VTE identification algorithms used for this study remain unknown, however, so the study findings should be interpreted in light of this limitation. The databases used for the analysis may not be representative of the US population as a whole; for example, this study used claims data from commercial and Medicare supplemental databases, which do not include Medicaid patients. Another limitation was that outpatient mechanical prophylaxis, such as graded compression stockings, was not captured due to over‐the‐counter availability. In addition, appropriateness of prophylaxis was not determined in this study, because these data could not be obtained from the claims database used. Further studies are warranted to obtain information on the incidence of VTE after hospitalization for medical illness in patients who received appropriate prophylaxis during hospitalization.
Finally, all dosages of a pharmacological agent were considered prophylactic only if a VTE event did not occur, with the exception of warfarin; any dose of warfarin was considered for prophylaxis, regardless of a VTE diagnosis. Warfarin may be used for purposes other than VTE prophylaxis (eg, prophylaxis for a thromboembolic cerebrovascular accident). The data source does not allow for identifying the exact reason for anticoagulation therapy with warfarin. Nonetheless, warfarin therapy will confer a decreased risk of VTE regardless of its purpose.
Results from this large cohort of medical patients indicate that symptomatic VTE risk is highest within the first 19 days after hospital admission (a period that may encompass both the duration of hospitalization as well as the period after discharge) with a considerable risk of VTE extending into the period after discharge. Receiving appropriate prophylaxis in‐hospital remains of great importance to prevent inpatient and likely post‐discharge VTE in patients with acute medical illness. In addition, given the time course of VTE events, with VTE incidence peaking at 8 days but with increased risk extending to 30 days, and the number of out‐of‐hospital VTE events incurred, the results of this study suggest that future research is warranted to investigate the risks and benefits of improving thromboprophylaxis practices in the period after hospitalization.
Acknowledgements
Funding Source: sanofi‐aventis U.S. provided funding to Thomson Reuters to perform this study. The authors received editorial/writing support from Tessa Hartog of Excerpta Medica in the preparation of the manuscript funded by sanofi‐aventis U.S.
Disclosure: Alpesh Amin has received research honorarium and is on the speakers bureau for sanofi‐aventis U.S. Otsuka Pharmaceutical, and Boehringer‐Ingelheim. Helen Varker, Nicole Princic, and Stephen Johnston are employees at Thomson Reuters, which received funding from sanofi‐aventis U.S. Jay Lin is an employee of Novosys Health, which received funding from sanofi‐aventis U.S. Stephen Thompson is an employee of sanofi‐aventis U.S.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S–453S.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160:809–815.
- . The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372.
- , , , . Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951–957.
- , , , et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study. Arch Intern Med. 2002;162:1245–1248.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–329.
- , , . Lack of thromboprophylaxis across the care continuum in US medical patients. Hosp Pract (Minneap). 2010;38:17–25.
- HCUP NIS Related Reports. Healthcare Cost and Utilization Project (HCUP), September 2008. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. Accessed June 2011.
- , . Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486.
- , , , , . Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471–1475.
- , , , . Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician's recommendations for at‐risk medical and surgical patients. J Hosp Med. 2009;4:E15–E21.
- , , , et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE start). J Thromb Thrombolysis. 2010;29:326–339.
- , , , et al. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103:736–748.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- American Society for Healthcare Engineering of the American Hospital Association. Overview of the Hospital Market, 2009. Available from: www.ashe.org/e2c/pdfs/energy/heg_ch2_background.pdf. Accessed June 2011.
- American Hospital Association. Fast Facts on US Hospitals, 2009. Available at: http://www.aha.org/aha/resource‐center/Statistics‐and‐Studies/fast‐facts.html. Accessed June 2011.
- American Hospital Association. AHA Annual Survey of Hospitals Database, 2009. Available from: http://www.ahadata.com/ahadata_app/index.jsp. Accessed June 2011.
- . The epidemiology of venous thromboembolism. Circulation. 2003;107(23 suppl 1):I4–I8.
- , , , et al. How valid is the ICD‐9‐CM based AHRQ patient safety indicator for postoperative venous thromboembolism? Med Care. 2009;47:1237–1243.
Patients who are hospitalized for acute medical illness are at an increased risk of developing venous thromboembolism (VTE), which comprises deep‐vein thrombosis (DVT) and pulmonary embolism (PE).13 In a recent real‐world study of 158,325 US medical patients by Spyropoulos et al,4 4.0% of patients developed DVT, 1.5% developed PE, and 0.2% developed both DVT and PE. Furthermore, results from a population‐based case‐control study indicate that hospitalization for medical illness accounted for a proportion of VTE events similar to that of hospitalization for surgery (22% and 24%, respectively).5
Thromboprophylaxis reduces VTE incidence in at‐risk medical patients and is recommended according to evidence‐based guidelines from the American College of Chest Physicians (ACCP).1 The ACCP guidelines advocate that acutely ill medical patients admitted to the hospital with congestive heart failure (CHF) or severe lung disease/chronic obstructive pulmonary disease (COPD) or those who are confined to bed and have one or more additional risk factors (including active cancer, previous VTE, sepsis, acute neurologic disease, or inflammatory bowel disease) receive pharmacological prophylaxis with lowmolecular weight heparin (LMWH), low‐dose unfractionated heparin (UFH), or fondaparinux.1 Although guidelines provide recommendations for the duration of prophylaxis after major orthopedic surgery, such recommendations are unavailable for medical patients. In clinical trials of acutely ill medical patients, prophylaxis regimens found to be effective were provided for a duration of hospitalization of 6‐14 days.68 The mean length of hospital stay for medical illnesses is decreasing and is currently shorter than 6‐14 days.9, 10
In clinical practice, the duration of VTE risk during and after hospitalization is not well understood in medical patients, particularly in the context of shortening hospital stays. Such information could, however, provide insight into whether current thromboprophylaxis practices reflect real‐world need. To gain a greater understanding of the period during which patients are at risk of VTE, this retrospective, observational study assessed the incidence and time course of symptomatic VTE events during and after hospitalization in a large population of US medical patients.
