User login
Update in intensive care medicine: Studies that challenged our practice in the last 5 years
We have seen significant growth in clinical research in critical care medicine in the last decade. Advances have been made in many important areas in this field; of these, advances in treating septic shock and acute respiratory distress syndrome (ARDS), and also in supportive therapies for critically ill patients (eg, sedatives, insulin), have perhaps received the most attention.
Of note, several once-established therapies in these areas have failed the test of time, as the result of evidence from more-recent clinical trials. For example, recent studies have shown that a pulmonary arterial catheter does not improve outcomes in patients with ARDS. Similarly, what used to be “optimal” fluid management in patients with ARDS is no longer considered appropriate.
In this review, we summarize eight major studies in critical care medicine published in the last 5 years, studies that have contributed to changes in our practice in the intensive care unit (ICU).
FLUID MANAGEMENT IN ARDS
Key points
- In patients with acute lung injury (ALI) and ARDS, fluid restriction is associated with better outcomes than a liberal fluid policy.
- A pulmonary arterial catheter is not necessary and, compared with a central venous catheter, may result in more complications in patients with ALI and ARDS.
Background
Fluid management practices in patients with ARDS have been extremely variable. Two different approaches are commonly used: the liberal or “wet” approach to optimize tissue perfusion and the “dry” approach, which focuses on reducing lung edema. Given that most deaths attributed to ARDS result from extrapulmonary organ failure, aggressive fluid restriction has been the less popular approach.
Additionally, although earlier studies and meta-analyses suggested that the use of a pulmonary arterial catheter was not associated with better outcomes in critically ill patients,1 controversy remained regarding the value of a pulmonary arterial catheter compared with a central venous catheter in guiding fluid management in patients with ARDS, and data were insufficient to prove one strategy better than the other.
The Fluids and Catheter Treatment Trial (FACTT)
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK; WIEDEMANN HP, WHEELER AP, BERNARD GR, ET AL. COMPARISON OF TWO FLUID-MANAGEMENT STRATEGIES IN ACUTE LUNG INJURY. N ENGL J MED 2006; 354:2564–2575.
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK; WHEELER AP, BERNARD GR, THOMPSON BT, ET AL. PULMONARY-ARTERY VERSUS CENTRAL VENOUS CATHETER TO GUIDE TREATMENT OF ACUTE LUNG INJURY. N ENGL J MED 2006; 354:2213–2224.
The Fluids and Catheter Treatment Trial (FACTT) compared two fluid strategies2 and also the utility of a pulmonary arterial catheter vs a central venous catheter3 in patients with ALI or ARDS.
This two-by-two factorial trial randomized 1,000 patients to be treated according to either a conservative (fluid-restrictive or “dry”) or a liberal (“wet”) fluid management strategy for 7 days. Additionally, they were randomly assigned to receive either a central venous catheter or a pulmonary arterial catheter. The trial thus had four treatment groups:
- Fluid-restricted and a central venous catheter, with a goal of keeping the central venous pressure below 4 mm Hg
- Fluid-restricted and a pulmonary arterial catheter: fluids were restricted and diuretics were given to keep the pulmonary artery occlusion pressure below 8 mm Hg
- Fluid-liberal and a central venous catheter: fluids were given to keep the central venous pressure between 10 and 14 mm Hg
- Fluid-liberal and a pulmonary arterial catheter: fluids were given to keep the pulmonary artery occlusion pressure between 14 and 18 mm Hg.
The primary end point was the mortality rate at 60 days. Secondary end points included the number of ventilator-free days and organ-failure-free days and parameters of lung physiology. All patients were managed with a low-tidal-volume strategy.
The ‘dry’ strategy was better
The cumulative fluid balance was −136 mL ± 491 mL in the “dry” group and 6,992 mL ± 502 mL in the “wet” group, a difference of more than 7 L (P < .0001). Of note, before randomization, the patients were already fluid-positive, with a mean total fluid balance of +2,700 mL).2
At 60 days, no statistically significant difference in mortality rate was seen between the fluid-management groups (25.5% in the dry group vs 28.4% in the wet group (P = .30). Nevertheless, patients in the dry group had better oxygenation indices and lung injury scores (including lower plateau airway pressure), resulting in more ventilator-free days (14.6 ± 0.5 vs 12.1 ± 0.5; P = .0002) and ICU-free days (13.4 ± 0.4 vs 11.2 ± 0.4; P = .0003).2
Although those in the dry-strategy group had a slightly lower cardiac index and mean arterial pressure, they did not have a higher incidence of shock.
More importantly, the dry group did not have a higher rate of nonpulmonary organ failure. Serum creatinine and blood urea nitrogen concentrations were slightly higher in this group, but this was not associated with a higher incidence of renal failure or the use of dialysis: 10% in the dry-strategy group vs 14% in the wet-strategy group; P = .0642).2
No advantage with a pulmonary arterial catheter
The mortality rate did not differ between the catheter groups. However, the patients who received a pulmonary arterial catheter stayed in the ICU 0.2 days longer and had twice as many nonfatal cardiac arrhythmias as those who received a central venous catheter.3
Comments
The liberal fluid-strategy group had fluid balances similar to those seen in previous National Institutes of Health ARDS Network trials in which fluid management was not controlled. This suggests that the liberal fluid strategy reflects usual clinical practice.
Although the goals used in this study (central venous pressure < 4 mm Hg or pulmonary artery occlusion pressure < 8 mm Hg) could be difficult to achieve in clinical practice, a conservative strategy of fluid management is preferred in patients with ALI or ARDS, given the benefits observed in this trial.
A pulmonary arterial catheter is not indicated to guide hemodynamic management of patients with ARDS.
CORTICOSTEROID USE IN ARDS
Key points
- In selected patients with ARDS, the prolonged use of corticosteroids may result in better oxygenation and a shorter duration of mechanical ventilation.
- Late use of corticosteroids in patients with ARDS (> 14 days after diagnosis) is not indicated and may increase the risk of death.
- The role of corticosteroids in early ARDS (< 7 days after diagnosis) remains controversial.
Background
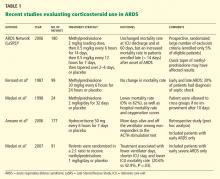
Systemic corticosteroid therapy was commonly used in ARDS patients in the 1970s and 1980s. However, a single-center study published in the late 1980s showed that a corticosteroid in high doses (methylprednisolone 30 mg/kg) resulted in more complications and was not associated with a lower mortality rate.4 On the other hand, a small study that included only patients with persistent ARDS (defined as ARDS lasting for more than 7 days) subsequently showed that oxygenation was significantly better and that fewer patients died while in the hospital with the use of methylprednisolone 2 mg/kg for 32 days.5
In view of these divergent findings, the ARDS Network decided to perform a study to help understand the role of corticosteroids in ARDS.
The Late Steroid Rescue Study (LaSRS)
STEINBERG KP, HUDSON LD, GOODMAN RB, ET AL; NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK. EFFICACY AND SAFETY OF CORTICOSTEROIDS FOR PERSISTENT ACUTE RESPIRATORY DISTRESS SYNDROME. N ENGL J MED 2006; 354:1671–1684.
The Late Steroid Rescue Study (LaSRS),6 a double-blind, multicenter trial, randomly assigned 180 patients with persistent ARDS (defined as ongoing disease 7–28 days after its onset) to receive methylprednisolone or placebo for 21 days.
Methylprednisolone was given in an initial dose of 2 mg/kg of predicted body weight followed by a dose of 0.5 mg/kg every 6 hours for 14 days and then a dose of 0.5 mg/kg every 12 hours for 7 days, and then it was tapered over 2 to 4 days and discontinued. It could be discontinued if 21 days of treatment were completed or if the patient was able to breathe without assistance.
The primary end point was the mortality rate at 60 days. Secondary end points included the number of ventilator-free days, organ-failure-free days, and complications and the levels of biomarkers of inflammation.
No reduction in mortality rates with steroids
The mortality rates did not differ significantly in the corticosteroid group vs the placebo group at 60 days:
- 29.2% with methylprednisolone (95% confidence interval [CI] 20.8–39.4)
- 28.6% with placebo (95% CI 20.3–38.6, P = 1.0).
Mortality rates at 180 days were also similar between the groups:
- 31.5% with methylprednisolone (95% CI 22.8–41.7)
- 31.9% with placebo (95% CI 23.2–42.0, P = 1.0).
In patients randomized between 7 and 13 days after the onset of ARDS, the mortality rates were lower in the methylprednisolone group than in the placebo group but the differences were not statistically significant. The mortality rate in this subgroup was 27% vs 36% (P = .26) at 60 days and was 27% vs 39% (P = .14) at 180 days.
However, in patients randomized more than 14 days after the onset of ARDS, the mortality rate was significantly higher in the methylprednisolone group than in the placebo group at 60 days (35% vs 8%, P = .02) and at 180 days (44% vs 12%, P = .01).
Some benefit in secondary outcomes
At day 28, methylprednisolone was associated with:
- More ventilator-free days (11.2 ± 9.4 vs 6.8 ± 8.5, P < .001)
- More shock-free days (20.7 ± 8.9 vs 17.9 ± 10.2, P = .04)
- More ICU-free days (8.9 ± 8.2 vs 6.7 ± 7.8, P = .02).
Similarly, pulmonary physiologic indices were better with methylprednisolone, specifically:
- The ratio of Pao2 to the fraction of inspired oxygen at days 3, 4, and 14 (P < .05)
- Plateau pressure at days 4, 5, and 7 (P < .05)
- Static compliance at days 7 and 14 (P < .05).
In terms of side effects, methylprednisolone was associated with more events associated with myopathy or neuropathy (9 vs 0, P = .001), but there were no differences in the number of serious infections or in glycemic control.
Comments
Although other recent studies suggested that corticosteroid use may be associated with a reduction in mortality rates,7–9 LaSRS did not confirm this effect. Although the doses and length of therapy were similar in these studies, LaSRS was much larger and included patients from the ARDS Network.
Nevertheless, LaSRS was criticized because of strict exclusion criteria and poor enrollment (only 5% of eligible patients were included). Additionally, it was conducted over a period of time when some ICU practices varied significantly (eg, low vs high tidal volume ventilation, tight vs loose glucose control).
INTERRUPTING SEDATION DURING MECHANICAL VENTILATION
Key points
- Daily awakening of mechanically ventilated patients is safe.
- Daily interruption of sedation in mechanically ventilated patients is associated with a shorter length of mechanical ventilation.
Background
Sedatives are a central component of critical care. Continuous infusions of narcotics, benzodiazepines, and anesthetic agents are frequently used to promote comfort in patients receiving mechanical ventilation.
Despite its widespread use in the ICU, there is little evidence that such sedation improves outcomes. Observational and randomized trials10–12 have shown that patients who receive continuous infusions of sedatives need to be on mechanical ventilation longer than those who receive intermittent dosing. Additionally, an earlier randomized controlled trial13 showed that daily interruption of sedative drug infusions decreased the duration of mechanical ventilation by almost 50% and resulted in a reduction in the length of stay in the ICU.
Despite these findings, many ICU physicians remain skeptical of the value of daily interruption of sedative medications and question the safety of this practice.
