Article
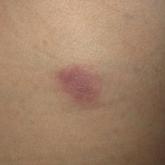
Cutaneous Gummatous Tuberculosis in a Kidney Transplant Patient
- Author:
- Nicole S. Evans, MD
- Ellen N. Pritchett, MD, MPH
- Krister Jones, MD
- Alden Doyle, MD, MPH
- Christina L. Chung, MD
- Herbert B. Allen, MD
- Carrie Ann R. Cusack, MD
Transplant patients are at increased risk for infection given their immunosuppressed state. Although rare, cutaneous tuberculosis should be...
Article
Purple-red papules on foot
- Author:
- John Patrick Welsh, MD
- Herbert B. Allen, MD
Does this patient’s Italian ancestry provide a clue to the diagnosis?
