User login
What drives intensification of antihypertensive therapy at discharge?
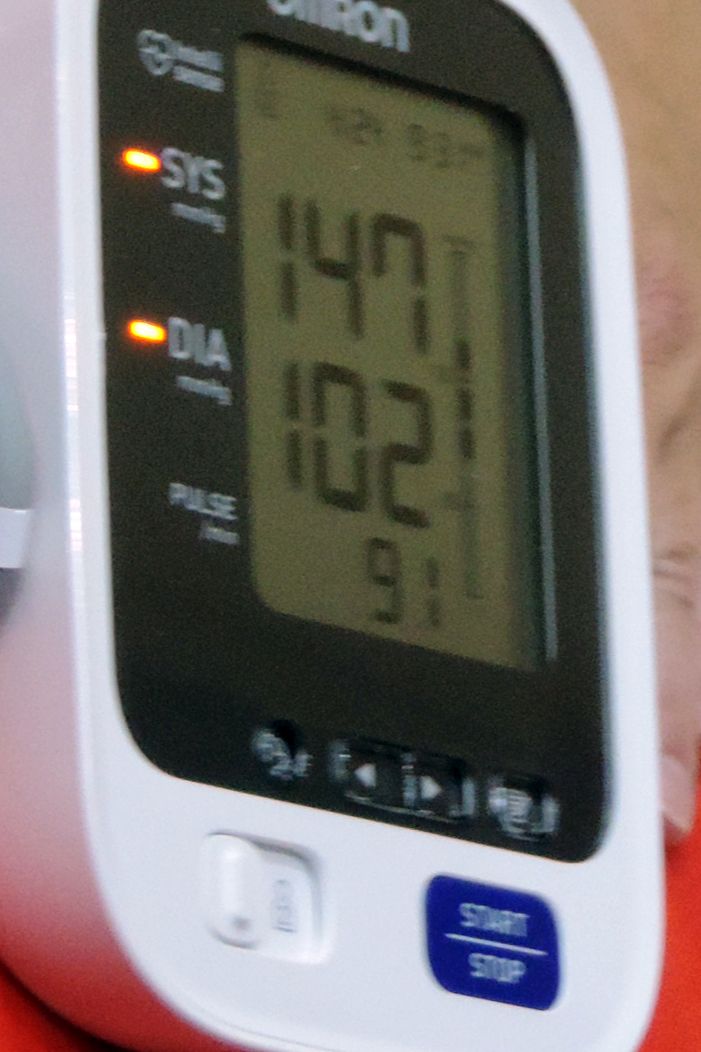
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
New single-dose influenza therapy effective among outpatients
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.