User login
Fluid restriction is superior in acute lung injury and ARDS
Although most clinicians tend to manage acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by giving more rather than less fluid,1,2 patients may actually fare better under a strategy of limited fluid intake and increased fluid excretion.
According to the results of the Fluids and Catheters Treatment Trial (FACTT),3 patients managed with fluid restriction (the “dry” or conservative strategy) spent significantly less time in the intensive care unit (ICU) and on mechanical ventilation than did patients who received a high fluid intake (the “wet” or liberal strategy). These benefits of the conservative strategy were attained without any increase in the mortality rate at 60 days or in nonpulmonary organ failure at 28 days.
In this article, I discuss the basis for the FACTT researchers’ conclusion that a conservative fluid strategy is preferable to a liberal fluid strategy in ALI/ARDS.
STUDY RATIONALE
One of the more enduring questions in critical care medicine is which fluid-management strategy is best for patients with ALI/ARDS.
The conservative strategy results in a lower vascular filling pressure, which in turn reduces pulmonary edema and improves gas exchange. The drawback to this strategy is that it may have a negative effect on cardiac output and nonpulmonary organ function.
The liberal strategy results in a higher vascular filling pressure, which may be beneficial in terms of cardiac output and nonpulmonary organ perfusion. However, this strategy does not reduce lung edema.
The evidence accumulated before FACTT did not favor one strategy over the other. However, most deaths among patients with ALI/ARDS are attributable to the failure of organs other than the lungs.4,5 As a result, aggressive fluid restriction has not been a common approach in hospitals throughout the United States.1,2
In an effort to resolve the controversy surrounding the management of ALI/ARDS and to broaden the scope of what we know about fluid balance, we undertook this multicenter, randomized, prospective clinical comparison of the two strategies. This study was conducted under the auspices of the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSnet).
STUDY DESIGN
Between June 8, 2000, and October 3, 2005, we screened more than 11,000 patients with ALI/ARDS at 20 centers in North America.
Eligibility
Eligible patients had experienced ALI/ARDS within the previous 48 hours, had been intubated for positive-pressure ventilation, had a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2) of less than 300, and exhibited bilateral infiltrates on chest radiography that were consistent with the presence of pulmonary edema without evidence of left atrial hypertension.6
Major exclusion criteria included the placement of a pulmonary artery catheter prior to randomization and the presence of certain illnesses that might have compromised the study results.
Patient population
The target enrollment of 1,000 patients was reached. These patients were randomized into one of four roughly equal groups based on the type of fluid-management strategy—conservative or liberal—and the type of catheter that was placed—pulmonary artery or central venous. (The ARDSnet researchers published the results of the catheter comparison in a separate article.7 Those results are not discussed here except to note that there were no statistically significant differences in outcomes between the two catheter groups.)
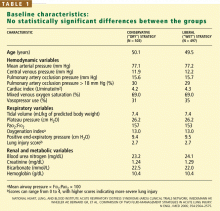
There were no statistically significant differences between the two groups with respect to baseline demographic characteristics. The conservative-strategy group consisted of 503 patients, of whom 52% were male and 65% were white; the mean age was 50.1 years. The liberal-strategy group consisted of 497 patients, of whom 55% were male and 63% were white; mean age was 49.5 years.
Management
Ventilation according to a low tidal volume strategy (6 mg/kg) was initiated within 1 hour after randomization. The pulmonary artery catheter or central venous catheter was inserted within 4 hours of randomization, and fluid management was started within 2 hours after catheter insertion. Fluid management was continued for 7 days or until 12 hours after extubation in patients who became able to breathe without assistance, whichever occurred first.
Target filling pressures. In the conservative-strategy group, the target filling pressures were low—a pulmonary artery occlusion pressure less than 8 mm Hg for those randomized to receive a pulmonary artery catheter, and a central venous pressure less than 4 mm Hg for those randomized to receive a central venous catheter. Barring adverse effects, patients were to undergo diuresis with furosemide (Lasix) until their goal was achieved, and then they would be maintained on that dosage through day 7. If we experienced difficulty in safely reaching these goals—say, if a patient developed hypoxemia, oliguria, or hypotension—we backed off the diuresis until the patient stabilized, and then we tried again. An inability to reach these filling pressure targets was not considered to be a treatment failure; our actual aim was to get as close to the target as possible as long as the patient tolerated the treatment.
In the liberal-strategy group, the target pressures were in the high-to-normal range—14 to 18 mm Hg for those with a pulmonary artery catheter and 10 to 14 mm Hg for those with a central venous catheter.
Patients with a pulmonary artery catheter who were hemodynamically stable after 3 days could be switched to a central venous catheter at the discretion of the clinician.
Monitoring. Patients were monitored once every 4 hours—more often if the clinician felt it necessary—for four variables:
- Pulmonary artery occlusion pressure or central venous pressure, depending on the type of catheter
- Shock, indicated by a mean arterial pressure of less than 60 mm Hg or the need for a vasopressor
- Oliguria, indicated by a urine output of less than 0.5 mL/kg/hour
- Ineffective circulation, represented by a cardiac index of less than 2.5 L/minute/cm2 in the pulmonary artery catheter group and by the presence of cold, mottled skin and a capillary-refilling time of more than 2 seconds in the central venous catheter group.
