User login
MSC product receives orphan designation
The US Food and Drug Administration (FDA) has granted orphan drug designation to CYP-001 for the treatment of acute graft-versus-host disease (GVHD).
CYP-001 is a mesenchymal stem cell (MSC) product manufactured using Cynata Therapeutics Limited’s proprietary Cymerus™ technology.
According to Cynata, this technology enables economic manufacture of MSCs at commercial scale through the production of an MSC precursor called a mesenchymoangioblast (MCA).
The Cymerus MCA platform provides a source of MSCs that is independent of donor limitations and provides a potential “off-the-shelf” stem cell platform for therapeutic use, Cynata says.
The company recently released results from the first cohort of patients in its ongoing phase 1 trial of CYP-001 for the treatment of steroid-resistant acute GVHD.
The trial includes adults who received an allogeneic hematopoietic stem cell transplant to treat a hematologic disorder and were then diagnosed with steroid-resistant, grade 2-4 GVHD.
The first 8 patients were enrolled in cohort A, where they received 2 infusions of CYP-001 at a dose of 1 million cells/kg, up to a maximum dose of 100 million cells. There was 1 week between the 2 CYP-001 infusions in each patient.
For these 8 patients, overall survival at day 100 was 87.5%.
The overall response rate by day 100 was 100%, with all 8 patients showing an improvement in the severity of GVHD by at least 1 grade compared to baseline.
The complete response rate by day 100 was 50%, meaning GVHD signs/symptoms completely resolved in 4 of the 8 patients.
No treatment-related serious adverse events or safety concerns have been identified to date.
The next 8 patients will be enrolled in cohort B and receive 2 infusions of CYP-001 at a dose of 2 million cells/kg, up to a maximum dose of 200 million cells. Recruitment of these patients is progressing.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to CYP-001 for the treatment of acute graft-versus-host disease (GVHD).
CYP-001 is a mesenchymal stem cell (MSC) product manufactured using Cynata Therapeutics Limited’s proprietary Cymerus™ technology.
According to Cynata, this technology enables economic manufacture of MSCs at commercial scale through the production of an MSC precursor called a mesenchymoangioblast (MCA).
The Cymerus MCA platform provides a source of MSCs that is independent of donor limitations and provides a potential “off-the-shelf” stem cell platform for therapeutic use, Cynata says.
The company recently released results from the first cohort of patients in its ongoing phase 1 trial of CYP-001 for the treatment of steroid-resistant acute GVHD.
The trial includes adults who received an allogeneic hematopoietic stem cell transplant to treat a hematologic disorder and were then diagnosed with steroid-resistant, grade 2-4 GVHD.
The first 8 patients were enrolled in cohort A, where they received 2 infusions of CYP-001 at a dose of 1 million cells/kg, up to a maximum dose of 100 million cells. There was 1 week between the 2 CYP-001 infusions in each patient.
For these 8 patients, overall survival at day 100 was 87.5%.
The overall response rate by day 100 was 100%, with all 8 patients showing an improvement in the severity of GVHD by at least 1 grade compared to baseline.
The complete response rate by day 100 was 50%, meaning GVHD signs/symptoms completely resolved in 4 of the 8 patients.
No treatment-related serious adverse events or safety concerns have been identified to date.
The next 8 patients will be enrolled in cohort B and receive 2 infusions of CYP-001 at a dose of 2 million cells/kg, up to a maximum dose of 200 million cells. Recruitment of these patients is progressing.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to CYP-001 for the treatment of acute graft-versus-host disease (GVHD).
CYP-001 is a mesenchymal stem cell (MSC) product manufactured using Cynata Therapeutics Limited’s proprietary Cymerus™ technology.
According to Cynata, this technology enables economic manufacture of MSCs at commercial scale through the production of an MSC precursor called a mesenchymoangioblast (MCA).
The Cymerus MCA platform provides a source of MSCs that is independent of donor limitations and provides a potential “off-the-shelf” stem cell platform for therapeutic use, Cynata says.
The company recently released results from the first cohort of patients in its ongoing phase 1 trial of CYP-001 for the treatment of steroid-resistant acute GVHD.
The trial includes adults who received an allogeneic hematopoietic stem cell transplant to treat a hematologic disorder and were then diagnosed with steroid-resistant, grade 2-4 GVHD.
The first 8 patients were enrolled in cohort A, where they received 2 infusions of CYP-001 at a dose of 1 million cells/kg, up to a maximum dose of 100 million cells. There was 1 week between the 2 CYP-001 infusions in each patient.
For these 8 patients, overall survival at day 100 was 87.5%.
The overall response rate by day 100 was 100%, with all 8 patients showing an improvement in the severity of GVHD by at least 1 grade compared to baseline.
The complete response rate by day 100 was 50%, meaning GVHD signs/symptoms completely resolved in 4 of the 8 patients.
No treatment-related serious adverse events or safety concerns have been identified to date.
The next 8 patients will be enrolled in cohort B and receive 2 infusions of CYP-001 at a dose of 2 million cells/kg, up to a maximum dose of 200 million cells. Recruitment of these patients is progressing.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
UCART19 can bridge to transplant in adults
LISBON—Preliminary data from a phase 1 trial suggest UCART19 can serve as a bridge to transplant in adults with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL).
This “universal,” donor-derived, chimeric antigen receptor (CAR) T-cell therapy produced complete responses (CRs) in 6 of 9 patients treated.
Five of the patients who were negative for minimal residual disease (MRD) proceeded to allogeneic hematopoietic stem cell transplant (allo-HSCT).
Two of the 5 patients were still alive and in MRD-negative remission at last follow-up, but 1 had relapsed and 2 died.