METHODS
Data and Patient Selection
This study employed linked administrative claims data and hospital billing data contained in the Thomson Reuters MarketScan Inpatient Drug Link File. This combines longitudinal patient‐level inpatient and outpatient medical and pharmaceutical claims data from the MarketScan Commercial claims data from the MarketScan Commercial Claims and Encounters (Commercial) and Medicare Supplemental and Coordination of Benefits (Medicare Supplemental) databases, with hospital discharge records detailing services used and drugs administered during a hospitalization, which are included in the Hospital Drug Database. The linked data sources enable analysis of a patient's experience before, during, and after a hospitalization. The present study was not designed to obtain bleeding rates.
The study cohort comprised patients considered to be at‐risk for VTE as a result of a medical hospitalization occurring between January 1, 2005, and December 31, 2008. At‐risk medical hospitalizations were those for which the primary diagnosis was for cancer, CHF, severe lung disease/COPD, or infectious disease (see Supporting Information, Appendix I, for International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes used to identify patients with medical illnesses). Included patients were required to be at least 18 years of age at the time of admission and were required to be continuously enrolled in their insurance benefits for at least 12 months before admission (the baseline period) and for at least 180 days after the admission date (the evaluation period) to ensure that all administrative claims data during that period were captured. Patients who died in‐hospital from any cause were exempted from the continuous enrollment criterion, as long as they had been continuously enrolled prior to inpatient death. Patients transferred from or discharged to another acute‐care facility were excluded because of the possibility for incomplete inpatient data capture. For patients who had multiple medical hospitalizations between January 1, 2005, and December 31, 2008, the hospitalization around which the analysis focused was randomly selected from the set of potential medical hospitalizations.
Prophylaxis
Pharmacological prophylaxis was identified via charge codes during hospitalization or via pharmacy claims after discharge for UFH, enoxaparin, dalteparin, warfarin, and fondaparinux. All dosages of a pharmacological agent were considered prophylactic only if there was no evidence of VTE during the admission, with the exception of warfarin (Supporting Information, Appendix II). Post‐discharge use of anticoagulation therapy was measured for up to 35 days after discharge from the hospital. Use of mechanical prophylaxis during hospitalization was identified via charge codes for graduated compression stockings and charge codes indicating use of intermittent pneumatic compression devices and/or venous foot pumps. The appropriateness of prophylaxis was not assessed.
Analysis
The risk of VTE was estimated across an evaluation period of 180 days by measuring VTE occurrence and person‐time exposure. Inpatient VTE occurrence was defined as any nonprimary diagnosis of DVT and/or PE during the at‐risk hospitalization. VTE after discharge was defined as an ICD‐9‐CM diagnosis code, whether primary or secondary, for DVT or PE in the evaluation period during an emergency room or inpatient admission, or on an outpatient claim with 1 or more of the following confirmatory events: an emergency room or inpatient admission for VTE within 2 days of the outpatient diagnosis; a prescription claim for enoxaparin, fondaparinux, or UFH within 15 days after diagnosis; or a prescription claim for warfarin within 15 days after diagnosis and no evidence of atrial fibrillation or atrial flutter in the 6 months before the outpatient diagnosis for DVT or PE. Person‐time exposure was measured as the number of days from the hospital admission date to the first occurrence of VTE, or censoring at a subsequent at‐risk hospitalization, death, or 180 days after admission.
Cumulative risk of VTE over the 180‐day evaluation period was calculated by the Kaplan‐Meier product limit method of survival analysis and displayed for deciles of cumulative risk at 180 days after the hospital admission date. The risk of VTE at each point of time during the evaluation period (the hazard function) was first calculated on a daily basis and then smoothed via LOESS regression, a locally weighted regression procedure.
RESULTS
Patient Demographics
A total of 11,139 medical patients were included in the analysis (Figure 1), with a mean standard deviation (SD) age of 67.6 13.9 years, and 51.6% were women (Table 1). Of the reasons for admission to the hospital, 51.5% of patients were admitted for severe lung disease/COPD, 20.1% were admitted for cancer, 15.3% were admitted for CHF, and 13.1% were admitted for severe infectious disease. Most patients were treated in an urban hospital (87.5%), in a hospital without teaching status (87.9%), and in the South Census region (74.1%). The majority of patients were treated in medium‐sized to large care facilities. Risk factors for VTE during the baseline period included hospitalization for a medical condition with a high risk for VTE (75.6%), a prior at‐risk hospitalization (18.6%), cancer therapy (10.0% of all medical patients combined and 18.5% of cancer patients), trauma (9.2%), and previous VTE (4.3%).

| Characteristic | Medical Patients (N = 11,139) |
|---|---|
| |
| Gender | |
| Men | 5389 (48.4) |
| Women | 5750 (51.6) |
| Reason for hospitalization | |
| Cancer | 2243 (20.1) |
| CHF | 1705 (15.3) |
| Severe lung disease/COPD | 5736 (51.5) |
| Severe infectious disease | 1455 (13.1) |
| Age group, years | |
| 1834 | 230 (2.1) |
| 3544 | 442 (4.0) |
| 4554 | 1188 (10.7) |
| 5564 | 2644 (23.7) |
| 6574 | 2657 (23.9) |
| 7584 | 2969 (26.7) |
| 85 years | 1009 (9.1) |
| Median age SD, years | 67.6 13.9 |
| Primary payer* | |
| Medicare | 6819 (61.2) |
| Commercial | 4320 (38.8) |
| Geographical area | |
| Northeast | 122 (1.1) |
| North Central | 2649 (23.8) |
| South | 8258 (74.1) |
| West | 110 (1.0) |
| Urban location | 9743 (87.5) |
| Teaching hospital | 1345 (12.1) |
| Licensed bed size | |
| 1199 | 1621 (14.6) |
| 200299 | 2869 (25.8) |
| 300499 | 4005 (36.0) |
| 500 | 2644 (23.7) |
VTE Prophylaxis
Patients stayed in hospital for a mean SD duration of 5.3 5.3 days, varying from 4.6 3.9 days in patients with CHF to 6.7 6.5 days in patients with infectious disease, during which 46.7% of patients received pharmacological VTE prophylaxis. Inpatient pharmacological prophylaxis rates ranged from 64.1% in patients with CHF to 30.7% in patients with cancer (Table 2). Overall, the most commonly used form of inpatient pharmacological prophylaxis was enoxaparin (26.8% of all patients), followed by UFH (13.5% of all patients). Mechanical prophylaxis was received by 12.2% of all patients. Mean SD VTE prophylaxis duration during hospitalization was 5.0 4.7 days, varying from 4.2 4.0 days in patients with cancer to 6.2 5.5 days in patients with infectious disease.