Depending on what the clinician found during monitoring, patients could receive a fluid bolus (if the filling pressure was too low), furosemide (if the filling pressure was too high), dobutamine (in certain rare circumstances), or nothing.
We monitored compliance with the protocol instructions twice each day—at a set time each morning and later in the day at a randomly selected time. An important aspect of this study is that we had no protocol instructions for managing shock. Individual clinicians were free to treat shock however they deemed best. In essence, then, our study was a comparison of liberal and conservative strategies during the nonshock phase of ALI/ARDS.
End points
The primary end point was the mortality rate at 60 days. Patients who were discharged earlier were assumed to be alive at 60 days.
The secondary end points were the number of ICU-free and ventilator-free days and the number of organ-failure-free days at day 28. Other end points included various indicators of lung physiology.
Statistical analysis
This intention-to-treat analysis was powered so that we had a 90% chance of detecting a 10% difference in mortality rate at day 60 (statistical significance: P < .05).
Protocol safeguards
Prior to treatment, we knew that some patients in the liberal-strategy group would not reach their filling-pressure targets despite the infusion of large amounts of fluid. To avoid “overdosing” these patients, we limited all patients to a maximum of three fluid boluses per 24 hours. Also, we withheld fluid boluses if a patient’s FIO2 level reached or exceeded 0.7 or if the cardiac index rose to 4.5 L/minute/cm2 or higher.
Diuretics were withheld when a patient had received a vasopressor or had emerged from shock within the preceding 12 hours. Also, diuretics were not given to any patient who had received a fluid bolus within the preceding 12 hours or when renal failure was present (these patients were given renal support therapy).
Finally, physicians and coordinators were instructed to assess each protocol instruction for safety and clinical validity before implementing the particular instruction. If, in their medical judgment, a particular protocol instruction should not be implemented, they were authorized to override the instruction and record the reason for doing so in the case report form.
RESULTS
Protocol compliance
Clinicians adhered to the protocol instructions during approximately 90% of the time.
Diuretic administration. In response to high filling pressures, patients in the conservative-strategy and liberal-strategy groups received furosemide during 41% and 10% of assessment periods, respectively (P < .0001). By day 7, the average patient in the conservative-strategy group had received a cumulative dose of approximately 1,000 mg of furosemide, while the average patient in the liberal-strategy group had received 500 mg.
Fluid administration. Low filling pressure prompted the administration of a fluid bolus to the liberal-strategy group during 15% of the assessment periods, compared with 6% in the conservative-strategy group (P < .0001).
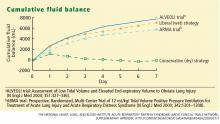
The conservative-strategy patients who were in shock at study entry had a net gain of approximately 3 L of fluid by day 7, while the liberal-strategy group had a gain of approximately 10 L. Among the patients who were shock-free at baseline, the conservative-strategy group had a net loss of almost 2 L at day 7 while the liberal-strategy group had a net gain of about 5 L.
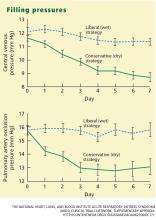
The pulmonary artery occlusion pressure fell from 15.6 mm Hg to just below 13 mm Hg in the conservative-strategy group by day 7, although there was a wide variation among individual patients. The pressure in the liberal-strategy group (15.7 mm Hg at baseline) was unchanged at day 7 (Figure 2).
Primary end point
Secondary end points
Through day 7, the average patient in the conservative-strategy group experienced significantly more ICU-free days (0.9 vs 0.6; P <.001) and more days free of central nervous system (CNS) failure (3.4 vs 2.9; P = .02). No significant differences were observed in the number of days free from coagulation abnormalities and renal or hepatic failure at day 28.
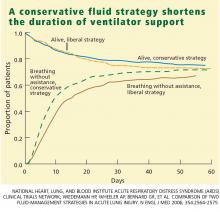
Through day 28, the average patient in the conservative-strategy group experienced significantly more ventilator-free days (14.6 vs 12.1; P < .001). The other 7-day results held up after 28 days, as the average conservative-strategy patient continued to experience more ICU-free days (13.4 vs 11.2; P < .001) and more days free of CNS failure (18.8 vs 17.2; P = .03). Again, no significant differences were observed in the number of days free of coagulation abnormalities and cardiovascular, renal, or hepatic failure.
It is not clear if the conservative strategy’s advantage in terms of more CNS-failure-free days was actually the result of the strategy itself or due to the fact that these patients were weaned off ventilation earlier and therefore received less sedation.
Other outcomes
Shock. One concern we had with the conservative strategy was that it might induce shock more frequently, but this did not occur. The percentage of patients who developed shock at least once during the 7-day treatment protocol was quite similar in the two groups. Also, it is interesting that patients who presented with no baseline shock had only about a 30% chance of developing shock during therapy. There was no significant difference in vasopressor use between the two groups.
Lung function. The conservative-strategy group had a significantly better Murray lung injury score at day 7: 2.03 vs 2.27 (P < .001).