The Awakening and Breathing Controlled (ABC) trial
GIRARD TD, KRESS JP, FUCHS BD, ET AL. EFFICACY AND SAFETY OF A PAIRED SEDATION AND VENTILATOR WEANING PROTOCOL FOR MECHANICALLY VENTILATED PATIENTS IN INTENSIVE CARE (AWAKENING AND BREATHING CONTROLLED TRIAL): A RANDOMISED CONTROLLED TRIAL. LANCET 2008; 371:126–134.
The Awakening and Breathing Controlled (ABC) trial14 was a multicenter, randomized controlled trial that included 336 patients who required at least 12 consecutive hours of mechanical ventilation. All patients had to be receiving patient-targeted sedation.
Those in the intervention group (n = 168) had their sedation interrupted every day, followed by a clinical assessment to determine whether they could be allowed to try breathing spontaneously. The control group (n = 168) also received a clinical assessment for a trial of spontaneous breathing, while their sedation was continued as usual.
In patients in the intervention group who failed the screening for a spontaneous breathing trial, the sedatives were resumed at half the previous dose. Criteria for failure on the spontaneous breathing trial included any of the following: anxiety, agitation, respiratory rate more than 35 breaths per minute for 5 minutes or longer, cardiac arrhythmia, oxygen saturation less than 88% for 5 minutes or longer, or two or more signs of respiratory distress, tachycardia, bradycardia, paradoxical breathing, accessory muscle use, diaphoresis, or marked dyspnea.
Interrupting sedation was superior
The combination of sedation interruption and a spontaneous breathing trial was superior to a spontaneous breathing trial alone. The mean number of ventilator-free days:
- 14.7 ± 0.9 with sedation interruption
- 11.6 ± 0.9 days with usual care (P = .02).
The median time to ICU discharge:
- 9.1 days with sedation interruption (interquartile range 5.1 to 17.8)
- 12.9 days with usual care (interquartile range 6.0 to 24.2, P = .01).
The mortality rate at 28 days:
- 28% with sedation interruption
- 35% with usual care (P = .21).
The mortality rate at 1 year:
- 44% with sedation interruption
- 58% with usual care (hazard ratio [HR] in the intervention group 0.68, 95% CI 0.50–0.92, P = .01).
Of note, patients in the intervention group had a higher rate of self-extubation (9.6% vs 3.6%, P = .03), but the rate of reintubation was similar between the groups (14% vs 13%, P = .47).
Comments
The addition of daily awakenings to spontaneous breathing trials results in a further reduction in the number of ICU days and increases the number of ventilator-free days.
Of note, the protocol allowed patients in the control group to undergo a spontaneous breathing trial while on sedatives (69% of the patients were receiving sedation at the time). Therefore, a bias effect in favor of the intervention group cannot be excluded. However, both groups had to meet criteria for readiness for spontaneous breathing.
The study demonstrates the safety of daily awakenings and confirms previous findings suggesting that a daily trial of spontaneous breathing results in better ICU outcomes.
GLUCOSE CONTROL IN THE ICU
Key points
- Although earlier studies suggested that intensive insulin therapy might be beneficial in critically ill patients, new findings show that strict glucose control can lead to complications without improving outcomes.
Background
A previous study15 found that intensive insulin therapy to maintain a blood glucose level between 80 and 110 mg/dL (compared with 180–200 mg/dL) reduced the mortality rate in surgical critical care patients. The mortality rate in the ICU was 4.6% with intensive insulin therapy vs 8.0% with conventional therapy (P < .04), and the effect was more robust for patients who remained longer than 5 days in the ICU (10.6% vs 20.2%).
Importantly, however, hypoglycemia (defined as blood glucose ≤ 40 mg/dL) occurred in 39 patients in the intensive-treatment group vs 6 patients in the conventional-treatment group.
The NICE-SUGAR trial
NICE-SUGAR STUDY INVESTIGATORS; FINFER S, CHITTOCK DR, SU SY, ET AL. INTENSIVE VERSUS CONVENTIONAL GLUCOSE CONTROL IN CRITICALLY ILL PATIENTS. N ENGL J MED 2009; 360:1283–1297.
The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial16 randomized 6,104 patients in medical and surgical ICUs to receive either intensive glucose control (blood glucose 81–108 mg/dL) with insulin therapy or conventional glucose control (blood glucose < 180 mg/dL). In the conventional-control group, insulin was discontinued if the blood glucose level dropped below 144 mg/dL.
A higher mortality rate with intensive glucose control
As expected, the intensive-control group achieved lower blood glucose levels: 115 vs 144 mg/dL.
Nevertheless, intensive glucose control was associated with a higher incidence of severe hypoglycemia, defined as a blood glucose level lower than 40 mg/dL: 6.8% vs 0.5%.
More importantly, compared with conventional insulin therapy, intensive glucose control was associated with a higher 90-day mortality rate: 27.5% vs 24.9% (odds ratio 1.14, 95% CI 1.02–1.28). These findings were similar in the subgroup of surgical patients (24.4% vs 19.8%, odds ratio 1.31, 95% CI 1.07–1.61).
Comments
Of note, the conventional-control group had more patients who discontinued the treatment protocol prematurely. Additionally, more patients in this group received corticosteroids.
These results widely differ from those of a previous study by van den Berghe et al,15 which showed that tight glycemic control is associated with a survival benefit. The differences in outcomes are probably largely related to different patient populations, as van den Berghe et al included patients who had undergone cardiac surgery, who were more likely to benefit from strict blood glucose control.
The VISEP trial
BRUNKHORST FM, ENGEL C, BLOOS F, ET AL; GERMAN COMPETENCE NETWORK SEPSIS (SEPNET). INTENSIVE INSULIN THERAPY AND PENTASTARCH RESUSCITATION IN SEVERE SEPSIS. N ENGL J MED 2008; 358:125–139.
The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial was a multicenter study designed to compare intensive insulin therapy (target blood glucose level 80–110 mg/dL) and conventional glucose control (target blood glucose level 180–200 mg/dL) in patients with severe sepsis.17 It also compared two fluids for volume resuscitation: 10% pentastarch vs modified Ringer's lactate. It included both medical and surgical patients.
Trial halted early for safety reasons
The mean morning blood glucose level was significantly lower in the intensive insulin group (112 vs 151 mg/dL).
Severe hypoglycemia (blood glucose ≤ 40 mg/dL) was more common in the group that received intensive insulin therapy (17% vs 4.1%, P < .001).
Mortality rates at 28 days did not differ significantly: 24.7% with intensive control vs 26.0% with conventional glucose control. The mortality rate at 90 days was 39.7% in the intensive therapy group and 35.4% in the conventional therapy group, but the difference was not statistically significant.
The intensive insulin arm of the trial was stopped after 488 patients were enrolled because of a higher rate of hypoglycemia (12.1% vs 2.1%) and of serious adverse events (10.9% vs 5.2%).
Additionally, the fluid resuscitation arm of the study was suspended at the first planned interim analysis because of a higher risk of organ failure in the 10% pentastarch group.
CORTICOSTEROID THERAPY IN SEPTIC SHOCK
Key points
- Corticosteroid therapy improves hemodynamic outcomes in patients with severe septic shock.
- Although meta-analyses suggest the mortality rate is lower with corticosteroid therapy, there is not enough evidence from randomized controlled trials to prove that the use of low-dose corticosteroids lowers the mortality rate in patients with septic shock.
- The corticotropin (ACTH) stimulation test should not be used to determine the need for corticosteroids in patients with septic shock.
Background
A previous multicenter study,18 performed in France, found that the use of corticosteroids in patients with septic shock resulted in lower rates of death at 28 days, in the ICU, and in the hospital and a shorter time to vasopressor withdrawal. Nevertheless, the beneficial effects were not observed in patients with adequate adrenal reserve (based on an ACTH stimulation test).
This study was criticized because of a high mortality rate in the placebo group.
The CORTICUS study
SPRUNG CL, ANNANE D, KEH D, ET AL; CORTICUS STUDY GROUP. HYDROCORTISONE THERAPY FOR PATIENTS WITH SEPTIC SHOCK. N ENGL J MED 2008; 358:111–124.
The Corticosteroid Therapy of Septic Shock (CORTICUS) study was a multicenter trial that randomly assigned 499 patients with septic shock to receive hydrocortisone (50 mg intravenously every 6 hours for 5 days, followed by a 6-day taper period) or placebo.19
Patients were eligible to be enrolled within 72 hours of onset of shock. Similar to previous studies, the CORTICUS trial classified patients on the basis of an ACTH stimulation test as having inadequate adrenal reserve (a cortisol increase of ≤ 9 μg/dL) or adequate adrenal reserve (a cortisol increase of > 9 μg/dL).
Faster reversal of shock with steroids
At baseline, the mean Simplified Acute Physiologic Score II (SAPS II) was 49 (the range of possible scores is 0 to 163; the higher the score the worse the organ dysfunction).
Hydrocortisone use resulted in a shorter duration of vasopressor use and a faster reversal of shock (3.3 days vs 5.8 days, P < .001).
This association was the same when patients were divided according to response to ACTH stimulation test. Time to reversal of shock in responders:
- 2.8 days with hydrocortisone
- 5.8 days with placebo (P < .001).
Time to reversal of shock in nonresponders:
- 3.9 days with hydrocortisone
- 6.0 days with placebo (P = .06).
Nevertheless, the treatment did not reduce the mortality rate at 28 days overall (34.3% vs 31.5% P = .51), or in the subgroups based on response to ACTH, or at any other time point. A post hoc analysis suggested that patients who had a systolic blood pressure of less than 90 mm Hg within 30 minutes of enrollment had a greater benefit in terms of mortality rate, but the effect was not statistically significant: the absolute difference was −11.2% (P = 0.28). Similarly, post hoc analyses also revealed a higher rate of death at 28 days in patients who received etomidate (Amidate) before randomization in both groups (P = .03).
Importantly, patients who received corticosteroids had a higher incidence of superinfections, including new episodes of sepsis or septic shock, with a combined odds ratio of 1.37 (95% CI 1.05–1.79).
Length of stay in the hospital or in the ICU was similar in patients who received corticosteroids and in those who received placebo. The ICU length of stay was 19 ± 31 days with hydrocortisone vs 18 ± 17 days with placebo (P = .51).
Comments
The CORTICUS trial showed that low-dose corticosteroid therapy results in faster reversal of shock in patients with severe septic shock. The hemodynamic benefits are present in all patients regardless of response to the ACTH stimulation test.
Nevertheless, contrary to previous findings,18 corticosteroid use was not associated with an improvement in mortality rates. Important differences exist between these two studies:
- The mortality rates in the placebo groups were significantly different (> 50% in the French study vs 30% in CORTICUS).
- The SAPS II scores were different in these two trials (55 vs 49), suggesting a greater severity of illness in the French study.
- The criteria for enrollment were different: the French study included patients who had a systolic blood pressure lower than 90 mm Hg for more than 1 hour despite fluid administration and vasopressor use, whereas the CORTICUS trial included patients who had a systolic blood pressure lower than 90 mm Hg for more than 1 hour despite fluid administration or vasopressor use.
