Article
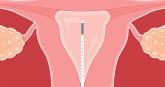
2023 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Michaelanne Shields, MD
Article
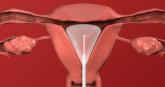
2022 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Marisa R. Adelman, MD
Insights into the COVID-19 vaccine’s effects on menstrual cycle irregularities; results of an RCT that examined the use of drospirenone 4 mg in a...
Article

2021 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Marisa R. Adelman, MD
Expert perspectives on a new cryotherapy device for endometrial ablation, the importance of quality of life issues in women with fibroids, and...
Article
2020 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Evangelia Lea Lazaris, MD
The evidence base on management strategies for AUB continues to grow, and recent studies suggest that therapy can be tailored...
Article
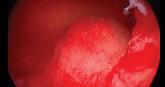
2019 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Marisa R. Adelman, MD
These experts discuss the factors that incur increased risk for malignant endometrial polyps, the relationship between chronic...
Article
2018 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Marisa R. Adelman, MD
Coverage and clinical perspective on recent AUB studies, including optimal procedure order for evaluation, cost-effectiveness of 4 treatment...
Article
2017 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Marissa Adelman, MD
As we move toward a value-based health care model, study data indicate that we consider obesity over age as a risk factor for endometrial...
News
2016 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
- Marisa Adelman, MD
Increasing evidence obligates a shift to less invasive surgery and surgical settings for abnormal uterine bleeding management. These experts offer...
News
2015 Update on abnormal uterine bleeding
- Author:
- Howard T. Sharp, MD
A focus on patient selection for surgical intervention, particularly endometrial ablation