A total of 4 patients died in this trial—1 from a dose-limiting toxicity (DLT), 1 from progressive disease, 1 from infection, and 1 from pulmonary hemorrhage.
“These early results for UCART19 are very encouraging, both in terms of manageable safety and the impressive complete molecular remission rate in these hard-to-treat adult patients with R/R B-ALL,” said principal investigator Reuben Benjamin, MBBS, PhD, a consultant hematologist at King’s College London in the UK.
Dr Benjamin presented these results, from the CALM trial, at the 44th Annual Meeting of the EBMT (abstract OS10-4*).
The CALM trial is sponsored by Servier. In 2015, Servier acquired exclusive rights from Cellectis for UCART19, which is being co-developed by Servier and Pfizer.
Patients and treatment
Dr Benjamin presented results in 9 patients with R/R B-ALL who had a median age of 23 (range, 18-49).
All patients had morphological disease or an MRD level of at least 1 x 10-3 (via flow cytometry and/or qPCR) at baseline. The median disease burden was 8% (range, 0-95).
Four patients had received 1 to 3 prior therapies, and 5 had received 4 or more prior treatments. Six patients had prior inotuzumab ozogamicin, 2 had prior blinatumomab, and 7 had prior allo-HSCT. The median time to relapse after allo-HSCT was 5.9 months (range, 4.1-11).
Patients underwent lymphodepletion with fludarabine, cyclophosphamide, and alemtuzumab (n=8) or fludarabine and cyclophosphamide (n=1).
They received UCART19 at 2 dose levels—6 x 106 total cells (n=6) or 6 to 8 x 107 total cells (n=3).
Toxicity
There were 2 DLTs, 1 at each dose level. At the lower dose, the DLT was grade 4 cytokine release syndrome (CRS) associated with grade 5 neutropenic sepsis. The patient with this DLT died at day 15 post-infusion.
At the higher dose level, the DLT was grade 4 prolonged cytopenia associated with infection and pulmonary hemorrhage. This patient died at day 82 post-infusion, 19 days after undergoing allo-HSCT.
In all, 8 patients had CRS. One patient had grade 1, 6 had grade 2, and 1 had grade 4 CRS. Seven patients had complete resolution of CRS.
Two patients had neurotoxic events, both grade 1.
Three patients had prolonged cytopenia, all grade 4. This was defined as persistent neutropenia and/or thrombocytopenia beyond day 42 after UCART19 infusion, except if the patient had >5% bone marrow blasts.
Two patients had neutropenic sepsis, grade 4 and grade 5.
Three patients had cytomegalovirus infection, all grade 2. Two patients had adenovirus infection, grade 1 and grade 3.
One patient had grade 1 acute cutaneous graft-versus-host disease.
Efficacy
Six of 9 patients achieved a CR, and 5 were MRD-negative by flow cytometry or qPCR. All 5 patients proceeded to allo-HSCT.
After transplant, 2 patients were still MRD-negative at 8 months, 1 patient relapsed after 6 months, and 2 patients died while MRD-negative. The deaths were due to infection and pulmonary hemorrhage.
Two other deaths were due to a DLT (neutropenic sepsis associated with CRS) and progressive disease.
Of the 5 patients who are alive, 2 are still MRD-negative after allo-HSCT. One of these patients relapsed after the first UCART19 infusion, received a second infusion, and achieved an MRD-negative CR that enabled transplant.
The remaining 3 patients who are still alive did not undergo transplant. One of these patients relapsed after achieving MRD-positive remission, and 1 relapsed after MRD-negative remission. The third patient relapsed and received a second UCART19 infusion. This patient has yet to respond to the second infusion but has minimal follow-up.
Recruitment in this study is ongoing at the higher dose level—6 to 8 x 107 total cells.
*Data in the abstract were updated in the presentation.
LISBON—Preliminary data from a phase 1 trial suggest UCART19 can serve as a bridge to transplant in adults with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL).
This “universal,” donor-derived, chimeric antigen receptor (CAR) T-cell therapy produced complete responses (CRs) in 6 of 9 patients treated.
Five of the patients who were negative for minimal residual disease (MRD) proceeded to allogeneic hematopoietic stem cell transplant (allo-HSCT).
Two of the 5 patients were still alive and in MRD-negative remission at last follow-up, but 1 had relapsed and 2 died.
A total of 4 patients died in this trial—1 from a dose-limiting toxicity (DLT), 1 from progressive disease, 1 from infection, and 1 from pulmonary hemorrhage.
“These early results for UCART19 are very encouraging, both in terms of manageable safety and the impressive complete molecular remission rate in these hard-to-treat adult patients with R/R B-ALL,” said principal investigator Reuben Benjamin, MBBS, PhD, a consultant hematologist at King’s College London in the UK.
Dr Benjamin presented these results, from the CALM trial, at the 44th Annual Meeting of the EBMT (abstract OS10-4*).
The CALM trial is sponsored by Servier. In 2015, Servier acquired exclusive rights from Cellectis for UCART19, which is being co-developed by Servier and Pfizer.
Patients and treatment
Dr Benjamin presented results in 9 patients with R/R B-ALL who had a median age of 23 (range, 18-49).
All patients had morphological disease or an MRD level of at least 1 x 10-3 (via flow cytometry and/or qPCR) at baseline. The median disease burden was 8% (range, 0-95).
Four patients had received 1 to 3 prior therapies, and 5 had received 4 or more prior treatments. Six patients had prior inotuzumab ozogamicin, 2 had prior blinatumomab, and 7 had prior allo-HSCT. The median time to relapse after allo-HSCT was 5.9 months (range, 4.1-11).