Tidal-volume scores (7.4 mL/kg in both groups at baseline) dropped at an equal rate and were virtually identical at day 7 (6.36 mL/kg in the conservative-strategy group and 6.34 in the liberal-strategy group), as expected.The plateau pressure, positive end-expiraory pressure, PaO2–FIO2 ratio, and oxygenation index were slightly but not significantly better in the conservative-strategy group at day 7.
Overall, lung function was considerably better in the conservative-strategy group.
Cardiovascular function. The mean arterial pressure was significantly lower in the conservative-strategy group at day 7 (81.00 vs 84.36 mm Hg; P = .03). It is interesting that both levels were higher than the baseline levels (77.1 and 77.2, respectively; not significant).
The stroke volume index and the cardiac index were slightly lower in the conservative-strategy group at day 7, but not significantly so. No differences were seen in heart rate and venous oxygen saturation levels.
Renal and metabolic function. At day 7, the conservative-strategy group had a significantly higher blood urea nitrogen level (33.62 vs 28.44 mg/dL; P = .009). No significant differences were seen between the groups in creatinine levels at day 7 and day 28.
At day 60, dialysis was needed by 10% of the conservative-strategy group and 14% of the liberal-strategy group (P = .06). The important finding here is that there was no trend toward a more frequent need for dialysis in the conservative-strategy group. Also, the average number of days on dialysis in the two groups was essentially the same (11.0 and 10.9, respectively).
Again, there was no difference in the number of renal-failure-free days at either day 7 or day 28.
Hematologic factors. At day 7, the conservative-strategy group had significantly higher hemoglobin (10.22 vs 9.65 g/dL) and albumin (2.30 vs 2.11 g/dL) levels and capillary osmotic pressure (19.18 vs 17.39 mm Hg), even though significantly more patients in the liberal-strategy group received transfusions through day 7 (39% vs 29%; P = .0007).
Safety. Although the number of adverse events—particularly, metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group (42 vs 19; P = .001), the overall incidence was low. No adverse event was associated with arrhythmia.
CONCLUSION
The two fluid-management protocols used in this study were designed to be prudent yet distinctly different. While designing our protocol, we were concerned on the one hand that despite our best efforts fluid balance would turn out to be very similar in the two groups; this did not happen. On the other hand, we were also worried that the fluid level in one of the two groups might turn out to be so bizarre that it would invalidate our study; this too did not occur. Therefore, we are pleased with the way the study was designed and conducted, and we are satisfied that the two protocols were legitimate.
As we went into our study, the literature contained only one other prospective trial that was in some way similar to ours. Mitchell et al9 conducted a randomized, prospective study of 101 critically ill patients, including 89 with pulmonary edema. A group of 52 patients were managed with a conservative strategy intended to reduce the amount of extravascular lung water; the other 49 patients were managed with a strategy similar to the liberal strategy used in our study. At the study’s end, the patients in the conservative-strategy group had a significantly lower amount of extravascular lung water and spent significantly fewer days on ventilation and in the ICU. No clinically significant adverse effects were associated with the conservative strategy. This small study was not highly powered, but it did show that aggressive fluid restriction conferred some benefit.
In our study, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and ICU stay without increasing nonpulmonary organ failures or increasing the risk of death within 60 days. Therefore, we recommend the conservative strategy for patients with ALI/ARDS.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132:485–489.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical TrialsNetwork. Supplementary appendix.http://content.nejm.org/cgi/data/NEJMoa062200/DC1/1.Accessed August 3, 2007.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP.Improved outcome based on fluid management in criticallyill patients requiring pulmonary artery catheterization.Am Rev Respir Dis 1992; 145:990–998.
Although most clinicians tend to manage acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by giving more rather than less fluid,1,2 patients may actually fare better under a strategy of limited fluid intake and increased fluid excretion.
According to the results of the Fluids and Catheters Treatment Trial (FACTT),3 patients managed with fluid restriction (the “dry” or conservative strategy) spent significantly less time in the intensive care unit (ICU) and on mechanical ventilation than did patients who received a high fluid intake (the “wet” or liberal strategy). These benefits of the conservative strategy were attained without any increase in the mortality rate at 60 days or in nonpulmonary organ failure at 28 days.
In this article, I discuss the basis for the FACTT researchers’ conclusion that a conservative fluid strategy is preferable to a liberal fluid strategy in ALI/ARDS.
STUDY RATIONALE
One of the more enduring questions in critical care medicine is which fluid-management strategy is best for patients with ALI/ARDS.
The conservative strategy results in a lower vascular filling pressure, which in turn reduces pulmonary edema and improves gas exchange. The drawback to this strategy is that it may have a negative effect on cardiac output and nonpulmonary organ function.
The liberal strategy results in a higher vascular filling pressure, which may be beneficial in terms of cardiac output and nonpulmonary organ perfusion. However, this strategy does not reduce lung edema.