| n (%) | Infectious Disease (n = 1455) | CHF (n = 1705) | Severe Lung Disease/COPD (n = 5736) | Cancer (n = 2243) | Any Medical (N = 11,139) |
|---|---|---|---|---|---|
| |||||
| Pharmacological prophylaxis during hospitalization* | 599 (41.2) | 1093 (64.1) | 2820 (49.2) | 688 (30.7) | 5200 (46.7) |
| Enoxaparin | 362 (24.9) | 466 (27.3) | 1877 (32.7) | 282 (12.6) | 2987 (26.8) |
| UFH | 191 (13.1) | 400 (23.5) | 527 (9.2) | 383 (17.1) | 1501 (13.5) |
| Warfarin | 135 (9.3) | 498 (29.2) | 622 (10.8) | 95 (4.2) | 1350 (12.1) |
| Dalteparin | 16 (1.1) | 21 (1.2) | 109 (1.9) | 16 (0.7) | 162 (1.5) |
| Fondaparinux | 5 (0.3) | 4 (0.2) | 22 (0.4) | 2 (0.1) | 33 (0.3) |
| Mechanical prophylaxis in hospital | 148 (10.2) | 65 (3.8) | 343 (6.0) | 803 (35.8) | 1359 (12.2) |
| Anticoagulation within 35 days after discharge | 104 (7.1) | 315 (18.5) | 397 (6.9) | 166 (7.4) | 982 (8.8) |
| Enoxaparin | 15 (1.0) | 14 (0.8) | 32 (0.6) | 25 (1.1) | 86 (0.8) |
| UFH | 17 (1.2) | 10 (0.6) | 23 (0.4) | 35 (1.6) | 85 (0.8) |
| Warfarin | 79 (5.4) | 302 (17.7) | 357 (6.2) | 116 (5.2) | 854 (7.7) |
| Dalteparin | 0 | 0 | 2 (<0.1) | 1 (<0.1) | 3 (<0.1) |
| Fondaparinux | 1 (0.1) | 0 | 0 | 2 (0.1) | 3 (<0.1) |
| Antiplatelet therapy within 35 days after discharge | 72 (4.9) | 217 (12.7) | 351 (6.1) | 53 (2.4) | 693 (6.2) |
In the 35 days after discharge, 8.8% of patients received anticoagulation therapy, most commonly warfarin (7.7%). The rate of outpatient prophylaxis was highest in patients hospitalized for CHF (18.5%) compared with other medical conditions (7%).
Time Course of VTE Risk and Hazard Function
Overall, there were 366 symptomatic VTE events, representing a VTE rate of 3.3%. These events comprised 241 DVT‐only events, 98 PE‐only events, and 27 events with evidence of both DVT and PE. In total, 43.4% of events occurred during hospitalization (Figure 2). The VTE rate was 5.7% in patients with cancer (30.5% of events occurring in hospital), 4.3% with infectious disease (61.9% in hospital), 3.1% with CHF (54.7% in hospital), and 2.1% with severe lung disease/COPD (42.6% in hospital). The highest number of VTE events, 97 events (62 DVT only, 26 PE only, and 9 events both DVT and PE), occurred in the first 9 days after the hospital admission date, of which 87.6% were during hospitalization. During days 10‐19, there were 82 VTE events (50 DVT only, 24 PE only, and 8 both DVT and PE), 70.7% of which occurred in the hospital. Over the following 10‐day periods, VTE incidence gradually declined (Figure 2) and fluctuated at a background level of 4‐8 events during each 10‐day interval from 120 to 180 days.

The cumulative probability of VTE among all patients was 0.035 (Figure 3A). Half of the VTE risk had accumulated by day 23, and 75% had accumulated by day 71. By day 30, the proportion of cumulative risk was 52.6% overall, and ranged from 41.9% with cancer to 72.9% with infectious disease (Figure 3).

The VTE hazard peaked at approximately 1.05 VTE events per 1000 person‐days on day 8 after the hospital admission date overall (Figure 4A). The cumulative hazard at the peak day was 18.2% of the total VTE hazard over the 180‐day evaluation period. The hazard peak ranged from day 7 in patients with severe lung disease/COPD to day 12 in patients with infectious disease (Figure 4B). The cumulative hazard at the peak day was 39.7% for patients with infectious disease, 29.2% for patients with CHF, and approximately 19% for cancer or severe lung disease/COPD. After the peak risk day, the VTE hazard function decreased until the curve reached an inflection point, at day 28, when the cumulative risk was 51.8% (Figure 4A). After the inflection point, the VTE hazard increased to 0.3 VTE events per 1000 person‐days at approximately day 40‐45 and then decreased to <0.2 events per 1000 person‐days. The timing of the inflection varied by approximately 1 week across the medical illnesses (ranging from day 25 for severe lung disease/COPD to day 33 for CHF), with the cumulative risk at the inflection point ranging from 41.9% with cancer to 72.9% with infectious disease.

DISCUSSION
The results from this large, real‐world study provide new insights into the duration of risk of symptomatic VTE in medical patients and demonstrate that the number of VTE events was highest during days 0‐19, with the peak of VTE hazard at day 8. Half of the total 180‐day cumulative risk had been incurred by day 23 after hospital admission, and the period of greatest increased risk extended up to at least 30 days. Importantly, more than half of VTE events occurred after discharge (56.6%). A particularly high proportion of VTE events (69.5%) had occurred after discharge in patients with cancer. Although it was assumed that most VTE events that could be reasonably attributed to an at‐risk hospitalization would occur within 90 days as shown previously,4, 11 the 180‐day evaluation period was used to examine whether there was a prolonged period of continually diminished VTE risk from 90 to 180 days. Thus, events occurring within the later portions of the evaluation period may or may not have been attributable to the index hospitalization, potentially reflecting a background rate of VTE as noted above. Although these events are included in our estimate of the 180‐day cumulative risk of VTE, interpretation of the study results excluding such events is possible by examining the cumulative risk that had been incurred at each time point during the evaluation period.