Patients underwent lymphodepletion with fludarabine, cyclophosphamide, and alemtuzumab (n=8) or fludarabine and cyclophosphamide (n=1).
They received UCART19 at 2 dose levels—6 x 106 total cells (n=6) or 6 to 8 x 107 total cells (n=3).
Toxicity
There were 2 DLTs, 1 at each dose level. At the lower dose, the DLT was grade 4 cytokine release syndrome (CRS) associated with grade 5 neutropenic sepsis. The patient with this DLT died at day 15 post-infusion.
At the higher dose level, the DLT was grade 4 prolonged cytopenia associated with infection and pulmonary hemorrhage. This patient died at day 82 post-infusion, 19 days after undergoing allo-HSCT.
In all, 8 patients had CRS. One patient had grade 1, 6 had grade 2, and 1 had grade 4 CRS. Seven patients had complete resolution of CRS.
Two patients had neurotoxic events, both grade 1.
Three patients had prolonged cytopenia, all grade 4. This was defined as persistent neutropenia and/or thrombocytopenia beyond day 42 after UCART19 infusion, except if the patient had >5% bone marrow blasts.
Two patients had neutropenic sepsis, grade 4 and grade 5.
Three patients had cytomegalovirus infection, all grade 2. Two patients had adenovirus infection, grade 1 and grade 3.
One patient had grade 1 acute cutaneous graft-versus-host disease.
Efficacy
Six of 9 patients achieved a CR, and 5 were MRD-negative by flow cytometry or qPCR. All 5 patients proceeded to allo-HSCT.
After transplant, 2 patients were still MRD-negative at 8 months, 1 patient relapsed after 6 months, and 2 patients died while MRD-negative. The deaths were due to infection and pulmonary hemorrhage.
Two other deaths were due to a DLT (neutropenic sepsis associated with CRS) and progressive disease.
Of the 5 patients who are alive, 2 are still MRD-negative after allo-HSCT. One of these patients relapsed after the first UCART19 infusion, received a second infusion, and achieved an MRD-negative CR that enabled transplant.
The remaining 3 patients who are still alive did not undergo transplant. One of these patients relapsed after achieving MRD-positive remission, and 1 relapsed after MRD-negative remission. The third patient relapsed and received a second UCART19 infusion. This patient has yet to respond to the second infusion but has minimal follow-up.
Recruitment in this study is ongoing at the higher dose level—6 to 8 x 107 total cells.
*Data in the abstract were updated in the presentation.
LISBON—Preliminary data from a phase 1 trial suggest UCART19 can serve as a bridge to transplant in adults with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL).
This “universal,” donor-derived, chimeric antigen receptor (CAR) T-cell therapy produced complete responses (CRs) in 6 of 9 patients treated.
Five of the patients who were negative for minimal residual disease (MRD) proceeded to allogeneic hematopoietic stem cell transplant (allo-HSCT).
Two of the 5 patients were still alive and in MRD-negative remission at last follow-up, but 1 had relapsed and 2 died.
A total of 4 patients died in this trial—1 from a dose-limiting toxicity (DLT), 1 from progressive disease, 1 from infection, and 1 from pulmonary hemorrhage.
“These early results for UCART19 are very encouraging, both in terms of manageable safety and the impressive complete molecular remission rate in these hard-to-treat adult patients with R/R B-ALL,” said principal investigator Reuben Benjamin, MBBS, PhD, a consultant hematologist at King’s College London in the UK.
Dr Benjamin presented these results, from the CALM trial, at the 44th Annual Meeting of the EBMT (abstract OS10-4*).
The CALM trial is sponsored by Servier. In 2015, Servier acquired exclusive rights from Cellectis for UCART19, which is being co-developed by Servier and Pfizer.
Patients and treatment
Dr Benjamin presented results in 9 patients with R/R B-ALL who had a median age of 23 (range, 18-49).
All patients had morphological disease or an MRD level of at least 1 x 10-3 (via flow cytometry and/or qPCR) at baseline. The median disease burden was 8% (range, 0-95).
Four patients had received 1 to 3 prior therapies, and 5 had received 4 or more prior treatments. Six patients had prior inotuzumab ozogamicin, 2 had prior blinatumomab, and 7 had prior allo-HSCT. The median time to relapse after allo-HSCT was 5.9 months (range, 4.1-11).
Patients underwent lymphodepletion with fludarabine, cyclophosphamide, and alemtuzumab (n=8) or fludarabine and cyclophosphamide (n=1).
They received UCART19 at 2 dose levels—6 x 106 total cells (n=6) or 6 to 8 x 107 total cells (n=3).
Toxicity
There were 2 DLTs, 1 at each dose level. At the lower dose, the DLT was grade 4 cytokine release syndrome (CRS) associated with grade 5 neutropenic sepsis. The patient with this DLT died at day 15 post-infusion.
At the higher dose level, the DLT was grade 4 prolonged cytopenia associated with infection and pulmonary hemorrhage. This patient died at day 82 post-infusion, 19 days after undergoing allo-HSCT.
In all, 8 patients had CRS. One patient had grade 1, 6 had grade 2, and 1 had grade 4 CRS. Seven patients had complete resolution of CRS.
Two patients had neurotoxic events, both grade 1.
Three patients had prolonged cytopenia, all grade 4. This was defined as persistent neutropenia and/or thrombocytopenia beyond day 42 after UCART19 infusion, except if the patient had >5% bone marrow blasts.
Two patients had neutropenic sepsis, grade 4 and grade 5.