- The time of enrollment was different: patients were enrolled much faster in the French study (within 8 hours) than in the CORTICUS trial (within 72 hours).
A recent meta-analysis of 17 randomized trials (including the CORTICUS study), found that, compared with those who received placebo, patients who received corticosteroids had a small reduction in the 28-day mortality rate (HR 0.84, 95% CI 0.71–1.00, P < .05).20 Of note, this meta-analysis has been criticized for possible publication bias and also for a large degree of heterogeneity in its results.21
VASOPRESSOR THERAPY IN SHOCK
Key points
- Vasopressin use in patients with severe septic shock is not associated with an improvement in mortality rates.
- Vasopressin should not be used as a first-line agent in patients with septic shock.
- Norepinephrine should be considered a first-line agent in patients with shock.
- Compared with norepinephrine, the use of dopamine in patients with shock is associated with similar mortality rates, although its use may result in a greater number of cardiac adverse events.
Background
Vasopressin gained popularity in critical care in the last 10 years because several small studies showed that adding it improves hemodynamics and results in a reduction in the doses of catecholamines in patients with refractory septic shock.22 Furthermore, the Surviving Sepsis Campaign guidelines recommended the use of vasopressin in patients who have refractory shock despite fluid resuscitation and the use of other “conventional” vasopressors.23
Despite these positive findings, it remained unknown if the use of vasopressin increases the survival rate in patients with septic shock.
The Vasopressin and Septic Shock Trial (VASST)
RUSSELL JA, WALLEY KR, SINGER J, ET AL; VASST INVESTIGATORS. VASOPRESSIN VERSUS NOREPINEPHRINE INFUSION IN PATIENTS WITH SEPTIC SHOCK. N ENGL J MED 2008; 358:877–887.
The Vasopressin and Septic Shock Trial (VASST)24 was a multicenter randomized, double-blind, controlled trial that included 778 patients with refractory septic shock. Refractory shock was defined as the lack of a response to a normal saline fluid bolus of 500 mL or the need for vasopressors (norepinephrine in doses of at least 5 μg/minute or its equivalent for 6 hours or more in the 24 hours before randomization).
Two subgroups were identified: those with severe septic shock (requiring norepinephrine in doses of 15 μg/minute or higher) and those with less-severe septic shock (needing norepinephrine in doses of 5 to 14 μg/minute). Patients with unstable coronary artery disease (acute myocardial infarction, angina) and severe congestive heart failure were excluded.
Patients were randomized to receive an intravenous infusion of vasopressin (0.01–0.03 U/minute) or norepinephrine (5–15 mg/minute) in addition to open-labeled vasopressors (excluding vasopressin). The primary outcome was the all-cause mortality rate at 28 days.
Results
At 28 days, fewer patients had died in the vasopressin group than in the norepinephrine group (35.4% vs 39.3%), but the difference was not statistically significant (P = .26). The trend was the same at 90 days (mortality rate 43.9% vs 49.6%, P = .11).
Subgroup analysis showed that in patients with less-severe septic shock, those who received vasopressin had a lower mortality rate at 28 days (26.5% vs 35.7%, P = .05; relative risk 0.74; 95% CI 0.55–1.01) and at 90 days (35.8% vs 46.1%, P = .04; relative risk 0.78, 95% CI 0.61–0.99).
There were no statistically significant differences in any of the other secondary outcomes or in serious adverse events.
Comments
The study has been criticized for several reasons:
- The mean arterial blood pressure at baseline before initiation of vasopressin was 72 mm Hg (and some argue that vasopressin was therefore not needed by the time it was started).
- The time from screening to infusion of the study drug was very long (12 hours).
- The observed mortality rate was lower than expected (37%).
Despite these considerations, the VASST trial showed that vasopressin is not associated with an increased number of adverse events in patients without active cardiovascular disease. The possible benefit in terms of the mortality rate in the subgroup of patients with less-severe septic shock requires further investigation.
Is dopamine equivalent to norepinephrine?
Previously, the Sepsis Occurrence in Acutely Ill Patients (SOAP) study, a multicenter, observational cohort study, found that dopamine use was associated with a higher all-cause mortality rate in the ICU compared with no dopamine.25 This finding had not been reproduced, as few well-designed studies had compared the effects of dopamine and norepinephrine.
The SOAP II study
DE BACKER D, BISTON P, DEVRIENDT J, ET AL; SOAP II INVESTIGATORS.. COMPARISON OF DOPAMINE AND NOREPINEPHRINE IN THE TREATMENT OF SHOCK. N ENGL J MED 2010; 362:779–789.
The SOAP II study,26 a multicenter, randomized trial, compared dopamine vs norepinephrine as first-line vasopressor therapy. In patients with refractory shock despite use of dopamine 20 μg/kg/minute or norepinephrine 0.19 μg/kg/minute, open-label norepinephrine, epinephrine, or vasopressin was added.
The primary outcome was the mortality rate at 28 days after randomization; secondary end points included the number of days without need for organ support and the occurrence of adverse events.
Results
A total of 1,679 patients were included; 858 were assigned to dopamine and 821 to norepinephrine. Most (1,044, 62%) of the patients had a diagnosis of septic shock.
No significant difference in mortality rates was noted at 28 days: 52.5% with dopamine vs 48.5% with norepinephrine (P = .10).
However, there were more arrhythmias in the patients treated with dopamine: 207 events (24.1%) vs 102 events (12.4%) (P < .001). The number of other adverse events such as renal failure, myocardial infarction, arterial occlusion, or skin necrosis was not different between the groups.
A subgroup analysis showed that dopamine was associated with more deaths at 28 days in patients with cardiogenic shock (P = .03) but not in patients with septic shock (P = .19) or with hypovolemic shock (P = .84).
Comments
The study was criticized because the patients may not have received adequate fluid resuscitation (the study considered adequate resuscitation to be equivalent to 1 L of crystalloids or 500 mL of colloids), as different degrees of volume depletion among patients make direct comparisons of vasopressor effects difficult.
Additionally, the study defined dopamine 20 μg/kg/minute as being equipotent with norepinephrine 0.19 μg/kg/minute. Comparisons of potency between drugs are difficult to establish, as there are no available data.
Nevertheless, this study further confirms previous findings suggesting that norepinephrine is not associated with more end-organ damage (such as renal failure or skin ischemia), and shows that dopamine may increase the number of adverse events, particularly in patients with cardiac disease.
- Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005; 294:1664–1670.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987; 317:1565–1570.
- Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998; 280:159–165.
- Steinberg KP, Hudson LD, Goodman RB, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354:1671–1684.
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007; 131:954–963.
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS results of a randomized controlled trial. 2007. Chest 2009; 136(suppl 5):e30.
- Annane D, Sébille V, Bellissant E; Ger-Inf-05 Study Group. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 2006; 34:22–30.
- Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998; 114:541–548.
- Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med 2006; 34:1326–1332.
- Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999; 27:2609–2615.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342:1471–1477.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871.
- Sprung CL, Annane D, Keh D, et al; CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124.
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009; 301:2362–2375.
- Minneci PC, Deans KJ, Natanson C. Corticosteroid therapy for severe sepsis and septic shock [letter]. JAMA 2009; 302:16443–1644.
- Kampmeier TG, Rehberg S, Westphal M, Lange M. Vasopressin in sepsis and septic shock. Minerva Anestesiol 2010; 76:844–850.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36:296–327.
- Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887.
- Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med 2006; 34:589–597.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
We have seen significant growth in clinical research in critical care medicine in the last decade. Advances have been made in many important areas in this field; of these, advances in treating septic shock and acute respiratory distress syndrome (ARDS), and also in supportive therapies for critically ill patients (eg, sedatives, insulin), have perhaps received the most attention.
Of note, several once-established therapies in these areas have failed the test of time, as the result of evidence from more-recent clinical trials. For example, recent studies have shown that a pulmonary arterial catheter does not improve outcomes in patients with ARDS. Similarly, what used to be “optimal” fluid management in patients with ARDS is no longer considered appropriate.
In this review, we summarize eight major studies in critical care medicine published in the last 5 years, studies that have contributed to changes in our practice in the intensive care unit (ICU).
FLUID MANAGEMENT IN ARDS
Key points
- In patients with acute lung injury (ALI) and ARDS, fluid restriction is associated with better outcomes than a liberal fluid policy.
- A pulmonary arterial catheter is not necessary and, compared with a central venous catheter, may result in more complications in patients with ALI and ARDS.
Background
Fluid management practices in patients with ARDS have been extremely variable. Two different approaches are commonly used: the liberal or “wet” approach to optimize tissue perfusion and the “dry” approach, which focuses on reducing lung edema. Given that most deaths attributed to ARDS result from extrapulmonary organ failure, aggressive fluid restriction has been the less popular approach.
Additionally, although earlier studies and meta-analyses suggested that the use of a pulmonary arterial catheter was not associated with better outcomes in critically ill patients,1 controversy remained regarding the value of a pulmonary arterial catheter compared with a central venous catheter in guiding fluid management in patients with ARDS, and data were insufficient to prove one strategy better than the other.
The Fluids and Catheter Treatment Trial (FACTT)
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK; WIEDEMANN HP, WHEELER AP, BERNARD GR, ET AL. COMPARISON OF TWO FLUID-MANAGEMENT STRATEGIES IN ACUTE LUNG INJURY. N ENGL J MED 2006; 354:2564–2575.
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK; WHEELER AP, BERNARD GR, THOMPSON BT, ET AL. PULMONARY-ARTERY VERSUS CENTRAL VENOUS CATHETER TO GUIDE TREATMENT OF ACUTE LUNG INJURY. N ENGL J MED 2006; 354:2213–2224.
The Fluids and Catheter Treatment Trial (FACTT) compared two fluid strategies2 and also the utility of a pulmonary arterial catheter vs a central venous catheter3 in patients with ALI or ARDS.
This two-by-two factorial trial randomized 1,000 patients to be treated according to either a conservative (fluid-restrictive or “dry”) or a liberal (“wet”) fluid management strategy for 7 days. Additionally, they were randomly assigned to receive either a central venous catheter or a pulmonary arterial catheter. The trial thus had four treatment groups:
- Fluid-restricted and a central venous catheter, with a goal of keeping the central venous pressure below 4 mm Hg
- Fluid-restricted and a pulmonary arterial catheter: fluids were restricted and diuretics were given to keep the pulmonary artery occlusion pressure below 8 mm Hg
- Fluid-liberal and a central venous catheter: fluids were given to keep the central venous pressure between 10 and 14 mm Hg
- Fluid-liberal and a pulmonary arterial catheter: fluids were given to keep the pulmonary artery occlusion pressure between 14 and 18 mm Hg.
The primary end point was the mortality rate at 60 days. Secondary end points included the number of ventilator-free days and organ-failure-free days and parameters of lung physiology. All patients were managed with a low-tidal-volume strategy.