Few other studies have assessed the duration of VTE risk in hospitalized medical patients. In a study by Spyropoulos et al,4 the median time to a DVT and/or PE event was 74 days, ranging from 62 days in severe infectious disease to 126 days in CHF. In another observational study that included patients who had recently been hospitalized but had not undergone surgery, 66.9% of patients who experienced DVT and/or PE events were diagnosed with DVT and/or PE within the first month after hospital discharge; 19.9% between months 1 and 2, and 13.2% between months 2 and 3.12
Fewer than half of the patients in the present study received thromboprophylaxis, which is consistent with other studies demonstrating the low prophylaxis rates in medical inpatients.9, 1315 In a recently published US study of discharge records that included 22,455 medical inpatients, prophylaxis rates were 59.4% in patients with CHF, 52.3% with cancer, 45.8% with severe lung disease/COPD, and 40.4% with infectious disease.14 Fewer than 10% of patients in the present study received prophylaxis after discharge, a result that is consistent with other studies.4, 9
The effect of extended prophylaxis in acutely ill medical patients with the LMWH enoxaparin beyond 6‐14 days has been investigated in the EXCLAIM study.16 This trial included approximately 5800 acutely ill medical patients at significant risk of developing VTE due to a recent reduction in mobility. Patients in the extended prophylaxis group had a lower risk of VTE (2.5% vs 4% for placebo; absolute risk reduction 1.5% [95.8% confidence interval 2.54% to 0.52%]), but had increased major bleeding events (0.8% vs 0.3% for placebo; absolute risk difference favoring placebo, 0.51% [95% confidence interval, 0.12% to 0.89%]). The patient populations with most benefit from an additional 28 days prophylaxis with enoxaparin, in addition to the usual short‐term prophylaxis of 10 days, were patients with restricted mobility (level 1; total bed rest/sedentary), elderly patients (age >75 years), and women. A limitation of the EXCLAIM trial is that estimates of efficacy and safety are difficult to interpret: after an interim analysis of adjudicated efficacy and safety outcomes, amendments were made to the original study protocol by changing eligibility criteria for patients with level 2 immobility (level 1 with bathroom privileges).16
The optimal duration of prophylaxis for medical patients has not been determined; prophylaxis is generally administered to at‐risk medical patients for the duration of hospitalization. In the current study, mean length of stay was 5.3 5.3 days overall. As hospital stays shorten, many medical patients who are prescribed inpatient prophylaxis alone are unlikely to receive the standard 6‐14 days of prophylaxis shown to be effective in clinical trials.68 Furthermore, the extended period of VTE risk in the present study and the finding that 56.6% of events occurred after discharge also suggest that current practices for inpatient prophylaxis alone may need to be evaluated.
This study reports real‐world data from a large, well‐defined population and obtains the incidence of symptomatic VTE events. Even though certain demographic data deviate from the national averagefor example, 74.1% of patients were treated in the South Census region, whereas this region is served by 37.6% of US hospitals17; 87.5% of hospitals had an urban location (compared with 60.1% of US hospitals18), and 85.4% of hospitals had a licensed bed size of at least 200 beds (compared with 28.2% of US hospitals, with the average US hospital having fewer than 100 beds19)these data may be beneficial in guiding policy and health care strategies for gaining understanding of the duration of risk for VTE.
Limitations of the study include characterization of the VTE risk period through examination of the cumulative risk and hazard of VTE across time, as the actual VTE risk period cannot be determined with exact precision. We used ICD‐9‐CM diagnosis coding to identify VTE. Since many cases of PE are asymptomatic and detected at autopsy,20 our approach may have missed such cases, as they would not have been recorded within the database. Furthermore, validation studies suggest that suboptimal specificity exists for ICD‐9‐CM diagnosis codes used to identify VTE.21 In an attempt to improve the specificity of our VTE identification algorithm, we required that post‐discharge VTE was recorded either during an emergency room or subsequent inpatient admission (which would be indicative of acute care for VTE) or on an outpatient claim with subsequent evidence of treatment for VTE. The true sensitivity and specificity of the VTE identification algorithms used for this study remain unknown, however, so the study findings should be interpreted in light of this limitation. The databases used for the analysis may not be representative of the US population as a whole; for example, this study used claims data from commercial and Medicare supplemental databases, which do not include Medicaid patients. Another limitation was that outpatient mechanical prophylaxis, such as graded compression stockings, was not captured due to over‐the‐counter availability. In addition, appropriateness of prophylaxis was not determined in this study, because these data could not be obtained from the claims database used. Further studies are warranted to obtain information on the incidence of VTE after hospitalization for medical illness in patients who received appropriate prophylaxis during hospitalization.
Finally, all dosages of a pharmacological agent were considered prophylactic only if a VTE event did not occur, with the exception of warfarin; any dose of warfarin was considered for prophylaxis, regardless of a VTE diagnosis. Warfarin may be used for purposes other than VTE prophylaxis (eg, prophylaxis for a thromboembolic cerebrovascular accident). The data source does not allow for identifying the exact reason for anticoagulation therapy with warfarin. Nonetheless, warfarin therapy will confer a decreased risk of VTE regardless of its purpose.
Results from this large cohort of medical patients indicate that symptomatic VTE risk is highest within the first 19 days after hospital admission (a period that may encompass both the duration of hospitalization as well as the period after discharge) with a considerable risk of VTE extending into the period after discharge. Receiving appropriate prophylaxis in‐hospital remains of great importance to prevent inpatient and likely post‐discharge VTE in patients with acute medical illness. In addition, given the time course of VTE events, with VTE incidence peaking at 8 days but with increased risk extending to 30 days, and the number of out‐of‐hospital VTE events incurred, the results of this study suggest that future research is warranted to investigate the risks and benefits of improving thromboprophylaxis practices in the period after hospitalization.
Acknowledgements
Funding Source: sanofi‐aventis U.S. provided funding to Thomson Reuters to perform this study. The authors received editorial/writing support from Tessa Hartog of Excerpta Medica in the preparation of the manuscript funded by sanofi‐aventis U.S.