Three patients had cytomegalovirus infection, all grade 2. Two patients had adenovirus infection, grade 1 and grade 3.
One patient had grade 1 acute cutaneous graft-versus-host disease.
Efficacy
Six of 9 patients achieved a CR, and 5 were MRD-negative by flow cytometry or qPCR. All 5 patients proceeded to allo-HSCT.
After transplant, 2 patients were still MRD-negative at 8 months, 1 patient relapsed after 6 months, and 2 patients died while MRD-negative. The deaths were due to infection and pulmonary hemorrhage.
Two other deaths were due to a DLT (neutropenic sepsis associated with CRS) and progressive disease.
Of the 5 patients who are alive, 2 are still MRD-negative after allo-HSCT. One of these patients relapsed after the first UCART19 infusion, received a second infusion, and achieved an MRD-negative CR that enabled transplant.
The remaining 3 patients who are still alive did not undergo transplant. One of these patients relapsed after achieving MRD-positive remission, and 1 relapsed after MRD-negative remission. The third patient relapsed and received a second UCART19 infusion. This patient has yet to respond to the second infusion but has minimal follow-up.
Recruitment in this study is ongoing at the higher dose level—6 to 8 x 107 total cells.
*Data in the abstract were updated in the presentation.
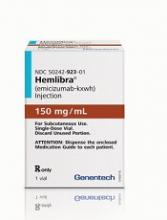
Deaths in patients on emicizumab
Two hemophilia organizations have notified the public of 5 deaths in adult patients receiving emicizumab (Hemlibra).
All 5 deaths—occurring in 2016 (n=1), 2017 (n=2), and this year (n=2)—were deemed unrelated to emicizumab by the investigator or treating physician.
The National Hemophilia Foundation and Hemophilia Federation of America reported these deaths after receiving notifications from Genentech.
The company said it has limited information about the circumstances of the deaths.
However, Genentech did say the 2016 death, 1 of the 2017 deaths, and 1 of the 2018 deaths occurred in patients receiving emicizumab via a compassionate use program.
Compassionate use of emicizumab has been available on a case-by-case basis, following a request to Roche from a patient’s treating physician, if the patient has a serious or life-threatening condition, has exhausted all other treatment options, and is unable to participate in a clinical trial.
The other death in 2017 occurred in a patient on the phase 3 HAVEN 1 trial and was reported along with the other results from that trial.
The remaining death in 2018 was in a patient receiving emicizumab via an expanded access protocol.
This protocol, which was reviewed by the US Food and Drug Administration, allowed US patients who were not participating in a clinical trial of emicizumab but who met eligibility criteria similar to key studies to have access to emicizumab prior to approval, which occurred in November 2017.
In response to the deaths, Genentech has pledged to share information on any adverse events that impact the overall benefit/risk profile of emicizumab.
“We are committed to providing timely and transparent updates on the safety profile of Hemlibra to health authorities, healthcare professionals, and the hemophilia community,” the company said.
For more information, patients and healthcare providers can call Genentech’s medical communications line at 1-800-821-8590.
Two hemophilia organizations have notified the public of 5 deaths in adult patients receiving emicizumab (Hemlibra).
All 5 deaths—occurring in 2016 (n=1), 2017 (n=2), and this year (n=2)—were deemed unrelated to emicizumab by the investigator or treating physician.
The National Hemophilia Foundation and Hemophilia Federation of America reported these deaths after receiving notifications from Genentech.
The company said it has limited information about the circumstances of the deaths.
However, Genentech did say the 2016 death, 1 of the 2017 deaths, and 1 of the 2018 deaths occurred in patients receiving emicizumab via a compassionate use program.
Compassionate use of emicizumab has been available on a case-by-case basis, following a request to Roche from a patient’s treating physician, if the patient has a serious or life-threatening condition, has exhausted all other treatment options, and is unable to participate in a clinical trial.
The other death in 2017 occurred in a patient on the phase 3 HAVEN 1 trial and was reported along with the other results from that trial.
The remaining death in 2018 was in a patient receiving emicizumab via an expanded access protocol.
This protocol, which was reviewed by the US Food and Drug Administration, allowed US patients who were not participating in a clinical trial of emicizumab but who met eligibility criteria similar to key studies to have access to emicizumab prior to approval, which occurred in November 2017.
In response to the deaths, Genentech has pledged to share information on any adverse events that impact the overall benefit/risk profile of emicizumab.
“We are committed to providing timely and transparent updates on the safety profile of Hemlibra to health authorities, healthcare professionals, and the hemophilia community,” the company said.
For more information, patients and healthcare providers can call Genentech’s medical communications line at 1-800-821-8590.
Two hemophilia organizations have notified the public of 5 deaths in adult patients receiving emicizumab (Hemlibra).
All 5 deaths—occurring in 2016 (n=1), 2017 (n=2), and this year (n=2)—were deemed unrelated to emicizumab by the investigator or treating physician.
The National Hemophilia Foundation and Hemophilia Federation of America reported these deaths after receiving notifications from Genentech.
The company said it has limited information about the circumstances of the deaths.
However, Genentech did say the 2016 death, 1 of the 2017 deaths, and 1 of the 2018 deaths occurred in patients receiving emicizumab via a compassionate use program.
Compassionate use of emicizumab has been available on a case-by-case basis, following a request to Roche from a patient’s treating physician, if the patient has a serious or life-threatening condition, has exhausted all other treatment options, and is unable to participate in a clinical trial.
The other death in 2017 occurred in a patient on the phase 3 HAVEN 1 trial and was reported along with the other results from that trial.
The remaining death in 2018 was in a patient receiving emicizumab via an expanded access protocol.