The ‘dry’ strategy was better
The cumulative fluid balance was −136 mL ± 491 mL in the “dry” group and 6,992 mL ± 502 mL in the “wet” group, a difference of more than 7 L (P < .0001). Of note, before randomization, the patients were already fluid-positive, with a mean total fluid balance of +2,700 mL).2
At 60 days, no statistically significant difference in mortality rate was seen between the fluid-management groups (25.5% in the dry group vs 28.4% in the wet group (P = .30). Nevertheless, patients in the dry group had better oxygenation indices and lung injury scores (including lower plateau airway pressure), resulting in more ventilator-free days (14.6 ± 0.5 vs 12.1 ± 0.5; P = .0002) and ICU-free days (13.4 ± 0.4 vs 11.2 ± 0.4; P = .0003).2
Although those in the dry-strategy group had a slightly lower cardiac index and mean arterial pressure, they did not have a higher incidence of shock.
More importantly, the dry group did not have a higher rate of nonpulmonary organ failure. Serum creatinine and blood urea nitrogen concentrations were slightly higher in this group, but this was not associated with a higher incidence of renal failure or the use of dialysis: 10% in the dry-strategy group vs 14% in the wet-strategy group; P = .0642).2
No advantage with a pulmonary arterial catheter
The mortality rate did not differ between the catheter groups. However, the patients who received a pulmonary arterial catheter stayed in the ICU 0.2 days longer and had twice as many nonfatal cardiac arrhythmias as those who received a central venous catheter.3
Comments
The liberal fluid-strategy group had fluid balances similar to those seen in previous National Institutes of Health ARDS Network trials in which fluid management was not controlled. This suggests that the liberal fluid strategy reflects usual clinical practice.
Although the goals used in this study (central venous pressure < 4 mm Hg or pulmonary artery occlusion pressure < 8 mm Hg) could be difficult to achieve in clinical practice, a conservative strategy of fluid management is preferred in patients with ALI or ARDS, given the benefits observed in this trial.
A pulmonary arterial catheter is not indicated to guide hemodynamic management of patients with ARDS.
CORTICOSTEROID USE IN ARDS
Key points
- In selected patients with ARDS, the prolonged use of corticosteroids may result in better oxygenation and a shorter duration of mechanical ventilation.
- Late use of corticosteroids in patients with ARDS (> 14 days after diagnosis) is not indicated and may increase the risk of death.
- The role of corticosteroids in early ARDS (< 7 days after diagnosis) remains controversial.
Background
Systemic corticosteroid therapy was commonly used in ARDS patients in the 1970s and 1980s. However, a single-center study published in the late 1980s showed that a corticosteroid in high doses (methylprednisolone 30 mg/kg) resulted in more complications and was not associated with a lower mortality rate.4 On the other hand, a small study that included only patients with persistent ARDS (defined as ARDS lasting for more than 7 days) subsequently showed that oxygenation was significantly better and that fewer patients died while in the hospital with the use of methylprednisolone 2 mg/kg for 32 days.5
In view of these divergent findings, the ARDS Network decided to perform a study to help understand the role of corticosteroids in ARDS.
The Late Steroid Rescue Study (LaSRS)
STEINBERG KP, HUDSON LD, GOODMAN RB, ET AL; NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK. EFFICACY AND SAFETY OF CORTICOSTEROIDS FOR PERSISTENT ACUTE RESPIRATORY DISTRESS SYNDROME. N ENGL J MED 2006; 354:1671–1684.
The Late Steroid Rescue Study (LaSRS),6 a double-blind, multicenter trial, randomly assigned 180 patients with persistent ARDS (defined as ongoing disease 7–28 days after its onset) to receive methylprednisolone or placebo for 21 days.
Methylprednisolone was given in an initial dose of 2 mg/kg of predicted body weight followed by a dose of 0.5 mg/kg every 6 hours for 14 days and then a dose of 0.5 mg/kg every 12 hours for 7 days, and then it was tapered over 2 to 4 days and discontinued. It could be discontinued if 21 days of treatment were completed or if the patient was able to breathe without assistance.
The primary end point was the mortality rate at 60 days. Secondary end points included the number of ventilator-free days, organ-failure-free days, and complications and the levels of biomarkers of inflammation.
No reduction in mortality rates with steroids
The mortality rates did not differ significantly in the corticosteroid group vs the placebo group at 60 days:
- 29.2% with methylprednisolone (95% confidence interval [CI] 20.8–39.4)
- 28.6% with placebo (95% CI 20.3–38.6, P = 1.0).
Mortality rates at 180 days were also similar between the groups:
- 31.5% with methylprednisolone (95% CI 22.8–41.7)
- 31.9% with placebo (95% CI 23.2–42.0, P = 1.0).
In patients randomized between 7 and 13 days after the onset of ARDS, the mortality rates were lower in the methylprednisolone group than in the placebo group but the differences were not statistically significant. The mortality rate in this subgroup was 27% vs 36% (P = .26) at 60 days and was 27% vs 39% (P = .14) at 180 days.
However, in patients randomized more than 14 days after the onset of ARDS, the mortality rate was significantly higher in the methylprednisolone group than in the placebo group at 60 days (35% vs 8%, P = .02) and at 180 days (44% vs 12%, P = .01).
Some benefit in secondary outcomes
At day 28, methylprednisolone was associated with:
- More ventilator-free days (11.2 ± 9.4 vs 6.8 ± 8.5, P < .001)
- More shock-free days (20.7 ± 8.9 vs 17.9 ± 10.2, P = .04)
- More ICU-free days (8.9 ± 8.2 vs 6.7 ± 7.8, P = .02).
Similarly, pulmonary physiologic indices were better with methylprednisolone, specifically:
- The ratio of Pao2 to the fraction of inspired oxygen at days 3, 4, and 14 (P < .05)
- Plateau pressure at days 4, 5, and 7 (P < .05)
- Static compliance at days 7 and 14 (P < .05).
In terms of side effects, methylprednisolone was associated with more events associated with myopathy or neuropathy (9 vs 0, P = .001), but there were no differences in the number of serious infections or in glycemic control.
Comments
Although other recent studies suggested that corticosteroid use may be associated with a reduction in mortality rates,7–9 LaSRS did not confirm this effect. Although the doses and length of therapy were similar in these studies, LaSRS was much larger and included patients from the ARDS Network.
Nevertheless, LaSRS was criticized because of strict exclusion criteria and poor enrollment (only 5% of eligible patients were included). Additionally, it was conducted over a period of time when some ICU practices varied significantly (eg, low vs high tidal volume ventilation, tight vs loose glucose control).
INTERRUPTING SEDATION DURING MECHANICAL VENTILATION
Key points
- Daily awakening of mechanically ventilated patients is safe.
- Daily interruption of sedation in mechanically ventilated patients is associated with a shorter length of mechanical ventilation.
Background
Sedatives are a central component of critical care. Continuous infusions of narcotics, benzodiazepines, and anesthetic agents are frequently used to promote comfort in patients receiving mechanical ventilation.
Despite its widespread use in the ICU, there is little evidence that such sedation improves outcomes. Observational and randomized trials10–12 have shown that patients who receive continuous infusions of sedatives need to be on mechanical ventilation longer than those who receive intermittent dosing. Additionally, an earlier randomized controlled trial13 showed that daily interruption of sedative drug infusions decreased the duration of mechanical ventilation by almost 50% and resulted in a reduction in the length of stay in the ICU.
Despite these findings, many ICU physicians remain skeptical of the value of daily interruption of sedative medications and question the safety of this practice.
The Awakening and Breathing Controlled (ABC) trial
GIRARD TD, KRESS JP, FUCHS BD, ET AL. EFFICACY AND SAFETY OF A PAIRED SEDATION AND VENTILATOR WEANING PROTOCOL FOR MECHANICALLY VENTILATED PATIENTS IN INTENSIVE CARE (AWAKENING AND BREATHING CONTROLLED TRIAL): A RANDOMISED CONTROLLED TRIAL. LANCET 2008; 371:126–134.
The Awakening and Breathing Controlled (ABC) trial14 was a multicenter, randomized controlled trial that included 336 patients who required at least 12 consecutive hours of mechanical ventilation. All patients had to be receiving patient-targeted sedation.
Those in the intervention group (n = 168) had their sedation interrupted every day, followed by a clinical assessment to determine whether they could be allowed to try breathing spontaneously. The control group (n = 168) also received a clinical assessment for a trial of spontaneous breathing, while their sedation was continued as usual.
In patients in the intervention group who failed the screening for a spontaneous breathing trial, the sedatives were resumed at half the previous dose. Criteria for failure on the spontaneous breathing trial included any of the following: anxiety, agitation, respiratory rate more than 35 breaths per minute for 5 minutes or longer, cardiac arrhythmia, oxygen saturation less than 88% for 5 minutes or longer, or two or more signs of respiratory distress, tachycardia, bradycardia, paradoxical breathing, accessory muscle use, diaphoresis, or marked dyspnea.
Interrupting sedation was superior
The combination of sedation interruption and a spontaneous breathing trial was superior to a spontaneous breathing trial alone. The mean number of ventilator-free days:
- 14.7 ± 0.9 with sedation interruption
- 11.6 ± 0.9 days with usual care (P = .02).
The median time to ICU discharge:
- 9.1 days with sedation interruption (interquartile range 5.1 to 17.8)
- 12.9 days with usual care (interquartile range 6.0 to 24.2, P = .01).
The mortality rate at 28 days:
- 28% with sedation interruption
- 35% with usual care (P = .21).
The mortality rate at 1 year:
- 44% with sedation interruption
- 58% with usual care (hazard ratio [HR] in the intervention group 0.68, 95% CI 0.50–0.92, P = .01).
Of note, patients in the intervention group had a higher rate of self-extubation (9.6% vs 3.6%, P = .03), but the rate of reintubation was similar between the groups (14% vs 13%, P = .47).
Comments
The addition of daily awakenings to spontaneous breathing trials results in a further reduction in the number of ICU days and increases the number of ventilator-free days.
Of note, the protocol allowed patients in the control group to undergo a spontaneous breathing trial while on sedatives (69% of the patients were receiving sedation at the time). Therefore, a bias effect in favor of the intervention group cannot be excluded. However, both groups had to meet criteria for readiness for spontaneous breathing.
The study demonstrates the safety of daily awakenings and confirms previous findings suggesting that a daily trial of spontaneous breathing results in better ICU outcomes.
GLUCOSE CONTROL IN THE ICU
Key points
- Although earlier studies suggested that intensive insulin therapy might be beneficial in critically ill patients, new findings show that strict glucose control can lead to complications without improving outcomes.
Background
A previous study15 found that intensive insulin therapy to maintain a blood glucose level between 80 and 110 mg/dL (compared with 180–200 mg/dL) reduced the mortality rate in surgical critical care patients. The mortality rate in the ICU was 4.6% with intensive insulin therapy vs 8.0% with conventional therapy (P < .04), and the effect was more robust for patients who remained longer than 5 days in the ICU (10.6% vs 20.2%).
Importantly, however, hypoglycemia (defined as blood glucose ≤ 40 mg/dL) occurred in 39 patients in the intensive-treatment group vs 6 patients in the conventional-treatment group.
The NICE-SUGAR trial
NICE-SUGAR STUDY INVESTIGATORS; FINFER S, CHITTOCK DR, SU SY, ET AL. INTENSIVE VERSUS CONVENTIONAL GLUCOSE CONTROL IN CRITICALLY ILL PATIENTS. N ENGL J MED 2009; 360:1283–1297.