Disclosure: Alpesh Amin has received research honorarium and is on the speakers bureau for sanofi‐aventis U.S. Otsuka Pharmaceutical, and Boehringer‐Ingelheim. Helen Varker, Nicole Princic, and Stephen Johnston are employees at Thomson Reuters, which received funding from sanofi‐aventis U.S. Jay Lin is an employee of Novosys Health, which received funding from sanofi‐aventis U.S. Stephen Thompson is an employee of sanofi‐aventis U.S.
Patients who are hospitalized for acute medical illness are at an increased risk of developing venous thromboembolism (VTE), which comprises deep‐vein thrombosis (DVT) and pulmonary embolism (PE).13 In a recent real‐world study of 158,325 US medical patients by Spyropoulos et al,4 4.0% of patients developed DVT, 1.5% developed PE, and 0.2% developed both DVT and PE. Furthermore, results from a population‐based case‐control study indicate that hospitalization for medical illness accounted for a proportion of VTE events similar to that of hospitalization for surgery (22% and 24%, respectively).5
Thromboprophylaxis reduces VTE incidence in at‐risk medical patients and is recommended according to evidence‐based guidelines from the American College of Chest Physicians (ACCP).1 The ACCP guidelines advocate that acutely ill medical patients admitted to the hospital with congestive heart failure (CHF) or severe lung disease/chronic obstructive pulmonary disease (COPD) or those who are confined to bed and have one or more additional risk factors (including active cancer, previous VTE, sepsis, acute neurologic disease, or inflammatory bowel disease) receive pharmacological prophylaxis with lowmolecular weight heparin (LMWH), low‐dose unfractionated heparin (UFH), or fondaparinux.1 Although guidelines provide recommendations for the duration of prophylaxis after major orthopedic surgery, such recommendations are unavailable for medical patients. In clinical trials of acutely ill medical patients, prophylaxis regimens found to be effective were provided for a duration of hospitalization of 6‐14 days.68 The mean length of hospital stay for medical illnesses is decreasing and is currently shorter than 6‐14 days.9, 10
In clinical practice, the duration of VTE risk during and after hospitalization is not well understood in medical patients, particularly in the context of shortening hospital stays. Such information could, however, provide insight into whether current thromboprophylaxis practices reflect real‐world need. To gain a greater understanding of the period during which patients are at risk of VTE, this retrospective, observational study assessed the incidence and time course of symptomatic VTE events during and after hospitalization in a large population of US medical patients.
METHODS
Data and Patient Selection
This study employed linked administrative claims data and hospital billing data contained in the Thomson Reuters MarketScan Inpatient Drug Link File. This combines longitudinal patient‐level inpatient and outpatient medical and pharmaceutical claims data from the MarketScan Commercial claims data from the MarketScan Commercial Claims and Encounters (Commercial) and Medicare Supplemental and Coordination of Benefits (Medicare Supplemental) databases, with hospital discharge records detailing services used and drugs administered during a hospitalization, which are included in the Hospital Drug Database. The linked data sources enable analysis of a patient's experience before, during, and after a hospitalization. The present study was not designed to obtain bleeding rates.
The study cohort comprised patients considered to be at‐risk for VTE as a result of a medical hospitalization occurring between January 1, 2005, and December 31, 2008. At‐risk medical hospitalizations were those for which the primary diagnosis was for cancer, CHF, severe lung disease/COPD, or infectious disease (see Supporting Information, Appendix I, for International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes used to identify patients with medical illnesses). Included patients were required to be at least 18 years of age at the time of admission and were required to be continuously enrolled in their insurance benefits for at least 12 months before admission (the baseline period) and for at least 180 days after the admission date (the evaluation period) to ensure that all administrative claims data during that period were captured. Patients who died in‐hospital from any cause were exempted from the continuous enrollment criterion, as long as they had been continuously enrolled prior to inpatient death. Patients transferred from or discharged to another acute‐care facility were excluded because of the possibility for incomplete inpatient data capture. For patients who had multiple medical hospitalizations between January 1, 2005, and December 31, 2008, the hospitalization around which the analysis focused was randomly selected from the set of potential medical hospitalizations.
Prophylaxis
Pharmacological prophylaxis was identified via charge codes during hospitalization or via pharmacy claims after discharge for UFH, enoxaparin, dalteparin, warfarin, and fondaparinux. All dosages of a pharmacological agent were considered prophylactic only if there was no evidence of VTE during the admission, with the exception of warfarin (Supporting Information, Appendix II). Post‐discharge use of anticoagulation therapy was measured for up to 35 days after discharge from the hospital. Use of mechanical prophylaxis during hospitalization was identified via charge codes for graduated compression stockings and charge codes indicating use of intermittent pneumatic compression devices and/or venous foot pumps. The appropriateness of prophylaxis was not assessed.
Analysis
The risk of VTE was estimated across an evaluation period of 180 days by measuring VTE occurrence and person‐time exposure. Inpatient VTE occurrence was defined as any nonprimary diagnosis of DVT and/or PE during the at‐risk hospitalization. VTE after discharge was defined as an ICD‐9‐CM diagnosis code, whether primary or secondary, for DVT or PE in the evaluation period during an emergency room or inpatient admission, or on an outpatient claim with 1 or more of the following confirmatory events: an emergency room or inpatient admission for VTE within 2 days of the outpatient diagnosis; a prescription claim for enoxaparin, fondaparinux, or UFH within 15 days after diagnosis; or a prescription claim for warfarin within 15 days after diagnosis and no evidence of atrial fibrillation or atrial flutter in the 6 months before the outpatient diagnosis for DVT or PE. Person‐time exposure was measured as the number of days from the hospital admission date to the first occurrence of VTE, or censoring at a subsequent at‐risk hospitalization, death, or 180 days after admission.
Cumulative risk of VTE over the 180‐day evaluation period was calculated by the Kaplan‐Meier product limit method of survival analysis and displayed for deciles of cumulative risk at 180 days after the hospital admission date. The risk of VTE at each point of time during the evaluation period (the hazard function) was first calculated on a daily basis and then smoothed via LOESS regression, a locally weighted regression procedure.