The evidence accumulated before FACTT did not favor one strategy over the other. However, most deaths among patients with ALI/ARDS are attributable to the failure of organs other than the lungs.4,5 As a result, aggressive fluid restriction has not been a common approach in hospitals throughout the United States.1,2
In an effort to resolve the controversy surrounding the management of ALI/ARDS and to broaden the scope of what we know about fluid balance, we undertook this multicenter, randomized, prospective clinical comparison of the two strategies. This study was conducted under the auspices of the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSnet).
STUDY DESIGN
Between June 8, 2000, and October 3, 2005, we screened more than 11,000 patients with ALI/ARDS at 20 centers in North America.
Eligibility
Eligible patients had experienced ALI/ARDS within the previous 48 hours, had been intubated for positive-pressure ventilation, had a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2) of less than 300, and exhibited bilateral infiltrates on chest radiography that were consistent with the presence of pulmonary edema without evidence of left atrial hypertension.6
Major exclusion criteria included the placement of a pulmonary artery catheter prior to randomization and the presence of certain illnesses that might have compromised the study results.
Patient population
The target enrollment of 1,000 patients was reached. These patients were randomized into one of four roughly equal groups based on the type of fluid-management strategy—conservative or liberal—and the type of catheter that was placed—pulmonary artery or central venous. (The ARDSnet researchers published the results of the catheter comparison in a separate article.7 Those results are not discussed here except to note that there were no statistically significant differences in outcomes between the two catheter groups.)
There were no statistically significant differences between the two groups with respect to baseline demographic characteristics. The conservative-strategy group consisted of 503 patients, of whom 52% were male and 65% were white; the mean age was 50.1 years. The liberal-strategy group consisted of 497 patients, of whom 55% were male and 63% were white; mean age was 49.5 years.
Management
Ventilation according to a low tidal volume strategy (6 mg/kg) was initiated within 1 hour after randomization. The pulmonary artery catheter or central venous catheter was inserted within 4 hours of randomization, and fluid management was started within 2 hours after catheter insertion. Fluid management was continued for 7 days or until 12 hours after extubation in patients who became able to breathe without assistance, whichever occurred first.
Target filling pressures. In the conservative-strategy group, the target filling pressures were low—a pulmonary artery occlusion pressure less than 8 mm Hg for those randomized to receive a pulmonary artery catheter, and a central venous pressure less than 4 mm Hg for those randomized to receive a central venous catheter. Barring adverse effects, patients were to undergo diuresis with furosemide (Lasix) until their goal was achieved, and then they would be maintained on that dosage through day 7. If we experienced difficulty in safely reaching these goals—say, if a patient developed hypoxemia, oliguria, or hypotension—we backed off the diuresis until the patient stabilized, and then we tried again. An inability to reach these filling pressure targets was not considered to be a treatment failure; our actual aim was to get as close to the target as possible as long as the patient tolerated the treatment.
In the liberal-strategy group, the target pressures were in the high-to-normal range—14 to 18 mm Hg for those with a pulmonary artery catheter and 10 to 14 mm Hg for those with a central venous catheter.
Patients with a pulmonary artery catheter who were hemodynamically stable after 3 days could be switched to a central venous catheter at the discretion of the clinician.
Monitoring. Patients were monitored once every 4 hours—more often if the clinician felt it necessary—for four variables:
- Pulmonary artery occlusion pressure or central venous pressure, depending on the type of catheter
- Shock, indicated by a mean arterial pressure of less than 60 mm Hg or the need for a vasopressor
- Oliguria, indicated by a urine output of less than 0.5 mL/kg/hour
- Ineffective circulation, represented by a cardiac index of less than 2.5 L/minute/cm2 in the pulmonary artery catheter group and by the presence of cold, mottled skin and a capillary-refilling time of more than 2 seconds in the central venous catheter group.
Depending on what the clinician found during monitoring, patients could receive a fluid bolus (if the filling pressure was too low), furosemide (if the filling pressure was too high), dobutamine (in certain rare circumstances), or nothing.
We monitored compliance with the protocol instructions twice each day—at a set time each morning and later in the day at a randomly selected time. An important aspect of this study is that we had no protocol instructions for managing shock. Individual clinicians were free to treat shock however they deemed best. In essence, then, our study was a comparison of liberal and conservative strategies during the nonshock phase of ALI/ARDS.
End points
The primary end point was the mortality rate at 60 days. Patients who were discharged earlier were assumed to be alive at 60 days.
The secondary end points were the number of ICU-free and ventilator-free days and the number of organ-failure-free days at day 28. Other end points included various indicators of lung physiology.
Statistical analysis
This intention-to-treat analysis was powered so that we had a 90% chance of detecting a 10% difference in mortality rate at day 60 (statistical significance: P < .05).
Protocol safeguards
Prior to treatment, we knew that some patients in the liberal-strategy group would not reach their filling-pressure targets despite the infusion of large amounts of fluid. To avoid “overdosing” these patients, we limited all patients to a maximum of three fluid boluses per 24 hours. Also, we withheld fluid boluses if a patient’s FIO2 level reached or exceeded 0.7 or if the cardiac index rose to 4.5 L/minute/cm2 or higher.
Diuretics were withheld when a patient had received a vasopressor or had emerged from shock within the preceding 12 hours. Also, diuretics were not given to any patient who had received a fluid bolus within the preceding 12 hours or when renal failure was present (these patients were given renal support therapy).