This protocol, which was reviewed by the US Food and Drug Administration, allowed US patients who were not participating in a clinical trial of emicizumab but who met eligibility criteria similar to key studies to have access to emicizumab prior to approval, which occurred in November 2017.
In response to the deaths, Genentech has pledged to share information on any adverse events that impact the overall benefit/risk profile of emicizumab.
“We are committed to providing timely and transparent updates on the safety profile of Hemlibra to health authorities, healthcare professionals, and the hemophilia community,” the company said.
For more information, patients and healthcare providers can call Genentech’s medical communications line at 1-800-821-8590.
CHMP supports expanded approval for fosaprepitant
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended changing the terms of marketing authorization for fosaprepitant (Ivemend).
The product is already approved in the European Union (EU) for the prevention of acute and delayed nausea and vomiting associated with moderately or highly emetogenic cancer chemotherapy in adults.
Now, the CHMP is recommending that fosaprepitant be authorized for the same indication in pediatric patients age 6 months and older.
As it is in adults, fosaprepitant would be given to children as part of combination therapy.
The CHMP’s opinion on fosaprepitant will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the EU. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
Merck Sharp & Dohme Corp., the company developing fosaprepitant, has conducted a phase 2 trial assessing the pharmacokinetics, pharmacodynamics, safety, and tolerability of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in children.
Patients ages 2 to 17 were randomized to receive 1 of 4 doses of fosaprepitant (0.4 mg/kg, 1.2 mg/kg, 3 mg/kg, and 5 mg/kg) or placebo in cycle 1. All patients also received ondansetron, with or without dexamethasone. Patients ages 0 to 11 were invited to participate in optional cycles 2 to 6, during which they received fosaprepitant at 3 mg/kg or 5 mg/kg.
Results from this trial have been posted on its clinicaltrials.gov page (NCT01697579).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended changing the terms of marketing authorization for fosaprepitant (Ivemend).
The product is already approved in the European Union (EU) for the prevention of acute and delayed nausea and vomiting associated with moderately or highly emetogenic cancer chemotherapy in adults.
Now, the CHMP is recommending that fosaprepitant be authorized for the same indication in pediatric patients age 6 months and older.
As it is in adults, fosaprepitant would be given to children as part of combination therapy.
The CHMP’s opinion on fosaprepitant will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the EU. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
Merck Sharp & Dohme Corp., the company developing fosaprepitant, has conducted a phase 2 trial assessing the pharmacokinetics, pharmacodynamics, safety, and tolerability of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in children.
Patients ages 2 to 17 were randomized to receive 1 of 4 doses of fosaprepitant (0.4 mg/kg, 1.2 mg/kg, 3 mg/kg, and 5 mg/kg) or placebo in cycle 1. All patients also received ondansetron, with or without dexamethasone. Patients ages 0 to 11 were invited to participate in optional cycles 2 to 6, during which they received fosaprepitant at 3 mg/kg or 5 mg/kg.
Results from this trial have been posted on its clinicaltrials.gov page (NCT01697579).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended changing the terms of marketing authorization for fosaprepitant (Ivemend).
The product is already approved in the European Union (EU) for the prevention of acute and delayed nausea and vomiting associated with moderately or highly emetogenic cancer chemotherapy in adults.
Now, the CHMP is recommending that fosaprepitant be authorized for the same indication in pediatric patients age 6 months and older.
As it is in adults, fosaprepitant would be given to children as part of combination therapy.
The CHMP’s opinion on fosaprepitant will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the EU. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
Merck Sharp & Dohme Corp., the company developing fosaprepitant, has conducted a phase 2 trial assessing the pharmacokinetics, pharmacodynamics, safety, and tolerability of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in children.
Patients ages 2 to 17 were randomized to receive 1 of 4 doses of fosaprepitant (0.4 mg/kg, 1.2 mg/kg, 3 mg/kg, and 5 mg/kg) or placebo in cycle 1. All patients also received ondansetron, with or without dexamethasone. Patients ages 0 to 11 were invited to participate in optional cycles 2 to 6, during which they received fosaprepitant at 3 mg/kg or 5 mg/kg.
Results from this trial have been posted on its clinicaltrials.gov page (NCT01697579).
Phase 1 results with UCART19 in kids
LISBON—Early results from a phase 1, pediatric trial of UCART19 expand upon results observed in children who received the therapy via a compassionate use program.
Two patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) received UCART19, a “universal,” donor-derived chimeric antigen receptor (CAR) T-cell therapy, via the program.
Both achieved remission and were still alive at last follow-up, more than 2 years after proceeding to transplant.
In the phase 1 trial, 5 of 6 B-ALL patients have achieved remission and gone on to transplant.
Three of the patients are still alive, and 2 are still negative for minimal residual disease (MRD) at 10 months and 11 months after UCART19 infusion.
However, 2 patients died of progression, and 1 died of transplant-related complications.
Paul Veys, MBBS, of Great Ormond Street Hospital (GOSH) in London, UK, presented these results, from the PALL trial, at the 44th Annual Meeting of the EBMT (abstract OS18-5*).
The trial is sponsored by Servier. In 2015, Servier acquired exclusive rights from Cellectis for UCART19, which is being co-developed by Servier and Pfizer.
Prior experience
Researchers previously reported results with UCART19 in 2 infants with relapsed/refractory B-ALL who had exhausted all other treatment options and received UCART19 via a compassionate use program.
Both patients achieved remission after UCART19 and proceeded to allogeneic hematopoietic stem cell transplant (allo-HSCT).