RESULTS
Patient Demographics
A total of 11,139 medical patients were included in the analysis (Figure 1), with a mean standard deviation (SD) age of 67.6 13.9 years, and 51.6% were women (Table 1). Of the reasons for admission to the hospital, 51.5% of patients were admitted for severe lung disease/COPD, 20.1% were admitted for cancer, 15.3% were admitted for CHF, and 13.1% were admitted for severe infectious disease. Most patients were treated in an urban hospital (87.5%), in a hospital without teaching status (87.9%), and in the South Census region (74.1%). The majority of patients were treated in medium‐sized to large care facilities. Risk factors for VTE during the baseline period included hospitalization for a medical condition with a high risk for VTE (75.6%), a prior at‐risk hospitalization (18.6%), cancer therapy (10.0% of all medical patients combined and 18.5% of cancer patients), trauma (9.2%), and previous VTE (4.3%).

| Characteristic | Medical Patients (N = 11,139) |
|---|---|
| |
| Gender | |
| Men | 5389 (48.4) |
| Women | 5750 (51.6) |
| Reason for hospitalization | |
| Cancer | 2243 (20.1) |
| CHF | 1705 (15.3) |
| Severe lung disease/COPD | 5736 (51.5) |
| Severe infectious disease | 1455 (13.1) |
| Age group, years | |
| 1834 | 230 (2.1) |
| 3544 | 442 (4.0) |
| 4554 | 1188 (10.7) |
| 5564 | 2644 (23.7) |
| 6574 | 2657 (23.9) |
| 7584 | 2969 (26.7) |
| 85 years | 1009 (9.1) |
| Median age SD, years | 67.6 13.9 |
| Primary payer* | |
| Medicare | 6819 (61.2) |
| Commercial | 4320 (38.8) |
| Geographical area | |
| Northeast | 122 (1.1) |
| North Central | 2649 (23.8) |
| South | 8258 (74.1) |
| West | 110 (1.0) |
| Urban location | 9743 (87.5) |
| Teaching hospital | 1345 (12.1) |
| Licensed bed size | |
| 1199 | 1621 (14.6) |
| 200299 | 2869 (25.8) |
| 300499 | 4005 (36.0) |
| 500 | 2644 (23.7) |
VTE Prophylaxis
Patients stayed in hospital for a mean SD duration of 5.3 5.3 days, varying from 4.6 3.9 days in patients with CHF to 6.7 6.5 days in patients with infectious disease, during which 46.7% of patients received pharmacological VTE prophylaxis. Inpatient pharmacological prophylaxis rates ranged from 64.1% in patients with CHF to 30.7% in patients with cancer (Table 2). Overall, the most commonly used form of inpatient pharmacological prophylaxis was enoxaparin (26.8% of all patients), followed by UFH (13.5% of all patients). Mechanical prophylaxis was received by 12.2% of all patients. Mean SD VTE prophylaxis duration during hospitalization was 5.0 4.7 days, varying from 4.2 4.0 days in patients with cancer to 6.2 5.5 days in patients with infectious disease.
| n (%) | Infectious Disease (n = 1455) | CHF (n = 1705) | Severe Lung Disease/COPD (n = 5736) | Cancer (n = 2243) | Any Medical (N = 11,139) |
|---|---|---|---|---|---|
| |||||
| Pharmacological prophylaxis during hospitalization* | 599 (41.2) | 1093 (64.1) | 2820 (49.2) | 688 (30.7) | 5200 (46.7) |
| Enoxaparin | 362 (24.9) | 466 (27.3) | 1877 (32.7) | 282 (12.6) | 2987 (26.8) |
| UFH | 191 (13.1) | 400 (23.5) | 527 (9.2) | 383 (17.1) | 1501 (13.5) |
| Warfarin | 135 (9.3) | 498 (29.2) | 622 (10.8) | 95 (4.2) | 1350 (12.1) |
| Dalteparin | 16 (1.1) | 21 (1.2) | 109 (1.9) | 16 (0.7) | 162 (1.5) |
| Fondaparinux | 5 (0.3) | 4 (0.2) | 22 (0.4) | 2 (0.1) | 33 (0.3) |
| Mechanical prophylaxis in hospital | 148 (10.2) | 65 (3.8) | 343 (6.0) | 803 (35.8) | 1359 (12.2) |
| Anticoagulation within 35 days after discharge | 104 (7.1) | 315 (18.5) | 397 (6.9) | 166 (7.4) | 982 (8.8) |
| Enoxaparin | 15 (1.0) | 14 (0.8) | 32 (0.6) | 25 (1.1) | 86 (0.8) |
| UFH | 17 (1.2) | 10 (0.6) | 23 (0.4) | 35 (1.6) | 85 (0.8) |
| Warfarin | 79 (5.4) | 302 (17.7) | 357 (6.2) | 116 (5.2) | 854 (7.7) |
| Dalteparin | 0 | 0 | 2 (<0.1) | 1 (<0.1) | 3 (<0.1) |
| Fondaparinux | 1 (0.1) | 0 | 0 | 2 (0.1) | 3 (<0.1) |
| Antiplatelet therapy within 35 days after discharge | 72 (4.9) | 217 (12.7) | 351 (6.1) | 53 (2.4) | 693 (6.2) |
In the 35 days after discharge, 8.8% of patients received anticoagulation therapy, most commonly warfarin (7.7%). The rate of outpatient prophylaxis was highest in patients hospitalized for CHF (18.5%) compared with other medical conditions (7%).
Time Course of VTE Risk and Hazard Function
Overall, there were 366 symptomatic VTE events, representing a VTE rate of 3.3%. These events comprised 241 DVT‐only events, 98 PE‐only events, and 27 events with evidence of both DVT and PE. In total, 43.4% of events occurred during hospitalization (Figure 2). The VTE rate was 5.7% in patients with cancer (30.5% of events occurring in hospital), 4.3% with infectious disease (61.9% in hospital), 3.1% with CHF (54.7% in hospital), and 2.1% with severe lung disease/COPD (42.6% in hospital). The highest number of VTE events, 97 events (62 DVT only, 26 PE only, and 9 events both DVT and PE), occurred in the first 9 days after the hospital admission date, of which 87.6% were during hospitalization. During days 10‐19, there were 82 VTE events (50 DVT only, 24 PE only, and 8 both DVT and PE), 70.7% of which occurred in the hospital. Over the following 10‐day periods, VTE incidence gradually declined (Figure 2) and fluctuated at a background level of 4‐8 events during each 10‐day interval from 120 to 180 days.