Finally, physicians and coordinators were instructed to assess each protocol instruction for safety and clinical validity before implementing the particular instruction. If, in their medical judgment, a particular protocol instruction should not be implemented, they were authorized to override the instruction and record the reason for doing so in the case report form.
RESULTS
Protocol compliance
Clinicians adhered to the protocol instructions during approximately 90% of the time.
Diuretic administration. In response to high filling pressures, patients in the conservative-strategy and liberal-strategy groups received furosemide during 41% and 10% of assessment periods, respectively (P < .0001). By day 7, the average patient in the conservative-strategy group had received a cumulative dose of approximately 1,000 mg of furosemide, while the average patient in the liberal-strategy group had received 500 mg.
Fluid administration. Low filling pressure prompted the administration of a fluid bolus to the liberal-strategy group during 15% of the assessment periods, compared with 6% in the conservative-strategy group (P < .0001).
The conservative-strategy patients who were in shock at study entry had a net gain of approximately 3 L of fluid by day 7, while the liberal-strategy group had a gain of approximately 10 L. Among the patients who were shock-free at baseline, the conservative-strategy group had a net loss of almost 2 L at day 7 while the liberal-strategy group had a net gain of about 5 L.
The pulmonary artery occlusion pressure fell from 15.6 mm Hg to just below 13 mm Hg in the conservative-strategy group by day 7, although there was a wide variation among individual patients. The pressure in the liberal-strategy group (15.7 mm Hg at baseline) was unchanged at day 7 (Figure 2).
Primary end point
Secondary end points
Through day 7, the average patient in the conservative-strategy group experienced significantly more ICU-free days (0.9 vs 0.6; P <.001) and more days free of central nervous system (CNS) failure (3.4 vs 2.9; P = .02). No significant differences were observed in the number of days free from coagulation abnormalities and renal or hepatic failure at day 28.
Through day 28, the average patient in the conservative-strategy group experienced significantly more ventilator-free days (14.6 vs 12.1; P < .001). The other 7-day results held up after 28 days, as the average conservative-strategy patient continued to experience more ICU-free days (13.4 vs 11.2; P < .001) and more days free of CNS failure (18.8 vs 17.2; P = .03). Again, no significant differences were observed in the number of days free of coagulation abnormalities and cardiovascular, renal, or hepatic failure.
It is not clear if the conservative strategy’s advantage in terms of more CNS-failure-free days was actually the result of the strategy itself or due to the fact that these patients were weaned off ventilation earlier and therefore received less sedation.
Other outcomes
Shock. One concern we had with the conservative strategy was that it might induce shock more frequently, but this did not occur. The percentage of patients who developed shock at least once during the 7-day treatment protocol was quite similar in the two groups. Also, it is interesting that patients who presented with no baseline shock had only about a 30% chance of developing shock during therapy. There was no significant difference in vasopressor use between the two groups.
Lung function. The conservative-strategy group had a significantly better Murray lung injury score at day 7: 2.03 vs 2.27 (P < .001).
Tidal-volume scores (7.4 mL/kg in both groups at baseline) dropped at an equal rate and were virtually identical at day 7 (6.36 mL/kg in the conservative-strategy group and 6.34 in the liberal-strategy group), as expected.The plateau pressure, positive end-expiraory pressure, PaO2–FIO2 ratio, and oxygenation index were slightly but not significantly better in the conservative-strategy group at day 7.
Overall, lung function was considerably better in the conservative-strategy group.
Cardiovascular function. The mean arterial pressure was significantly lower in the conservative-strategy group at day 7 (81.00 vs 84.36 mm Hg; P = .03). It is interesting that both levels were higher than the baseline levels (77.1 and 77.2, respectively; not significant).
The stroke volume index and the cardiac index were slightly lower in the conservative-strategy group at day 7, but not significantly so. No differences were seen in heart rate and venous oxygen saturation levels.
Renal and metabolic function. At day 7, the conservative-strategy group had a significantly higher blood urea nitrogen level (33.62 vs 28.44 mg/dL; P = .009). No significant differences were seen between the groups in creatinine levels at day 7 and day 28.
At day 60, dialysis was needed by 10% of the conservative-strategy group and 14% of the liberal-strategy group (P = .06). The important finding here is that there was no trend toward a more frequent need for dialysis in the conservative-strategy group. Also, the average number of days on dialysis in the two groups was essentially the same (11.0 and 10.9, respectively).
Again, there was no difference in the number of renal-failure-free days at either day 7 or day 28.
Hematologic factors. At day 7, the conservative-strategy group had significantly higher hemoglobin (10.22 vs 9.65 g/dL) and albumin (2.30 vs 2.11 g/dL) levels and capillary osmotic pressure (19.18 vs 17.39 mm Hg), even though significantly more patients in the liberal-strategy group received transfusions through day 7 (39% vs 29%; P = .0007).
Safety. Although the number of adverse events—particularly, metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group (42 vs 19; P = .001), the overall incidence was low. No adverse event was associated with arrhythmia.