When these results were published, in January 2017, both patients were still alive and leukemia-free at last follow-up—12 months and 18 months after UCART19 infusion.
Dr Veys provided an update, noting that these patients were still alive and in remission at 24 months and 30 months after allo-HSCT.
Phase 1 patients and treatment
Thus far, the phase 1 trial has enrolled and treated 6 patients with relapsed B-ALL. They had a median age of 3.75 (range, 0.8-16.4).
All patients had morphological disease or an MRD level of at least 1 x 10-3 (via flow cytometry and/or qPCR) at baseline.
One patient had received 1 prior therapy, 2 had 3 prior therapies, and 3 had 4 or more prior therapies. Two patients had prior inotuzumab ozogamicin, and 2 had prior allo-HSCT. Both had relapsed within 6 months of allo-HSCT.
Five patients had less than 10% bone marrow blasts prior to lymphodepletion, and 1 had greater than 50% blasts.
Patients underwent lymphodepletion with fludarabine, cyclophosphamide, and alemtuzumab (n=5) or fludarabine and cyclophosphamide (n=1).
The patients received UCART19 at doses of 2 x 107 total cells or 1.1 to 2.3 x 106 cells/kg.
Toxicity
All 6 patients developed cytokine release syndrome (CRS), including grade 1 (n=1), grade 2 (n=4), and grade 3 (n-1) CRS. However, all 6 cases resolved completely.
Three patients had neurotoxic events, 2 grade 1 and 1 grade 2. One patient had grade 3 febrile neutropenia.
Three patients had grade 4 prolonged cytopenia. This was defined as persistent neutropenia and/or thrombocytopenia beyond day 42 after UCART19 infusion, except if the patient had >5% bone marrow blasts.
One patient had grade 1 adenovirus infection, 1 had grade 3 cytomegalovirus infection, 2 had grade 3 BK virus hemorrhagic cystitis, and 1 had grade 4 metapneumovirus infection.
One patient had grade 1 acute cutaneous graft-versus-host disease.
Efficacy
All 6 patients achieved a complete response at day 28 to 42 after UCART19 infusion. Five patients achieved MRD negativity according to flow cytometry, and 3 were MRD-negative according to PCR.
The 5 flow-MRD-negative patients went on to receive an allo-HSCT between 49 days and 62 days after UCART19 infusion. Conditioning consisted of total body irradiation and fludarabine, with or without cyclophosphamide and antithymocyte globulin. All of these patients received a dose of rituximab as well, which was intended to target any remaining UCART19 cells.
Two patients relapsed 3 months after transplant and died at 7 months and 8 months after UCART19 infusion. One of these patients was CD19-, and 1 was CD19+, but both were MRD-positive by PCR prior to receiving their transplant.
A third patient died 2.5 months after allo-HSCT from transplant-related complications, including thrombotic microangiopathy, BK hemorrhagic cystitis, and nephritis.
The remaining 3 patients are still alive at 1.5 months, 10 months, and 11 months after UCART19 infusion. Two are still MRD-negative, and 1 is MRD-positive. The MRD-positive patient has not undergone allo-HSCT.
*Data in the abstract were updated in the presentation.
LISBON—Early results from a phase 1, pediatric trial of UCART19 expand upon results observed in children who received the therapy via a compassionate use program.
Two patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) received UCART19, a “universal,” donor-derived chimeric antigen receptor (CAR) T-cell therapy, via the program.
Both achieved remission and were still alive at last follow-up, more than 2 years after proceeding to transplant.
In the phase 1 trial, 5 of 6 B-ALL patients have achieved remission and gone on to transplant.
Three of the patients are still alive, and 2 are still negative for minimal residual disease (MRD) at 10 months and 11 months after UCART19 infusion.
However, 2 patients died of progression, and 1 died of transplant-related complications.
Paul Veys, MBBS, of Great Ormond Street Hospital (GOSH) in London, UK, presented these results, from the PALL trial, at the 44th Annual Meeting of the EBMT (abstract OS18-5*).
The trial is sponsored by Servier. In 2015, Servier acquired exclusive rights from Cellectis for UCART19, which is being co-developed by Servier and Pfizer.
Prior experience
Researchers previously reported results with UCART19 in 2 infants with relapsed/refractory B-ALL who had exhausted all other treatment options and received UCART19 via a compassionate use program.
Both patients achieved remission after UCART19 and proceeded to allogeneic hematopoietic stem cell transplant (allo-HSCT).
When these results were published, in January 2017, both patients were still alive and leukemia-free at last follow-up—12 months and 18 months after UCART19 infusion.
Dr Veys provided an update, noting that these patients were still alive and in remission at 24 months and 30 months after allo-HSCT.
Phase 1 patients and treatment
Thus far, the phase 1 trial has enrolled and treated 6 patients with relapsed B-ALL. They had a median age of 3.75 (range, 0.8-16.4).
All patients had morphological disease or an MRD level of at least 1 x 10-3 (via flow cytometry and/or qPCR) at baseline.
One patient had received 1 prior therapy, 2 had 3 prior therapies, and 3 had 4 or more prior therapies. Two patients had prior inotuzumab ozogamicin, and 2 had prior allo-HSCT. Both had relapsed within 6 months of allo-HSCT.
Five patients had less than 10% bone marrow blasts prior to lymphodepletion, and 1 had greater than 50% blasts.
Patients underwent lymphodepletion with fludarabine, cyclophosphamide, and alemtuzumab (n=5) or fludarabine and cyclophosphamide (n=1).
The patients received UCART19 at doses of 2 x 107 total cells or 1.1 to 2.3 x 106 cells/kg.