The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial16 randomized 6,104 patients in medical and surgical ICUs to receive either intensive glucose control (blood glucose 81–108 mg/dL) with insulin therapy or conventional glucose control (blood glucose < 180 mg/dL). In the conventional-control group, insulin was discontinued if the blood glucose level dropped below 144 mg/dL.
A higher mortality rate with intensive glucose control
As expected, the intensive-control group achieved lower blood glucose levels: 115 vs 144 mg/dL.
Nevertheless, intensive glucose control was associated with a higher incidence of severe hypoglycemia, defined as a blood glucose level lower than 40 mg/dL: 6.8% vs 0.5%.
More importantly, compared with conventional insulin therapy, intensive glucose control was associated with a higher 90-day mortality rate: 27.5% vs 24.9% (odds ratio 1.14, 95% CI 1.02–1.28). These findings were similar in the subgroup of surgical patients (24.4% vs 19.8%, odds ratio 1.31, 95% CI 1.07–1.61).
Comments
Of note, the conventional-control group had more patients who discontinued the treatment protocol prematurely. Additionally, more patients in this group received corticosteroids.
These results widely differ from those of a previous study by van den Berghe et al,15 which showed that tight glycemic control is associated with a survival benefit. The differences in outcomes are probably largely related to different patient populations, as van den Berghe et al included patients who had undergone cardiac surgery, who were more likely to benefit from strict blood glucose control.
The VISEP trial
BRUNKHORST FM, ENGEL C, BLOOS F, ET AL; GERMAN COMPETENCE NETWORK SEPSIS (SEPNET). INTENSIVE INSULIN THERAPY AND PENTASTARCH RESUSCITATION IN SEVERE SEPSIS. N ENGL J MED 2008; 358:125–139.
The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial was a multicenter study designed to compare intensive insulin therapy (target blood glucose level 80–110 mg/dL) and conventional glucose control (target blood glucose level 180–200 mg/dL) in patients with severe sepsis.17 It also compared two fluids for volume resuscitation: 10% pentastarch vs modified Ringer's lactate. It included both medical and surgical patients.
Trial halted early for safety reasons
The mean morning blood glucose level was significantly lower in the intensive insulin group (112 vs 151 mg/dL).
Severe hypoglycemia (blood glucose ≤ 40 mg/dL) was more common in the group that received intensive insulin therapy (17% vs 4.1%, P < .001).
Mortality rates at 28 days did not differ significantly: 24.7% with intensive control vs 26.0% with conventional glucose control. The mortality rate at 90 days was 39.7% in the intensive therapy group and 35.4% in the conventional therapy group, but the difference was not statistically significant.
The intensive insulin arm of the trial was stopped after 488 patients were enrolled because of a higher rate of hypoglycemia (12.1% vs 2.1%) and of serious adverse events (10.9% vs 5.2%).
Additionally, the fluid resuscitation arm of the study was suspended at the first planned interim analysis because of a higher risk of organ failure in the 10% pentastarch group.
CORTICOSTEROID THERAPY IN SEPTIC SHOCK
Key points
- Corticosteroid therapy improves hemodynamic outcomes in patients with severe septic shock.
- Although meta-analyses suggest the mortality rate is lower with corticosteroid therapy, there is not enough evidence from randomized controlled trials to prove that the use of low-dose corticosteroids lowers the mortality rate in patients with septic shock.
- The corticotropin (ACTH) stimulation test should not be used to determine the need for corticosteroids in patients with septic shock.
Background
A previous multicenter study,18 performed in France, found that the use of corticosteroids in patients with septic shock resulted in lower rates of death at 28 days, in the ICU, and in the hospital and a shorter time to vasopressor withdrawal. Nevertheless, the beneficial effects were not observed in patients with adequate adrenal reserve (based on an ACTH stimulation test).
This study was criticized because of a high mortality rate in the placebo group.
The CORTICUS study
SPRUNG CL, ANNANE D, KEH D, ET AL; CORTICUS STUDY GROUP. HYDROCORTISONE THERAPY FOR PATIENTS WITH SEPTIC SHOCK. N ENGL J MED 2008; 358:111–124.
The Corticosteroid Therapy of Septic Shock (CORTICUS) study was a multicenter trial that randomly assigned 499 patients with septic shock to receive hydrocortisone (50 mg intravenously every 6 hours for 5 days, followed by a 6-day taper period) or placebo.19
Patients were eligible to be enrolled within 72 hours of onset of shock. Similar to previous studies, the CORTICUS trial classified patients on the basis of an ACTH stimulation test as having inadequate adrenal reserve (a cortisol increase of ≤ 9 μg/dL) or adequate adrenal reserve (a cortisol increase of > 9 μg/dL).
Faster reversal of shock with steroids
At baseline, the mean Simplified Acute Physiologic Score II (SAPS II) was 49 (the range of possible scores is 0 to 163; the higher the score the worse the organ dysfunction).
Hydrocortisone use resulted in a shorter duration of vasopressor use and a faster reversal of shock (3.3 days vs 5.8 days, P < .001).
This association was the same when patients were divided according to response to ACTH stimulation test. Time to reversal of shock in responders:
- 2.8 days with hydrocortisone
- 5.8 days with placebo (P < .001).
Time to reversal of shock in nonresponders:
- 3.9 days with hydrocortisone
- 6.0 days with placebo (P = .06).
Nevertheless, the treatment did not reduce the mortality rate at 28 days overall (34.3% vs 31.5% P = .51), or in the subgroups based on response to ACTH, or at any other time point. A post hoc analysis suggested that patients who had a systolic blood pressure of less than 90 mm Hg within 30 minutes of enrollment had a greater benefit in terms of mortality rate, but the effect was not statistically significant: the absolute difference was −11.2% (P = 0.28). Similarly, post hoc analyses also revealed a higher rate of death at 28 days in patients who received etomidate (Amidate) before randomization in both groups (P = .03).
Importantly, patients who received corticosteroids had a higher incidence of superinfections, including new episodes of sepsis or septic shock, with a combined odds ratio of 1.37 (95% CI 1.05–1.79).
Length of stay in the hospital or in the ICU was similar in patients who received corticosteroids and in those who received placebo. The ICU length of stay was 19 ± 31 days with hydrocortisone vs 18 ± 17 days with placebo (P = .51).
Comments
The CORTICUS trial showed that low-dose corticosteroid therapy results in faster reversal of shock in patients with severe septic shock. The hemodynamic benefits are present in all patients regardless of response to the ACTH stimulation test.
Nevertheless, contrary to previous findings,18 corticosteroid use was not associated with an improvement in mortality rates. Important differences exist between these two studies:
- The mortality rates in the placebo groups were significantly different (> 50% in the French study vs 30% in CORTICUS).
- The SAPS II scores were different in these two trials (55 vs 49), suggesting a greater severity of illness in the French study.
- The criteria for enrollment were different: the French study included patients who had a systolic blood pressure lower than 90 mm Hg for more than 1 hour despite fluid administration and vasopressor use, whereas the CORTICUS trial included patients who had a systolic blood pressure lower than 90 mm Hg for more than 1 hour despite fluid administration or vasopressor use.
- The time of enrollment was different: patients were enrolled much faster in the French study (within 8 hours) than in the CORTICUS trial (within 72 hours).
A recent meta-analysis of 17 randomized trials (including the CORTICUS study), found that, compared with those who received placebo, patients who received corticosteroids had a small reduction in the 28-day mortality rate (HR 0.84, 95% CI 0.71–1.00, P < .05).20 Of note, this meta-analysis has been criticized for possible publication bias and also for a large degree of heterogeneity in its results.21
VASOPRESSOR THERAPY IN SHOCK
Key points
- Vasopressin use in patients with severe septic shock is not associated with an improvement in mortality rates.
- Vasopressin should not be used as a first-line agent in patients with septic shock.
- Norepinephrine should be considered a first-line agent in patients with shock.
- Compared with norepinephrine, the use of dopamine in patients with shock is associated with similar mortality rates, although its use may result in a greater number of cardiac adverse events.
Background
Vasopressin gained popularity in critical care in the last 10 years because several small studies showed that adding it improves hemodynamics and results in a reduction in the doses of catecholamines in patients with refractory septic shock.22 Furthermore, the Surviving Sepsis Campaign guidelines recommended the use of vasopressin in patients who have refractory shock despite fluid resuscitation and the use of other “conventional” vasopressors.23
Despite these positive findings, it remained unknown if the use of vasopressin increases the survival rate in patients with septic shock.
The Vasopressin and Septic Shock Trial (VASST)
RUSSELL JA, WALLEY KR, SINGER J, ET AL; VASST INVESTIGATORS. VASOPRESSIN VERSUS NOREPINEPHRINE INFUSION IN PATIENTS WITH SEPTIC SHOCK. N ENGL J MED 2008; 358:877–887.
The Vasopressin and Septic Shock Trial (VASST)24 was a multicenter randomized, double-blind, controlled trial that included 778 patients with refractory septic shock. Refractory shock was defined as the lack of a response to a normal saline fluid bolus of 500 mL or the need for vasopressors (norepinephrine in doses of at least 5 μg/minute or its equivalent for 6 hours or more in the 24 hours before randomization).
Two subgroups were identified: those with severe septic shock (requiring norepinephrine in doses of 15 μg/minute or higher) and those with less-severe septic shock (needing norepinephrine in doses of 5 to 14 μg/minute). Patients with unstable coronary artery disease (acute myocardial infarction, angina) and severe congestive heart failure were excluded.
Patients were randomized to receive an intravenous infusion of vasopressin (0.01–0.03 U/minute) or norepinephrine (5–15 mg/minute) in addition to open-labeled vasopressors (excluding vasopressin). The primary outcome was the all-cause mortality rate at 28 days.
Results
At 28 days, fewer patients had died in the vasopressin group than in the norepinephrine group (35.4% vs 39.3%), but the difference was not statistically significant (P = .26). The trend was the same at 90 days (mortality rate 43.9% vs 49.6%, P = .11).
Subgroup analysis showed that in patients with less-severe septic shock, those who received vasopressin had a lower mortality rate at 28 days (26.5% vs 35.7%, P = .05; relative risk 0.74; 95% CI 0.55–1.01) and at 90 days (35.8% vs 46.1%, P = .04; relative risk 0.78, 95% CI 0.61–0.99).
There were no statistically significant differences in any of the other secondary outcomes or in serious adverse events.
Comments
The study has been criticized for several reasons:
- The mean arterial blood pressure at baseline before initiation of vasopressin was 72 mm Hg (and some argue that vasopressin was therefore not needed by the time it was started).
- The time from screening to infusion of the study drug was very long (12 hours).
- The observed mortality rate was lower than expected (37%).
Despite these considerations, the VASST trial showed that vasopressin is not associated with an increased number of adverse events in patients without active cardiovascular disease. The possible benefit in terms of the mortality rate in the subgroup of patients with less-severe septic shock requires further investigation.
Is dopamine equivalent to norepinephrine?
Previously, the Sepsis Occurrence in Acutely Ill Patients (SOAP) study, a multicenter, observational cohort study, found that dopamine use was associated with a higher all-cause mortality rate in the ICU compared with no dopamine.25 This finding had not been reproduced, as few well-designed studies had compared the effects of dopamine and norepinephrine.