CONCLUSION
The two fluid-management protocols used in this study were designed to be prudent yet distinctly different. While designing our protocol, we were concerned on the one hand that despite our best efforts fluid balance would turn out to be very similar in the two groups; this did not happen. On the other hand, we were also worried that the fluid level in one of the two groups might turn out to be so bizarre that it would invalidate our study; this too did not occur. Therefore, we are pleased with the way the study was designed and conducted, and we are satisfied that the two protocols were legitimate.
As we went into our study, the literature contained only one other prospective trial that was in some way similar to ours. Mitchell et al9 conducted a randomized, prospective study of 101 critically ill patients, including 89 with pulmonary edema. A group of 52 patients were managed with a conservative strategy intended to reduce the amount of extravascular lung water; the other 49 patients were managed with a strategy similar to the liberal strategy used in our study. At the study’s end, the patients in the conservative-strategy group had a significantly lower amount of extravascular lung water and spent significantly fewer days on ventilation and in the ICU. No clinically significant adverse effects were associated with the conservative strategy. This small study was not highly powered, but it did show that aggressive fluid restriction conferred some benefit.
In our study, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and ICU stay without increasing nonpulmonary organ failures or increasing the risk of death within 60 days. Therefore, we recommend the conservative strategy for patients with ALI/ARDS.
Although most clinicians tend to manage acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by giving more rather than less fluid,1,2 patients may actually fare better under a strategy of limited fluid intake and increased fluid excretion.
According to the results of the Fluids and Catheters Treatment Trial (FACTT),3 patients managed with fluid restriction (the “dry” or conservative strategy) spent significantly less time in the intensive care unit (ICU) and on mechanical ventilation than did patients who received a high fluid intake (the “wet” or liberal strategy). These benefits of the conservative strategy were attained without any increase in the mortality rate at 60 days or in nonpulmonary organ failure at 28 days.
In this article, I discuss the basis for the FACTT researchers’ conclusion that a conservative fluid strategy is preferable to a liberal fluid strategy in ALI/ARDS.
STUDY RATIONALE
One of the more enduring questions in critical care medicine is which fluid-management strategy is best for patients with ALI/ARDS.
The conservative strategy results in a lower vascular filling pressure, which in turn reduces pulmonary edema and improves gas exchange. The drawback to this strategy is that it may have a negative effect on cardiac output and nonpulmonary organ function.
The liberal strategy results in a higher vascular filling pressure, which may be beneficial in terms of cardiac output and nonpulmonary organ perfusion. However, this strategy does not reduce lung edema.
The evidence accumulated before FACTT did not favor one strategy over the other. However, most deaths among patients with ALI/ARDS are attributable to the failure of organs other than the lungs.4,5 As a result, aggressive fluid restriction has not been a common approach in hospitals throughout the United States.1,2
In an effort to resolve the controversy surrounding the management of ALI/ARDS and to broaden the scope of what we know about fluid balance, we undertook this multicenter, randomized, prospective clinical comparison of the two strategies. This study was conducted under the auspices of the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSnet).
STUDY DESIGN
Between June 8, 2000, and October 3, 2005, we screened more than 11,000 patients with ALI/ARDS at 20 centers in North America.
Eligibility
Eligible patients had experienced ALI/ARDS within the previous 48 hours, had been intubated for positive-pressure ventilation, had a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2) of less than 300, and exhibited bilateral infiltrates on chest radiography that were consistent with the presence of pulmonary edema without evidence of left atrial hypertension.6
Major exclusion criteria included the placement of a pulmonary artery catheter prior to randomization and the presence of certain illnesses that might have compromised the study results.
Patient population
The target enrollment of 1,000 patients was reached. These patients were randomized into one of four roughly equal groups based on the type of fluid-management strategy—conservative or liberal—and the type of catheter that was placed—pulmonary artery or central venous. (The ARDSnet researchers published the results of the catheter comparison in a separate article.7 Those results are not discussed here except to note that there were no statistically significant differences in outcomes between the two catheter groups.)
There were no statistically significant differences between the two groups with respect to baseline demographic characteristics. The conservative-strategy group consisted of 503 patients, of whom 52% were male and 65% were white; the mean age was 50.1 years. The liberal-strategy group consisted of 497 patients, of whom 55% were male and 63% were white; mean age was 49.5 years.
Management
Ventilation according to a low tidal volume strategy (6 mg/kg) was initiated within 1 hour after randomization. The pulmonary artery catheter or central venous catheter was inserted within 4 hours of randomization, and fluid management was started within 2 hours after catheter insertion. Fluid management was continued for 7 days or until 12 hours after extubation in patients who became able to breathe without assistance, whichever occurred first.
Target filling pressures. In the conservative-strategy group, the target filling pressures were low—a pulmonary artery occlusion pressure less than 8 mm Hg for those randomized to receive a pulmonary artery catheter, and a central venous pressure less than 4 mm Hg for those randomized to receive a central venous catheter. Barring adverse effects, patients were to undergo diuresis with furosemide (Lasix) until their goal was achieved, and then they would be maintained on that dosage through day 7. If we experienced difficulty in safely reaching these goals—say, if a patient developed hypoxemia, oliguria, or hypotension—we backed off the diuresis until the patient stabilized, and then we tried again. An inability to reach these filling pressure targets was not considered to be a treatment failure; our actual aim was to get as close to the target as possible as long as the patient tolerated the treatment.