Toxicity
All 6 patients developed cytokine release syndrome (CRS), including grade 1 (n=1), grade 2 (n=4), and grade 3 (n-1) CRS. However, all 6 cases resolved completely.
Three patients had neurotoxic events, 2 grade 1 and 1 grade 2. One patient had grade 3 febrile neutropenia.
Three patients had grade 4 prolonged cytopenia. This was defined as persistent neutropenia and/or thrombocytopenia beyond day 42 after UCART19 infusion, except if the patient had >5% bone marrow blasts.
One patient had grade 1 adenovirus infection, 1 had grade 3 cytomegalovirus infection, 2 had grade 3 BK virus hemorrhagic cystitis, and 1 had grade 4 metapneumovirus infection.
One patient had grade 1 acute cutaneous graft-versus-host disease.
Efficacy
All 6 patients achieved a complete response at day 28 to 42 after UCART19 infusion. Five patients achieved MRD negativity according to flow cytometry, and 3 were MRD-negative according to PCR.
The 5 flow-MRD-negative patients went on to receive an allo-HSCT between 49 days and 62 days after UCART19 infusion. Conditioning consisted of total body irradiation and fludarabine, with or without cyclophosphamide and antithymocyte globulin. All of these patients received a dose of rituximab as well, which was intended to target any remaining UCART19 cells.
Two patients relapsed 3 months after transplant and died at 7 months and 8 months after UCART19 infusion. One of these patients was CD19-, and 1 was CD19+, but both were MRD-positive by PCR prior to receiving their transplant.
A third patient died 2.5 months after allo-HSCT from transplant-related complications, including thrombotic microangiopathy, BK hemorrhagic cystitis, and nephritis.
The remaining 3 patients are still alive at 1.5 months, 10 months, and 11 months after UCART19 infusion. Two are still MRD-negative, and 1 is MRD-positive. The MRD-positive patient has not undergone allo-HSCT.
*Data in the abstract were updated in the presentation.
LISBON—Early results from a phase 1, pediatric trial of UCART19 expand upon results observed in children who received the therapy via a compassionate use program.
Two patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) received UCART19, a “universal,” donor-derived chimeric antigen receptor (CAR) T-cell therapy, via the program.
Both achieved remission and were still alive at last follow-up, more than 2 years after proceeding to transplant.
In the phase 1 trial, 5 of 6 B-ALL patients have achieved remission and gone on to transplant.
Three of the patients are still alive, and 2 are still negative for minimal residual disease (MRD) at 10 months and 11 months after UCART19 infusion.
However, 2 patients died of progression, and 1 died of transplant-related complications.
Paul Veys, MBBS, of Great Ormond Street Hospital (GOSH) in London, UK, presented these results, from the PALL trial, at the 44th Annual Meeting of the EBMT (abstract OS18-5*).
The trial is sponsored by Servier. In 2015, Servier acquired exclusive rights from Cellectis for UCART19, which is being co-developed by Servier and Pfizer.
Prior experience
Researchers previously reported results with UCART19 in 2 infants with relapsed/refractory B-ALL who had exhausted all other treatment options and received UCART19 via a compassionate use program.
Both patients achieved remission after UCART19 and proceeded to allogeneic hematopoietic stem cell transplant (allo-HSCT).
When these results were published, in January 2017, both patients were still alive and leukemia-free at last follow-up—12 months and 18 months after UCART19 infusion.
Dr Veys provided an update, noting that these patients were still alive and in remission at 24 months and 30 months after allo-HSCT.
Phase 1 patients and treatment
Thus far, the phase 1 trial has enrolled and treated 6 patients with relapsed B-ALL. They had a median age of 3.75 (range, 0.8-16.4).
All patients had morphological disease or an MRD level of at least 1 x 10-3 (via flow cytometry and/or qPCR) at baseline.
One patient had received 1 prior therapy, 2 had 3 prior therapies, and 3 had 4 or more prior therapies. Two patients had prior inotuzumab ozogamicin, and 2 had prior allo-HSCT. Both had relapsed within 6 months of allo-HSCT.
Five patients had less than 10% bone marrow blasts prior to lymphodepletion, and 1 had greater than 50% blasts.
Patients underwent lymphodepletion with fludarabine, cyclophosphamide, and alemtuzumab (n=5) or fludarabine and cyclophosphamide (n=1).
The patients received UCART19 at doses of 2 x 107 total cells or 1.1 to 2.3 x 106 cells/kg.
Toxicity
All 6 patients developed cytokine release syndrome (CRS), including grade 1 (n=1), grade 2 (n=4), and grade 3 (n-1) CRS. However, all 6 cases resolved completely.
Three patients had neurotoxic events, 2 grade 1 and 1 grade 2. One patient had grade 3 febrile neutropenia.
Three patients had grade 4 prolonged cytopenia. This was defined as persistent neutropenia and/or thrombocytopenia beyond day 42 after UCART19 infusion, except if the patient had >5% bone marrow blasts.
One patient had grade 1 adenovirus infection, 1 had grade 3 cytomegalovirus infection, 2 had grade 3 BK virus hemorrhagic cystitis, and 1 had grade 4 metapneumovirus infection.
One patient had grade 1 acute cutaneous graft-versus-host disease.
Efficacy
All 6 patients achieved a complete response at day 28 to 42 after UCART19 infusion. Five patients achieved MRD negativity according to flow cytometry, and 3 were MRD-negative according to PCR.
The 5 flow-MRD-negative patients went on to receive an allo-HSCT between 49 days and 62 days after UCART19 infusion. Conditioning consisted of total body irradiation and fludarabine, with or without cyclophosphamide and antithymocyte globulin. All of these patients received a dose of rituximab as well, which was intended to target any remaining UCART19 cells.
