Article
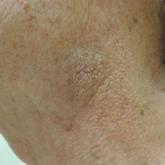
Minimally Hyperpigmented Plaque With Skin Thickening on the Neck
- Author:
- Henry Tomlinson, MD
- Barbara B. Wilson, MD
A 74-year-old man with a history of melanoma and basal cell carcinoma presented for an annual skin examination and displayed asymptomatic stable...
Article

What’s Eating You? The South African Fattail Scorpion Revisited
- Author:
- Henry Tomlinson, MD
- Dirk M. Elston, MD
Worldwide, there are more than 3250 deaths a year related to scorpion stings. With the increasing popularity of exotic and dangerous pets,...
Article

Aquatic Antagonists: Lionfish (Pterois volitans)
- Author:
- Henry Tomlinson, MD
- Dirk M. Elston, MD
Lionfish (Pterois volitans) are an invasive species originally from the Indian and Pacific oceans and the Red Sea that now are found all...