The SOAP II study
DE BACKER D, BISTON P, DEVRIENDT J, ET AL; SOAP II INVESTIGATORS.. COMPARISON OF DOPAMINE AND NOREPINEPHRINE IN THE TREATMENT OF SHOCK. N ENGL J MED 2010; 362:779–789.
The SOAP II study,26 a multicenter, randomized trial, compared dopamine vs norepinephrine as first-line vasopressor therapy. In patients with refractory shock despite use of dopamine 20 μg/kg/minute or norepinephrine 0.19 μg/kg/minute, open-label norepinephrine, epinephrine, or vasopressin was added.
The primary outcome was the mortality rate at 28 days after randomization; secondary end points included the number of days without need for organ support and the occurrence of adverse events.
Results
A total of 1,679 patients were included; 858 were assigned to dopamine and 821 to norepinephrine. Most (1,044, 62%) of the patients had a diagnosis of septic shock.
No significant difference in mortality rates was noted at 28 days: 52.5% with dopamine vs 48.5% with norepinephrine (P = .10).
However, there were more arrhythmias in the patients treated with dopamine: 207 events (24.1%) vs 102 events (12.4%) (P < .001). The number of other adverse events such as renal failure, myocardial infarction, arterial occlusion, or skin necrosis was not different between the groups.
A subgroup analysis showed that dopamine was associated with more deaths at 28 days in patients with cardiogenic shock (P = .03) but not in patients with septic shock (P = .19) or with hypovolemic shock (P = .84).
Comments
The study was criticized because the patients may not have received adequate fluid resuscitation (the study considered adequate resuscitation to be equivalent to 1 L of crystalloids or 500 mL of colloids), as different degrees of volume depletion among patients make direct comparisons of vasopressor effects difficult.
Additionally, the study defined dopamine 20 μg/kg/minute as being equipotent with norepinephrine 0.19 μg/kg/minute. Comparisons of potency between drugs are difficult to establish, as there are no available data.
Nevertheless, this study further confirms previous findings suggesting that norepinephrine is not associated with more end-organ damage (such as renal failure or skin ischemia), and shows that dopamine may increase the number of adverse events, particularly in patients with cardiac disease.
We have seen significant growth in clinical research in critical care medicine in the last decade. Advances have been made in many important areas in this field; of these, advances in treating septic shock and acute respiratory distress syndrome (ARDS), and also in supportive therapies for critically ill patients (eg, sedatives, insulin), have perhaps received the most attention.
Of note, several once-established therapies in these areas have failed the test of time, as the result of evidence from more-recent clinical trials. For example, recent studies have shown that a pulmonary arterial catheter does not improve outcomes in patients with ARDS. Similarly, what used to be “optimal” fluid management in patients with ARDS is no longer considered appropriate.
In this review, we summarize eight major studies in critical care medicine published in the last 5 years, studies that have contributed to changes in our practice in the intensive care unit (ICU).
FLUID MANAGEMENT IN ARDS
Key points
- In patients with acute lung injury (ALI) and ARDS, fluid restriction is associated with better outcomes than a liberal fluid policy.
- A pulmonary arterial catheter is not necessary and, compared with a central venous catheter, may result in more complications in patients with ALI and ARDS.
Background
Fluid management practices in patients with ARDS have been extremely variable. Two different approaches are commonly used: the liberal or “wet” approach to optimize tissue perfusion and the “dry” approach, which focuses on reducing lung edema. Given that most deaths attributed to ARDS result from extrapulmonary organ failure, aggressive fluid restriction has been the less popular approach.
Additionally, although earlier studies and meta-analyses suggested that the use of a pulmonary arterial catheter was not associated with better outcomes in critically ill patients,1 controversy remained regarding the value of a pulmonary arterial catheter compared with a central venous catheter in guiding fluid management in patients with ARDS, and data were insufficient to prove one strategy better than the other.
The Fluids and Catheter Treatment Trial (FACTT)
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK; WIEDEMANN HP, WHEELER AP, BERNARD GR, ET AL. COMPARISON OF TWO FLUID-MANAGEMENT STRATEGIES IN ACUTE LUNG INJURY. N ENGL J MED 2006; 354:2564–2575.
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK; WHEELER AP, BERNARD GR, THOMPSON BT, ET AL. PULMONARY-ARTERY VERSUS CENTRAL VENOUS CATHETER TO GUIDE TREATMENT OF ACUTE LUNG INJURY. N ENGL J MED 2006; 354:2213–2224.
The Fluids and Catheter Treatment Trial (FACTT) compared two fluid strategies2 and also the utility of a pulmonary arterial catheter vs a central venous catheter3 in patients with ALI or ARDS.
This two-by-two factorial trial randomized 1,000 patients to be treated according to either a conservative (fluid-restrictive or “dry”) or a liberal (“wet”) fluid management strategy for 7 days. Additionally, they were randomly assigned to receive either a central venous catheter or a pulmonary arterial catheter. The trial thus had four treatment groups:
- Fluid-restricted and a central venous catheter, with a goal of keeping the central venous pressure below 4 mm Hg
- Fluid-restricted and a pulmonary arterial catheter: fluids were restricted and diuretics were given to keep the pulmonary artery occlusion pressure below 8 mm Hg
- Fluid-liberal and a central venous catheter: fluids were given to keep the central venous pressure between 10 and 14 mm Hg
- Fluid-liberal and a pulmonary arterial catheter: fluids were given to keep the pulmonary artery occlusion pressure between 14 and 18 mm Hg.
The primary end point was the mortality rate at 60 days. Secondary end points included the number of ventilator-free days and organ-failure-free days and parameters of lung physiology. All patients were managed with a low-tidal-volume strategy.
The ‘dry’ strategy was better
The cumulative fluid balance was −136 mL ± 491 mL in the “dry” group and 6,992 mL ± 502 mL in the “wet” group, a difference of more than 7 L (P < .0001). Of note, before randomization, the patients were already fluid-positive, with a mean total fluid balance of +2,700 mL).2
At 60 days, no statistically significant difference in mortality rate was seen between the fluid-management groups (25.5% in the dry group vs 28.4% in the wet group (P = .30). Nevertheless, patients in the dry group had better oxygenation indices and lung injury scores (including lower plateau airway pressure), resulting in more ventilator-free days (14.6 ± 0.5 vs 12.1 ± 0.5; P = .0002) and ICU-free days (13.4 ± 0.4 vs 11.2 ± 0.4; P = .0003).2
Although those in the dry-strategy group had a slightly lower cardiac index and mean arterial pressure, they did not have a higher incidence of shock.
More importantly, the dry group did not have a higher rate of nonpulmonary organ failure. Serum creatinine and blood urea nitrogen concentrations were slightly higher in this group, but this was not associated with a higher incidence of renal failure or the use of dialysis: 10% in the dry-strategy group vs 14% in the wet-strategy group; P = .0642).2
No advantage with a pulmonary arterial catheter
The mortality rate did not differ between the catheter groups. However, the patients who received a pulmonary arterial catheter stayed in the ICU 0.2 days longer and had twice as many nonfatal cardiac arrhythmias as those who received a central venous catheter.3
Comments
The liberal fluid-strategy group had fluid balances similar to those seen in previous National Institutes of Health ARDS Network trials in which fluid management was not controlled. This suggests that the liberal fluid strategy reflects usual clinical practice.
Although the goals used in this study (central venous pressure < 4 mm Hg or pulmonary artery occlusion pressure < 8 mm Hg) could be difficult to achieve in clinical practice, a conservative strategy of fluid management is preferred in patients with ALI or ARDS, given the benefits observed in this trial.
A pulmonary arterial catheter is not indicated to guide hemodynamic management of patients with ARDS.
CORTICOSTEROID USE IN ARDS
Key points
- In selected patients with ARDS, the prolonged use of corticosteroids may result in better oxygenation and a shorter duration of mechanical ventilation.
- Late use of corticosteroids in patients with ARDS (> 14 days after diagnosis) is not indicated and may increase the risk of death.
- The role of corticosteroids in early ARDS (< 7 days after diagnosis) remains controversial.
Background
Systemic corticosteroid therapy was commonly used in ARDS patients in the 1970s and 1980s. However, a single-center study published in the late 1980s showed that a corticosteroid in high doses (methylprednisolone 30 mg/kg) resulted in more complications and was not associated with a lower mortality rate.4 On the other hand, a small study that included only patients with persistent ARDS (defined as ARDS lasting for more than 7 days) subsequently showed that oxygenation was significantly better and that fewer patients died while in the hospital with the use of methylprednisolone 2 mg/kg for 32 days.5
In view of these divergent findings, the ARDS Network decided to perform a study to help understand the role of corticosteroids in ARDS.
The Late Steroid Rescue Study (LaSRS)
STEINBERG KP, HUDSON LD, GOODMAN RB, ET AL; NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) CLINICAL TRIALS NETWORK. EFFICACY AND SAFETY OF CORTICOSTEROIDS FOR PERSISTENT ACUTE RESPIRATORY DISTRESS SYNDROME. N ENGL J MED 2006; 354:1671–1684.
The Late Steroid Rescue Study (LaSRS),6 a double-blind, multicenter trial, randomly assigned 180 patients with persistent ARDS (defined as ongoing disease 7–28 days after its onset) to receive methylprednisolone or placebo for 21 days.
Methylprednisolone was given in an initial dose of 2 mg/kg of predicted body weight followed by a dose of 0.5 mg/kg every 6 hours for 14 days and then a dose of 0.5 mg/kg every 12 hours for 7 days, and then it was tapered over 2 to 4 days and discontinued. It could be discontinued if 21 days of treatment were completed or if the patient was able to breathe without assistance.
The primary end point was the mortality rate at 60 days. Secondary end points included the number of ventilator-free days, organ-failure-free days, and complications and the levels of biomarkers of inflammation.
No reduction in mortality rates with steroids
The mortality rates did not differ significantly in the corticosteroid group vs the placebo group at 60 days:
- 29.2% with methylprednisolone (95% confidence interval [CI] 20.8–39.4)
- 28.6% with placebo (95% CI 20.3–38.6, P = 1.0).
Mortality rates at 180 days were also similar between the groups:
- 31.5% with methylprednisolone (95% CI 22.8–41.7)
- 31.9% with placebo (95% CI 23.2–42.0, P = 1.0).
In patients randomized between 7 and 13 days after the onset of ARDS, the mortality rates were lower in the methylprednisolone group than in the placebo group but the differences were not statistically significant. The mortality rate in this subgroup was 27% vs 36% (P = .26) at 60 days and was 27% vs 39% (P = .14) at 180 days.
However, in patients randomized more than 14 days after the onset of ARDS, the mortality rate was significantly higher in the methylprednisolone group than in the placebo group at 60 days (35% vs 8%, P = .02) and at 180 days (44% vs 12%, P = .01).
Some benefit in secondary outcomes
At day 28, methylprednisolone was associated with:
- More ventilator-free days (11.2 ± 9.4 vs 6.8 ± 8.5, P < .001)
- More shock-free days (20.7 ± 8.9 vs 17.9 ± 10.2, P = .04)
- More ICU-free days (8.9 ± 8.2 vs 6.7 ± 7.8, P = .02).
