User login
Inpatient Management of Urinary Tract Infections in Infants and Young Children
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
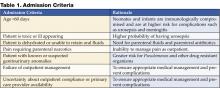
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
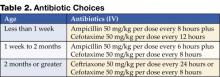
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
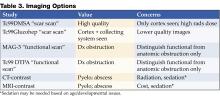
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.