In the liberal-strategy group, the target pressures were in the high-to-normal range—14 to 18 mm Hg for those with a pulmonary artery catheter and 10 to 14 mm Hg for those with a central venous catheter.
Patients with a pulmonary artery catheter who were hemodynamically stable after 3 days could be switched to a central venous catheter at the discretion of the clinician.
Monitoring. Patients were monitored once every 4 hours—more often if the clinician felt it necessary—for four variables:
- Pulmonary artery occlusion pressure or central venous pressure, depending on the type of catheter
- Shock, indicated by a mean arterial pressure of less than 60 mm Hg or the need for a vasopressor
- Oliguria, indicated by a urine output of less than 0.5 mL/kg/hour
- Ineffective circulation, represented by a cardiac index of less than 2.5 L/minute/cm2 in the pulmonary artery catheter group and by the presence of cold, mottled skin and a capillary-refilling time of more than 2 seconds in the central venous catheter group.
Depending on what the clinician found during monitoring, patients could receive a fluid bolus (if the filling pressure was too low), furosemide (if the filling pressure was too high), dobutamine (in certain rare circumstances), or nothing.
We monitored compliance with the protocol instructions twice each day—at a set time each morning and later in the day at a randomly selected time. An important aspect of this study is that we had no protocol instructions for managing shock. Individual clinicians were free to treat shock however they deemed best. In essence, then, our study was a comparison of liberal and conservative strategies during the nonshock phase of ALI/ARDS.
End points
The primary end point was the mortality rate at 60 days. Patients who were discharged earlier were assumed to be alive at 60 days.
The secondary end points were the number of ICU-free and ventilator-free days and the number of organ-failure-free days at day 28. Other end points included various indicators of lung physiology.
Statistical analysis
This intention-to-treat analysis was powered so that we had a 90% chance of detecting a 10% difference in mortality rate at day 60 (statistical significance: P < .05).
Protocol safeguards
Prior to treatment, we knew that some patients in the liberal-strategy group would not reach their filling-pressure targets despite the infusion of large amounts of fluid. To avoid “overdosing” these patients, we limited all patients to a maximum of three fluid boluses per 24 hours. Also, we withheld fluid boluses if a patient’s FIO2 level reached or exceeded 0.7 or if the cardiac index rose to 4.5 L/minute/cm2 or higher.
Diuretics were withheld when a patient had received a vasopressor or had emerged from shock within the preceding 12 hours. Also, diuretics were not given to any patient who had received a fluid bolus within the preceding 12 hours or when renal failure was present (these patients were given renal support therapy).
Finally, physicians and coordinators were instructed to assess each protocol instruction for safety and clinical validity before implementing the particular instruction. If, in their medical judgment, a particular protocol instruction should not be implemented, they were authorized to override the instruction and record the reason for doing so in the case report form.
RESULTS
Protocol compliance
Clinicians adhered to the protocol instructions during approximately 90% of the time.
Diuretic administration. In response to high filling pressures, patients in the conservative-strategy and liberal-strategy groups received furosemide during 41% and 10% of assessment periods, respectively (P < .0001). By day 7, the average patient in the conservative-strategy group had received a cumulative dose of approximately 1,000 mg of furosemide, while the average patient in the liberal-strategy group had received 500 mg.
Fluid administration. Low filling pressure prompted the administration of a fluid bolus to the liberal-strategy group during 15% of the assessment periods, compared with 6% in the conservative-strategy group (P < .0001).
The conservative-strategy patients who were in shock at study entry had a net gain of approximately 3 L of fluid by day 7, while the liberal-strategy group had a gain of approximately 10 L. Among the patients who were shock-free at baseline, the conservative-strategy group had a net loss of almost 2 L at day 7 while the liberal-strategy group had a net gain of about 5 L.
The pulmonary artery occlusion pressure fell from 15.6 mm Hg to just below 13 mm Hg in the conservative-strategy group by day 7, although there was a wide variation among individual patients. The pressure in the liberal-strategy group (15.7 mm Hg at baseline) was unchanged at day 7 (Figure 2).
Primary end point
Secondary end points
Through day 7, the average patient in the conservative-strategy group experienced significantly more ICU-free days (0.9 vs 0.6; P <.001) and more days free of central nervous system (CNS) failure (3.4 vs 2.9; P = .02). No significant differences were observed in the number of days free from coagulation abnormalities and renal or hepatic failure at day 28.
Through day 28, the average patient in the conservative-strategy group experienced significantly more ventilator-free days (14.6 vs 12.1; P < .001). The other 7-day results held up after 28 days, as the average conservative-strategy patient continued to experience more ICU-free days (13.4 vs 11.2; P < .001) and more days free of CNS failure (18.8 vs 17.2; P = .03). Again, no significant differences were observed in the number of days free of coagulation abnormalities and cardiovascular, renal, or hepatic failure.