Similarly, pulmonary physiologic indices were better with methylprednisolone, specifically:
- The ratio of Pao2 to the fraction of inspired oxygen at days 3, 4, and 14 (P < .05)
- Plateau pressure at days 4, 5, and 7 (P < .05)
- Static compliance at days 7 and 14 (P < .05).
In terms of side effects, methylprednisolone was associated with more events associated with myopathy or neuropathy (9 vs 0, P = .001), but there were no differences in the number of serious infections or in glycemic control.
Comments
Although other recent studies suggested that corticosteroid use may be associated with a reduction in mortality rates,7–9 LaSRS did not confirm this effect. Although the doses and length of therapy were similar in these studies, LaSRS was much larger and included patients from the ARDS Network.
Nevertheless, LaSRS was criticized because of strict exclusion criteria and poor enrollment (only 5% of eligible patients were included). Additionally, it was conducted over a period of time when some ICU practices varied significantly (eg, low vs high tidal volume ventilation, tight vs loose glucose control).
INTERRUPTING SEDATION DURING MECHANICAL VENTILATION
Key points
- Daily awakening of mechanically ventilated patients is safe.
- Daily interruption of sedation in mechanically ventilated patients is associated with a shorter length of mechanical ventilation.
Background
Sedatives are a central component of critical care. Continuous infusions of narcotics, benzodiazepines, and anesthetic agents are frequently used to promote comfort in patients receiving mechanical ventilation.
Despite its widespread use in the ICU, there is little evidence that such sedation improves outcomes. Observational and randomized trials10–12 have shown that patients who receive continuous infusions of sedatives need to be on mechanical ventilation longer than those who receive intermittent dosing. Additionally, an earlier randomized controlled trial13 showed that daily interruption of sedative drug infusions decreased the duration of mechanical ventilation by almost 50% and resulted in a reduction in the length of stay in the ICU.
Despite these findings, many ICU physicians remain skeptical of the value of daily interruption of sedative medications and question the safety of this practice.
The Awakening and Breathing Controlled (ABC) trial
GIRARD TD, KRESS JP, FUCHS BD, ET AL. EFFICACY AND SAFETY OF A PAIRED SEDATION AND VENTILATOR WEANING PROTOCOL FOR MECHANICALLY VENTILATED PATIENTS IN INTENSIVE CARE (AWAKENING AND BREATHING CONTROLLED TRIAL): A RANDOMISED CONTROLLED TRIAL. LANCET 2008; 371:126–134.
The Awakening and Breathing Controlled (ABC) trial14 was a multicenter, randomized controlled trial that included 336 patients who required at least 12 consecutive hours of mechanical ventilation. All patients had to be receiving patient-targeted sedation.
Those in the intervention group (n = 168) had their sedation interrupted every day, followed by a clinical assessment to determine whether they could be allowed to try breathing spontaneously. The control group (n = 168) also received a clinical assessment for a trial of spontaneous breathing, while their sedation was continued as usual.
In patients in the intervention group who failed the screening for a spontaneous breathing trial, the sedatives were resumed at half the previous dose. Criteria for failure on the spontaneous breathing trial included any of the following: anxiety, agitation, respiratory rate more than 35 breaths per minute for 5 minutes or longer, cardiac arrhythmia, oxygen saturation less than 88% for 5 minutes or longer, or two or more signs of respiratory distress, tachycardia, bradycardia, paradoxical breathing, accessory muscle use, diaphoresis, or marked dyspnea.
Interrupting sedation was superior
The combination of sedation interruption and a spontaneous breathing trial was superior to a spontaneous breathing trial alone. The mean number of ventilator-free days:
- 14.7 ± 0.9 with sedation interruption
- 11.6 ± 0.9 days with usual care (P = .02).
The median time to ICU discharge:
- 9.1 days with sedation interruption (interquartile range 5.1 to 17.8)
- 12.9 days with usual care (interquartile range 6.0 to 24.2, P = .01).
The mortality rate at 28 days:
- 28% with sedation interruption
- 35% with usual care (P = .21).
The mortality rate at 1 year:
- 44% with sedation interruption
- 58% with usual care (hazard ratio [HR] in the intervention group 0.68, 95% CI 0.50–0.92, P = .01).
Of note, patients in the intervention group had a higher rate of self-extubation (9.6% vs 3.6%, P = .03), but the rate of reintubation was similar between the groups (14% vs 13%, P = .47).
Comments
The addition of daily awakenings to spontaneous breathing trials results in a further reduction in the number of ICU days and increases the number of ventilator-free days.
Of note, the protocol allowed patients in the control group to undergo a spontaneous breathing trial while on sedatives (69% of the patients were receiving sedation at the time). Therefore, a bias effect in favor of the intervention group cannot be excluded. However, both groups had to meet criteria for readiness for spontaneous breathing.
The study demonstrates the safety of daily awakenings and confirms previous findings suggesting that a daily trial of spontaneous breathing results in better ICU outcomes.
GLUCOSE CONTROL IN THE ICU
Key points
- Although earlier studies suggested that intensive insulin therapy might be beneficial in critically ill patients, new findings show that strict glucose control can lead to complications without improving outcomes.
Background
A previous study15 found that intensive insulin therapy to maintain a blood glucose level between 80 and 110 mg/dL (compared with 180–200 mg/dL) reduced the mortality rate in surgical critical care patients. The mortality rate in the ICU was 4.6% with intensive insulin therapy vs 8.0% with conventional therapy (P < .04), and the effect was more robust for patients who remained longer than 5 days in the ICU (10.6% vs 20.2%).
Importantly, however, hypoglycemia (defined as blood glucose ≤ 40 mg/dL) occurred in 39 patients in the intensive-treatment group vs 6 patients in the conventional-treatment group.
The NICE-SUGAR trial
NICE-SUGAR STUDY INVESTIGATORS; FINFER S, CHITTOCK DR, SU SY, ET AL. INTENSIVE VERSUS CONVENTIONAL GLUCOSE CONTROL IN CRITICALLY ILL PATIENTS. N ENGL J MED 2009; 360:1283–1297.
The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial16 randomized 6,104 patients in medical and surgical ICUs to receive either intensive glucose control (blood glucose 81–108 mg/dL) with insulin therapy or conventional glucose control (blood glucose < 180 mg/dL). In the conventional-control group, insulin was discontinued if the blood glucose level dropped below 144 mg/dL.
A higher mortality rate with intensive glucose control
As expected, the intensive-control group achieved lower blood glucose levels: 115 vs 144 mg/dL.
Nevertheless, intensive glucose control was associated with a higher incidence of severe hypoglycemia, defined as a blood glucose level lower than 40 mg/dL: 6.8% vs 0.5%.
More importantly, compared with conventional insulin therapy, intensive glucose control was associated with a higher 90-day mortality rate: 27.5% vs 24.9% (odds ratio 1.14, 95% CI 1.02–1.28). These findings were similar in the subgroup of surgical patients (24.4% vs 19.8%, odds ratio 1.31, 95% CI 1.07–1.61).
Comments
Of note, the conventional-control group had more patients who discontinued the treatment protocol prematurely. Additionally, more patients in this group received corticosteroids.
These results widely differ from those of a previous study by van den Berghe et al,15 which showed that tight glycemic control is associated with a survival benefit. The differences in outcomes are probably largely related to different patient populations, as van den Berghe et al included patients who had undergone cardiac surgery, who were more likely to benefit from strict blood glucose control.
The VISEP trial
BRUNKHORST FM, ENGEL C, BLOOS F, ET AL; GERMAN COMPETENCE NETWORK SEPSIS (SEPNET). INTENSIVE INSULIN THERAPY AND PENTASTARCH RESUSCITATION IN SEVERE SEPSIS. N ENGL J MED 2008; 358:125–139.
The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial was a multicenter study designed to compare intensive insulin therapy (target blood glucose level 80–110 mg/dL) and conventional glucose control (target blood glucose level 180–200 mg/dL) in patients with severe sepsis.17 It also compared two fluids for volume resuscitation: 10% pentastarch vs modified Ringer's lactate. It included both medical and surgical patients.
Trial halted early for safety reasons
The mean morning blood glucose level was significantly lower in the intensive insulin group (112 vs 151 mg/dL).
Severe hypoglycemia (blood glucose ≤ 40 mg/dL) was more common in the group that received intensive insulin therapy (17% vs 4.1%, P < .001).
Mortality rates at 28 days did not differ significantly: 24.7% with intensive control vs 26.0% with conventional glucose control. The mortality rate at 90 days was 39.7% in the intensive therapy group and 35.4% in the conventional therapy group, but the difference was not statistically significant.
The intensive insulin arm of the trial was stopped after 488 patients were enrolled because of a higher rate of hypoglycemia (12.1% vs 2.1%) and of serious adverse events (10.9% vs 5.2%).
Additionally, the fluid resuscitation arm of the study was suspended at the first planned interim analysis because of a higher risk of organ failure in the 10% pentastarch group.
CORTICOSTEROID THERAPY IN SEPTIC SHOCK
Key points
- Corticosteroid therapy improves hemodynamic outcomes in patients with severe septic shock.
- Although meta-analyses suggest the mortality rate is lower with corticosteroid therapy, there is not enough evidence from randomized controlled trials to prove that the use of low-dose corticosteroids lowers the mortality rate in patients with septic shock.
- The corticotropin (ACTH) stimulation test should not be used to determine the need for corticosteroids in patients with septic shock.
Background
A previous multicenter study,18 performed in France, found that the use of corticosteroids in patients with septic shock resulted in lower rates of death at 28 days, in the ICU, and in the hospital and a shorter time to vasopressor withdrawal. Nevertheless, the beneficial effects were not observed in patients with adequate adrenal reserve (based on an ACTH stimulation test).
This study was criticized because of a high mortality rate in the placebo group.
The CORTICUS study
SPRUNG CL, ANNANE D, KEH D, ET AL; CORTICUS STUDY GROUP. HYDROCORTISONE THERAPY FOR PATIENTS WITH SEPTIC SHOCK. N ENGL J MED 2008; 358:111–124.
The Corticosteroid Therapy of Septic Shock (CORTICUS) study was a multicenter trial that randomly assigned 499 patients with septic shock to receive hydrocortisone (50 mg intravenously every 6 hours for 5 days, followed by a 6-day taper period) or placebo.19
Patients were eligible to be enrolled within 72 hours of onset of shock. Similar to previous studies, the CORTICUS trial classified patients on the basis of an ACTH stimulation test as having inadequate adrenal reserve (a cortisol increase of ≤ 9 μg/dL) or adequate adrenal reserve (a cortisol increase of > 9 μg/dL).
Faster reversal of shock with steroids
At baseline, the mean Simplified Acute Physiologic Score II (SAPS II) was 49 (the range of possible scores is 0 to 163; the higher the score the worse the organ dysfunction).
Hydrocortisone use resulted in a shorter duration of vasopressor use and a faster reversal of shock (3.3 days vs 5.8 days, P < .001).
This association was the same when patients were divided according to response to ACTH stimulation test. Time to reversal of shock in responders:
- 2.8 days with hydrocortisone
- 5.8 days with placebo (P < .001).