The cumulative probability of VTE among all patients was 0.035 (Figure 3A). Half of the VTE risk had accumulated by day 23, and 75% had accumulated by day 71. By day 30, the proportion of cumulative risk was 52.6% overall, and ranged from 41.9% with cancer to 72.9% with infectious disease (Figure 3).

The VTE hazard peaked at approximately 1.05 VTE events per 1000 person‐days on day 8 after the hospital admission date overall (Figure 4A). The cumulative hazard at the peak day was 18.2% of the total VTE hazard over the 180‐day evaluation period. The hazard peak ranged from day 7 in patients with severe lung disease/COPD to day 12 in patients with infectious disease (Figure 4B). The cumulative hazard at the peak day was 39.7% for patients with infectious disease, 29.2% for patients with CHF, and approximately 19% for cancer or severe lung disease/COPD. After the peak risk day, the VTE hazard function decreased until the curve reached an inflection point, at day 28, when the cumulative risk was 51.8% (Figure 4A). After the inflection point, the VTE hazard increased to 0.3 VTE events per 1000 person‐days at approximately day 40‐45 and then decreased to <0.2 events per 1000 person‐days. The timing of the inflection varied by approximately 1 week across the medical illnesses (ranging from day 25 for severe lung disease/COPD to day 33 for CHF), with the cumulative risk at the inflection point ranging from 41.9% with cancer to 72.9% with infectious disease.

DISCUSSION
The results from this large, real‐world study provide new insights into the duration of risk of symptomatic VTE in medical patients and demonstrate that the number of VTE events was highest during days 0‐19, with the peak of VTE hazard at day 8. Half of the total 180‐day cumulative risk had been incurred by day 23 after hospital admission, and the period of greatest increased risk extended up to at least 30 days. Importantly, more than half of VTE events occurred after discharge (56.6%). A particularly high proportion of VTE events (69.5%) had occurred after discharge in patients with cancer. Although it was assumed that most VTE events that could be reasonably attributed to an at‐risk hospitalization would occur within 90 days as shown previously,4, 11 the 180‐day evaluation period was used to examine whether there was a prolonged period of continually diminished VTE risk from 90 to 180 days. Thus, events occurring within the later portions of the evaluation period may or may not have been attributable to the index hospitalization, potentially reflecting a background rate of VTE as noted above. Although these events are included in our estimate of the 180‐day cumulative risk of VTE, interpretation of the study results excluding such events is possible by examining the cumulative risk that had been incurred at each time point during the evaluation period.
Few other studies have assessed the duration of VTE risk in hospitalized medical patients. In a study by Spyropoulos et al,4 the median time to a DVT and/or PE event was 74 days, ranging from 62 days in severe infectious disease to 126 days in CHF. In another observational study that included patients who had recently been hospitalized but had not undergone surgery, 66.9% of patients who experienced DVT and/or PE events were diagnosed with DVT and/or PE within the first month after hospital discharge; 19.9% between months 1 and 2, and 13.2% between months 2 and 3.12
Fewer than half of the patients in the present study received thromboprophylaxis, which is consistent with other studies demonstrating the low prophylaxis rates in medical inpatients.9, 1315 In a recently published US study of discharge records that included 22,455 medical inpatients, prophylaxis rates were 59.4% in patients with CHF, 52.3% with cancer, 45.8% with severe lung disease/COPD, and 40.4% with infectious disease.14 Fewer than 10% of patients in the present study received prophylaxis after discharge, a result that is consistent with other studies.4, 9
The effect of extended prophylaxis in acutely ill medical patients with the LMWH enoxaparin beyond 6‐14 days has been investigated in the EXCLAIM study.16 This trial included approximately 5800 acutely ill medical patients at significant risk of developing VTE due to a recent reduction in mobility. Patients in the extended prophylaxis group had a lower risk of VTE (2.5% vs 4% for placebo; absolute risk reduction 1.5% [95.8% confidence interval 2.54% to 0.52%]), but had increased major bleeding events (0.8% vs 0.3% for placebo; absolute risk difference favoring placebo, 0.51% [95% confidence interval, 0.12% to 0.89%]). The patient populations with most benefit from an additional 28 days prophylaxis with enoxaparin, in addition to the usual short‐term prophylaxis of 10 days, were patients with restricted mobility (level 1; total bed rest/sedentary), elderly patients (age >75 years), and women. A limitation of the EXCLAIM trial is that estimates of efficacy and safety are difficult to interpret: after an interim analysis of adjudicated efficacy and safety outcomes, amendments were made to the original study protocol by changing eligibility criteria for patients with level 2 immobility (level 1 with bathroom privileges).16
The optimal duration of prophylaxis for medical patients has not been determined; prophylaxis is generally administered to at‐risk medical patients for the duration of hospitalization. In the current study, mean length of stay was 5.3 5.3 days overall. As hospital stays shorten, many medical patients who are prescribed inpatient prophylaxis alone are unlikely to receive the standard 6‐14 days of prophylaxis shown to be effective in clinical trials.68 Furthermore, the extended period of VTE risk in the present study and the finding that 56.6% of events occurred after discharge also suggest that current practices for inpatient prophylaxis alone may need to be evaluated.
This study reports real‐world data from a large, well‐defined population and obtains the incidence of symptomatic VTE events. Even though certain demographic data deviate from the national averagefor example, 74.1% of patients were treated in the South Census region, whereas this region is served by 37.6% of US hospitals17; 87.5% of hospitals had an urban location (compared with 60.1% of US hospitals18), and 85.4% of hospitals had a licensed bed size of at least 200 beds (compared with 28.2% of US hospitals, with the average US hospital having fewer than 100 beds19)these data may be beneficial in guiding policy and health care strategies for gaining understanding of the duration of risk for VTE.