It is not clear if the conservative strategy’s advantage in terms of more CNS-failure-free days was actually the result of the strategy itself or due to the fact that these patients were weaned off ventilation earlier and therefore received less sedation.
Other outcomes
Shock. One concern we had with the conservative strategy was that it might induce shock more frequently, but this did not occur. The percentage of patients who developed shock at least once during the 7-day treatment protocol was quite similar in the two groups. Also, it is interesting that patients who presented with no baseline shock had only about a 30% chance of developing shock during therapy. There was no significant difference in vasopressor use between the two groups.
Lung function. The conservative-strategy group had a significantly better Murray lung injury score at day 7: 2.03 vs 2.27 (P < .001).
Tidal-volume scores (7.4 mL/kg in both groups at baseline) dropped at an equal rate and were virtually identical at day 7 (6.36 mL/kg in the conservative-strategy group and 6.34 in the liberal-strategy group), as expected.The plateau pressure, positive end-expiraory pressure, PaO2–FIO2 ratio, and oxygenation index were slightly but not significantly better in the conservative-strategy group at day 7.
Overall, lung function was considerably better in the conservative-strategy group.
Cardiovascular function. The mean arterial pressure was significantly lower in the conservative-strategy group at day 7 (81.00 vs 84.36 mm Hg; P = .03). It is interesting that both levels were higher than the baseline levels (77.1 and 77.2, respectively; not significant).
The stroke volume index and the cardiac index were slightly lower in the conservative-strategy group at day 7, but not significantly so. No differences were seen in heart rate and venous oxygen saturation levels.
Renal and metabolic function. At day 7, the conservative-strategy group had a significantly higher blood urea nitrogen level (33.62 vs 28.44 mg/dL; P = .009). No significant differences were seen between the groups in creatinine levels at day 7 and day 28.
At day 60, dialysis was needed by 10% of the conservative-strategy group and 14% of the liberal-strategy group (P = .06). The important finding here is that there was no trend toward a more frequent need for dialysis in the conservative-strategy group. Also, the average number of days on dialysis in the two groups was essentially the same (11.0 and 10.9, respectively).
Again, there was no difference in the number of renal-failure-free days at either day 7 or day 28.
Hematologic factors. At day 7, the conservative-strategy group had significantly higher hemoglobin (10.22 vs 9.65 g/dL) and albumin (2.30 vs 2.11 g/dL) levels and capillary osmotic pressure (19.18 vs 17.39 mm Hg), even though significantly more patients in the liberal-strategy group received transfusions through day 7 (39% vs 29%; P = .0007).
Safety. Although the number of adverse events—particularly, metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group (42 vs 19; P = .001), the overall incidence was low. No adverse event was associated with arrhythmia.
CONCLUSION
The two fluid-management protocols used in this study were designed to be prudent yet distinctly different. While designing our protocol, we were concerned on the one hand that despite our best efforts fluid balance would turn out to be very similar in the two groups; this did not happen. On the other hand, we were also worried that the fluid level in one of the two groups might turn out to be so bizarre that it would invalidate our study; this too did not occur. Therefore, we are pleased with the way the study was designed and conducted, and we are satisfied that the two protocols were legitimate.
As we went into our study, the literature contained only one other prospective trial that was in some way similar to ours. Mitchell et al9 conducted a randomized, prospective study of 101 critically ill patients, including 89 with pulmonary edema. A group of 52 patients were managed with a conservative strategy intended to reduce the amount of extravascular lung water; the other 49 patients were managed with a strategy similar to the liberal strategy used in our study. At the study’s end, the patients in the conservative-strategy group had a significantly lower amount of extravascular lung water and spent significantly fewer days on ventilation and in the ICU. No clinically significant adverse effects were associated with the conservative strategy. This small study was not highly powered, but it did show that aggressive fluid restriction conferred some benefit.
In our study, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and ICU stay without increasing nonpulmonary organ failures or increasing the risk of death within 60 days. Therefore, we recommend the conservative strategy for patients with ALI/ARDS.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132:485–489.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical TrialsNetwork. Supplementary appendix.http://content.nejm.org/cgi/data/NEJMoa062200/DC1/1.Accessed August 3, 2007.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP.Improved outcome based on fluid management in criticallyill patients requiring pulmonary artery catheterization.Am Rev Respir Dis 1992; 145:990–998.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132:485–489.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical TrialsNetwork. Supplementary appendix.http://content.nejm.org/cgi/data/NEJMoa062200/DC1/1.Accessed August 3, 2007.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP.Improved outcome based on fluid management in criticallyill patients requiring pulmonary artery catheterization.Am Rev Respir Dis 1992; 145:990–998.
KEY POINTS
- In the conservative-strategy group, the target filling pressures were a pulmonary artery occlusion pressure less than 8 mm Hg for those with a pulmonary artery catheter and a central venous pressure less than 4 mm Hg for those with only a central venous catheter. Pressures were brought into these ranges by diuresis.
- The conservative-strategy group did not experience more frequent need for dialysis or more shock.
- Although the number of adverse events—particularly ,metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group, the overall incidence was low.