Time to reversal of shock in nonresponders:
- 3.9 days with hydrocortisone
- 6.0 days with placebo (P = .06).
Nevertheless, the treatment did not reduce the mortality rate at 28 days overall (34.3% vs 31.5% P = .51), or in the subgroups based on response to ACTH, or at any other time point. A post hoc analysis suggested that patients who had a systolic blood pressure of less than 90 mm Hg within 30 minutes of enrollment had a greater benefit in terms of mortality rate, but the effect was not statistically significant: the absolute difference was −11.2% (P = 0.28). Similarly, post hoc analyses also revealed a higher rate of death at 28 days in patients who received etomidate (Amidate) before randomization in both groups (P = .03).
Importantly, patients who received corticosteroids had a higher incidence of superinfections, including new episodes of sepsis or septic shock, with a combined odds ratio of 1.37 (95% CI 1.05–1.79).
Length of stay in the hospital or in the ICU was similar in patients who received corticosteroids and in those who received placebo. The ICU length of stay was 19 ± 31 days with hydrocortisone vs 18 ± 17 days with placebo (P = .51).
Comments
The CORTICUS trial showed that low-dose corticosteroid therapy results in faster reversal of shock in patients with severe septic shock. The hemodynamic benefits are present in all patients regardless of response to the ACTH stimulation test.
Nevertheless, contrary to previous findings,18 corticosteroid use was not associated with an improvement in mortality rates. Important differences exist between these two studies:
- The mortality rates in the placebo groups were significantly different (> 50% in the French study vs 30% in CORTICUS).
- The SAPS II scores were different in these two trials (55 vs 49), suggesting a greater severity of illness in the French study.
- The criteria for enrollment were different: the French study included patients who had a systolic blood pressure lower than 90 mm Hg for more than 1 hour despite fluid administration and vasopressor use, whereas the CORTICUS trial included patients who had a systolic blood pressure lower than 90 mm Hg for more than 1 hour despite fluid administration or vasopressor use.
- The time of enrollment was different: patients were enrolled much faster in the French study (within 8 hours) than in the CORTICUS trial (within 72 hours).
A recent meta-analysis of 17 randomized trials (including the CORTICUS study), found that, compared with those who received placebo, patients who received corticosteroids had a small reduction in the 28-day mortality rate (HR 0.84, 95% CI 0.71–1.00, P < .05).20 Of note, this meta-analysis has been criticized for possible publication bias and also for a large degree of heterogeneity in its results.21
VASOPRESSOR THERAPY IN SHOCK
Key points
- Vasopressin use in patients with severe septic shock is not associated with an improvement in mortality rates.
- Vasopressin should not be used as a first-line agent in patients with septic shock.
- Norepinephrine should be considered a first-line agent in patients with shock.
- Compared with norepinephrine, the use of dopamine in patients with shock is associated with similar mortality rates, although its use may result in a greater number of cardiac adverse events.
Background
Vasopressin gained popularity in critical care in the last 10 years because several small studies showed that adding it improves hemodynamics and results in a reduction in the doses of catecholamines in patients with refractory septic shock.22 Furthermore, the Surviving Sepsis Campaign guidelines recommended the use of vasopressin in patients who have refractory shock despite fluid resuscitation and the use of other “conventional” vasopressors.23
Despite these positive findings, it remained unknown if the use of vasopressin increases the survival rate in patients with septic shock.
The Vasopressin and Septic Shock Trial (VASST)
RUSSELL JA, WALLEY KR, SINGER J, ET AL; VASST INVESTIGATORS. VASOPRESSIN VERSUS NOREPINEPHRINE INFUSION IN PATIENTS WITH SEPTIC SHOCK. N ENGL J MED 2008; 358:877–887.
The Vasopressin and Septic Shock Trial (VASST)24 was a multicenter randomized, double-blind, controlled trial that included 778 patients with refractory septic shock. Refractory shock was defined as the lack of a response to a normal saline fluid bolus of 500 mL or the need for vasopressors (norepinephrine in doses of at least 5 μg/minute or its equivalent for 6 hours or more in the 24 hours before randomization).
Two subgroups were identified: those with severe septic shock (requiring norepinephrine in doses of 15 μg/minute or higher) and those with less-severe septic shock (needing norepinephrine in doses of 5 to 14 μg/minute). Patients with unstable coronary artery disease (acute myocardial infarction, angina) and severe congestive heart failure were excluded.
Patients were randomized to receive an intravenous infusion of vasopressin (0.01–0.03 U/minute) or norepinephrine (5–15 mg/minute) in addition to open-labeled vasopressors (excluding vasopressin). The primary outcome was the all-cause mortality rate at 28 days.
Results
At 28 days, fewer patients had died in the vasopressin group than in the norepinephrine group (35.4% vs 39.3%), but the difference was not statistically significant (P = .26). The trend was the same at 90 days (mortality rate 43.9% vs 49.6%, P = .11).
Subgroup analysis showed that in patients with less-severe septic shock, those who received vasopressin had a lower mortality rate at 28 days (26.5% vs 35.7%, P = .05; relative risk 0.74; 95% CI 0.55–1.01) and at 90 days (35.8% vs 46.1%, P = .04; relative risk 0.78, 95% CI 0.61–0.99).
There were no statistically significant differences in any of the other secondary outcomes or in serious adverse events.
Comments
The study has been criticized for several reasons:
- The mean arterial blood pressure at baseline before initiation of vasopressin was 72 mm Hg (and some argue that vasopressin was therefore not needed by the time it was started).
- The time from screening to infusion of the study drug was very long (12 hours).
- The observed mortality rate was lower than expected (37%).
Despite these considerations, the VASST trial showed that vasopressin is not associated with an increased number of adverse events in patients without active cardiovascular disease. The possible benefit in terms of the mortality rate in the subgroup of patients with less-severe septic shock requires further investigation.
Is dopamine equivalent to norepinephrine?
Previously, the Sepsis Occurrence in Acutely Ill Patients (SOAP) study, a multicenter, observational cohort study, found that dopamine use was associated with a higher all-cause mortality rate in the ICU compared with no dopamine.25 This finding had not been reproduced, as few well-designed studies had compared the effects of dopamine and norepinephrine.
The SOAP II study
DE BACKER D, BISTON P, DEVRIENDT J, ET AL; SOAP II INVESTIGATORS.. COMPARISON OF DOPAMINE AND NOREPINEPHRINE IN THE TREATMENT OF SHOCK. N ENGL J MED 2010; 362:779–789.
The SOAP II study,26 a multicenter, randomized trial, compared dopamine vs norepinephrine as first-line vasopressor therapy. In patients with refractory shock despite use of dopamine 20 μg/kg/minute or norepinephrine 0.19 μg/kg/minute, open-label norepinephrine, epinephrine, or vasopressin was added.
The primary outcome was the mortality rate at 28 days after randomization; secondary end points included the number of days without need for organ support and the occurrence of adverse events.
Results
A total of 1,679 patients were included; 858 were assigned to dopamine and 821 to norepinephrine. Most (1,044, 62%) of the patients had a diagnosis of septic shock.
No significant difference in mortality rates was noted at 28 days: 52.5% with dopamine vs 48.5% with norepinephrine (P = .10).
However, there were more arrhythmias in the patients treated with dopamine: 207 events (24.1%) vs 102 events (12.4%) (P < .001). The number of other adverse events such as renal failure, myocardial infarction, arterial occlusion, or skin necrosis was not different between the groups.
A subgroup analysis showed that dopamine was associated with more deaths at 28 days in patients with cardiogenic shock (P = .03) but not in patients with septic shock (P = .19) or with hypovolemic shock (P = .84).
Comments
The study was criticized because the patients may not have received adequate fluid resuscitation (the study considered adequate resuscitation to be equivalent to 1 L of crystalloids or 500 mL of colloids), as different degrees of volume depletion among patients make direct comparisons of vasopressor effects difficult.
Additionally, the study defined dopamine 20 μg/kg/minute as being equipotent with norepinephrine 0.19 μg/kg/minute. Comparisons of potency between drugs are difficult to establish, as there are no available data.
Nevertheless, this study further confirms previous findings suggesting that norepinephrine is not associated with more end-organ damage (such as renal failure or skin ischemia), and shows that dopamine may increase the number of adverse events, particularly in patients with cardiac disease.
- Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005; 294:1664–1670.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987; 317:1565–1570.
- Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998; 280:159–165.
- Steinberg KP, Hudson LD, Goodman RB, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354:1671–1684.
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007; 131:954–963.
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS results of a randomized controlled trial. 2007. Chest 2009; 136(suppl 5):e30.
- Annane D, Sébille V, Bellissant E; Ger-Inf-05 Study Group. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 2006; 34:22–30.
- Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998; 114:541–548.
- Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med 2006; 34:1326–1332.
- Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999; 27:2609–2615.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342:1471–1477.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871.
- Sprung CL, Annane D, Keh D, et al; CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124.
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009; 301:2362–2375.
- Minneci PC, Deans KJ, Natanson C. Corticosteroid therapy for severe sepsis and septic shock [letter]. JAMA 2009; 302:16443–1644.
- Kampmeier TG, Rehberg S, Westphal M, Lange M. Vasopressin in sepsis and septic shock. Minerva Anestesiol 2010; 76:844–850.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36:296–327.
- Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887.
- Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med 2006; 34:589–597.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005; 294:1664–1670.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987; 317:1565–1570.
- Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998; 280:159–165.
- Steinberg KP, Hudson LD, Goodman RB, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354:1671–1684.
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007; 131:954–963.
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS results of a randomized controlled trial. 2007. Chest 2009; 136(suppl 5):e30.
- Annane D, Sébille V, Bellissant E; Ger-Inf-05 Study Group. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 2006; 34:22–30.
- Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998; 114:541–548.
- Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med 2006; 34:1326–1332.
- Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999; 27:2609–2615.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342:1471–1477.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871.
- Sprung CL, Annane D, Keh D, et al; CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124.
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009; 301:2362–2375.
- Minneci PC, Deans KJ, Natanson C. Corticosteroid therapy for severe sepsis and septic shock [letter]. JAMA 2009; 302:16443–1644.
- Kampmeier TG, Rehberg S, Westphal M, Lange M. Vasopressin in sepsis and septic shock. Minerva Anestesiol 2010; 76:844–850.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36:296–327.
- Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887.
- Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med 2006; 34:589–597.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
KEY POINTS
- In patients with acute respiratory distress syndrome (ARDS), fluid restriction is associated with better outcomes. A pulmonary arterial catheter is not indicated in the routine management of ARDS. Corticosteroid use can result in improved oxygenation but may be associated with worse outcomes if treatment is started late, ie, more than 14 days after the onset of the disease.
- Intensive insulin therapy is associated with hypoglycemia and may be associated with complications in medical patients.
- In patients with septic shock, corticosteroid therapy is associated with faster shock reversal, but its effects on mortality rates remain controversial. Vasopressin improves hemodynamic variables but is not associated with a lower mortality rate.
- Daily interruption of sedation and early awakening of mechanically ventilated patients result in better outcomes.
- Compared with norepinephrine, dopamine is associated with more cardiac adverse events in patients with shock.