Limitations of the study include characterization of the VTE risk period through examination of the cumulative risk and hazard of VTE across time, as the actual VTE risk period cannot be determined with exact precision. We used ICD‐9‐CM diagnosis coding to identify VTE. Since many cases of PE are asymptomatic and detected at autopsy,20 our approach may have missed such cases, as they would not have been recorded within the database. Furthermore, validation studies suggest that suboptimal specificity exists for ICD‐9‐CM diagnosis codes used to identify VTE.21 In an attempt to improve the specificity of our VTE identification algorithm, we required that post‐discharge VTE was recorded either during an emergency room or subsequent inpatient admission (which would be indicative of acute care for VTE) or on an outpatient claim with subsequent evidence of treatment for VTE. The true sensitivity and specificity of the VTE identification algorithms used for this study remain unknown, however, so the study findings should be interpreted in light of this limitation. The databases used for the analysis may not be representative of the US population as a whole; for example, this study used claims data from commercial and Medicare supplemental databases, which do not include Medicaid patients. Another limitation was that outpatient mechanical prophylaxis, such as graded compression stockings, was not captured due to over‐the‐counter availability. In addition, appropriateness of prophylaxis was not determined in this study, because these data could not be obtained from the claims database used. Further studies are warranted to obtain information on the incidence of VTE after hospitalization for medical illness in patients who received appropriate prophylaxis during hospitalization.
Finally, all dosages of a pharmacological agent were considered prophylactic only if a VTE event did not occur, with the exception of warfarin; any dose of warfarin was considered for prophylaxis, regardless of a VTE diagnosis. Warfarin may be used for purposes other than VTE prophylaxis (eg, prophylaxis for a thromboembolic cerebrovascular accident). The data source does not allow for identifying the exact reason for anticoagulation therapy with warfarin. Nonetheless, warfarin therapy will confer a decreased risk of VTE regardless of its purpose.
Results from this large cohort of medical patients indicate that symptomatic VTE risk is highest within the first 19 days after hospital admission (a period that may encompass both the duration of hospitalization as well as the period after discharge) with a considerable risk of VTE extending into the period after discharge. Receiving appropriate prophylaxis in‐hospital remains of great importance to prevent inpatient and likely post‐discharge VTE in patients with acute medical illness. In addition, given the time course of VTE events, with VTE incidence peaking at 8 days but with increased risk extending to 30 days, and the number of out‐of‐hospital VTE events incurred, the results of this study suggest that future research is warranted to investigate the risks and benefits of improving thromboprophylaxis practices in the period after hospitalization.
Acknowledgements
Funding Source: sanofi‐aventis U.S. provided funding to Thomson Reuters to perform this study. The authors received editorial/writing support from Tessa Hartog of Excerpta Medica in the preparation of the manuscript funded by sanofi‐aventis U.S.
Disclosure: Alpesh Amin has received research honorarium and is on the speakers bureau for sanofi‐aventis U.S. Otsuka Pharmaceutical, and Boehringer‐Ingelheim. Helen Varker, Nicole Princic, and Stephen Johnston are employees at Thomson Reuters, which received funding from sanofi‐aventis U.S. Jay Lin is an employee of Novosys Health, which received funding from sanofi‐aventis U.S. Stephen Thompson is an employee of sanofi‐aventis U.S.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S–453S.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160:809–815.
- . The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372.
- , , , . Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951–957.
- , , , et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study. Arch Intern Med. 2002;162:1245–1248.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–329.
- , , . Lack of thromboprophylaxis across the care continuum in US medical patients. Hosp Pract (Minneap). 2010;38:17–25.
- HCUP NIS Related Reports. Healthcare Cost and Utilization Project (HCUP), September 2008. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. Accessed June 2011.
- , . Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486.
- , , , , . Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471–1475.
- , , , . Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician's recommendations for at‐risk medical and surgical patients. J Hosp Med. 2009;4:E15–E21.
- , , , et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE start). J Thromb Thrombolysis. 2010;29:326–339.
- , , , et al. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103:736–748.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- American Society for Healthcare Engineering of the American Hospital Association. Overview of the Hospital Market, 2009. Available from: www.ashe.org/e2c/pdfs/energy/heg_ch2_background.pdf. Accessed June 2011.
- American Hospital Association. Fast Facts on US Hospitals, 2009. Available at: http://www.aha.org/aha/resource‐center/Statistics‐and‐Studies/fast‐facts.html. Accessed June 2011.
- American Hospital Association. AHA Annual Survey of Hospitals Database, 2009. Available from: http://www.ahadata.com/ahadata_app/index.jsp. Accessed June 2011.
- . The epidemiology of venous thromboembolism. Circulation. 2003;107(23 suppl 1):I4–I8.
- , , , et al. How valid is the ICD‐9‐CM based AHRQ patient safety indicator for postoperative venous thromboembolism? Med Care. 2009;47:1237–1243.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S–453S.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160:809–815.
- . The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372.
- , , , . Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951–957.
- , , , et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study. Arch Intern Med. 2002;162:1245–1248.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–329.
- , , . Lack of thromboprophylaxis across the care continuum in US medical patients. Hosp Pract (Minneap). 2010;38:17–25.
- HCUP NIS Related Reports. Healthcare Cost and Utilization Project (HCUP), September 2008. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. Accessed June 2011.
- , . Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486.
- , , , , . Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471–1475.
- , , , . Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician's recommendations for at‐risk medical and surgical patients. J Hosp Med. 2009;4:E15–E21.
- , , , et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE start). J Thromb Thrombolysis. 2010;29:326–339.
- , , , et al. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103:736–748.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- American Society for Healthcare Engineering of the American Hospital Association. Overview of the Hospital Market, 2009. Available from: www.ashe.org/e2c/pdfs/energy/heg_ch2_background.pdf. Accessed June 2011.
- American Hospital Association. Fast Facts on US Hospitals, 2009. Available at: http://www.aha.org/aha/resource‐center/Statistics‐and‐Studies/fast‐facts.html. Accessed June 2011.
- American Hospital Association. AHA Annual Survey of Hospitals Database, 2009. Available from: http://www.ahadata.com/ahadata_app/index.jsp. Accessed June 2011.
- . The epidemiology of venous thromboembolism. Circulation. 2003;107(23 suppl 1):I4–I8.
- , , , et al. How valid is the ICD‐9‐CM based AHRQ patient safety indicator for postoperative venous thromboembolism? Med Care. 2009;47:1237–1243.
Copyright © 2011 Society of Hospital Medicine