Two patients relapsed 3 months after transplant and died at 7 months and 8 months after UCART19 infusion. One of these patients was CD19-, and 1 was CD19+, but both were MRD-positive by PCR prior to receiving their transplant.
A third patient died 2.5 months after allo-HSCT from transplant-related complications, including thrombotic microangiopathy, BK hemorrhagic cystitis, and nephritis.
The remaining 3 patients are still alive at 1.5 months, 10 months, and 11 months after UCART19 infusion. Two are still MRD-negative, and 1 is MRD-positive. The MRD-positive patient has not undergone allo-HSCT.
*Data in the abstract were updated in the presentation.
CHMP rejects plitidepsin again
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of marketing authorization for plitidepsin (Aplidin).
PharmaMar is seeking approval for plitidepsin to treat adults with multiple myeloma (MM) who have received at least 3 prior treatments, including bortezomib and either lenalidomide or thalidomide.
Plitidepsin is intended to be used in combination with dexamethasone.
This is the second time the CHMP has recommended against authorizing plitidepsin for this indication. The first time was last December.
At that time, PharmaMar asked the CHMP to re-examine its opinion, and the CHMP obliged. The committee confirmed its negative opinion of plitidepsin last week.
The CHMP’s review
Upon its initial review, the CHMP was concerned that data from the main study of plitidepsin in MM—the phase 3 ADMYRE trial—did not demonstrate a sufficient benefit of plitidepsin plus dexamethasone, compared to dexamethasone alone, in MM patients who had received 3 to 6 prior therapies.
The CHMP noted that the data showed a modest increase in progression free-survival (PFS)—around 1 month—with plitidepsin. According to study investigators, the median PFS was 3.8 months in patients who received plitidepsin and 1.9 months in those who received dexamethasone alone. According to an independent review committee, the median PFS was 2.6 months and 1.7 months, respectively.
The CHMP also said improvement in overall survival (OS) was not sufficiently demonstrated in this trial. The median OS was 11.6 months in the plitidepsin arm and 6.4 months in the dexamethasone arm.
Finally, the CHMP noted that severe adverse events were reported more frequently in patients who received plitidepsin. The most common grade 3/4 treatment-related adverse events (in the plitidepsin and dexamethasone arms, respectively) were fatigue (10.8% vs 1.2%), myalgia (5.4% vs 0%), and nausea (3.6% vs 1.2%).
Rates of treatment discontinuation were 9% in the plitidepsin arm, 6.5% in the dexamethasone arm, and 13.5% among patients who crossed over from the dexamethasone arm to the plitidepsin arm. Patients were allowed to cross over if they progressed after at least 8 weeks of treatment.
Based on these data, the CHMP was of the opinion that the benefits of plitidepsin did not outweigh its risks, so the committee recommended refusal of marketing authorization. After re-examination, the CHMP remained of the same opinion.
The European Commission (EC) has the final say on the marketing authorization application for plitidepsin. Though it is not required to do so, the EC typically follows the CHMP’s advice. The EC makes its decision within 67 days of the CHMP’s opinion.
PharmaMar has not announced whether it plans to continue developing plitidepsin for MM patients if the EC refuses to authorize the drug, and the company did not respond to a request for comment.
About plitidepsin
Plitidepsin is an investigational anticancer agent of marine origin, originally obtained from the ascidian Aplidium albicans. The drug specifically binds to eEF1A2 and targets the non-canonical role of this protein, resulting in cancer cell death via apoptosis.
Plitidepsin is currently in clinical development for hematologic malignancies.
In a phase 1b trial (NCT02100657), researchers are evaluating plitidepsin in combination with bortezomib and dexamethasone for patients with relapsed/refractory MM.
In a phase 2 trial (NCT03117361), researchers are investigating plitidepsin in combination with bortezomib and dexamethasone for patients with MM that is refractory to both lenalidomide and bortezomib.
Plitidepsin has received orphan drug designation in the European Union and the US.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of marketing authorization for plitidepsin (Aplidin).
PharmaMar is seeking approval for plitidepsin to treat adults with multiple myeloma (MM) who have received at least 3 prior treatments, including bortezomib and either lenalidomide or thalidomide.
Plitidepsin is intended to be used in combination with dexamethasone.
This is the second time the CHMP has recommended against authorizing plitidepsin for this indication. The first time was last December.
At that time, PharmaMar asked the CHMP to re-examine its opinion, and the CHMP obliged. The committee confirmed its negative opinion of plitidepsin last week.
The CHMP’s review
Upon its initial review, the CHMP was concerned that data from the main study of plitidepsin in MM—the phase 3 ADMYRE trial—did not demonstrate a sufficient benefit of plitidepsin plus dexamethasone, compared to dexamethasone alone, in MM patients who had received 3 to 6 prior therapies.
The CHMP noted that the data showed a modest increase in progression free-survival (PFS)—around 1 month—with plitidepsin. According to study investigators, the median PFS was 3.8 months in patients who received plitidepsin and 1.9 months in those who received dexamethasone alone. According to an independent review committee, the median PFS was 2.6 months and 1.7 months, respectively.
The CHMP also said improvement in overall survival (OS) was not sufficiently demonstrated in this trial. The median OS was 11.6 months in the plitidepsin arm and 6.4 months in the dexamethasone arm.
Finally, the CHMP noted that severe adverse events were reported more frequently in patients who received plitidepsin. The most common grade 3/4 treatment-related adverse events (in the plitidepsin and dexamethasone arms, respectively) were fatigue (10.8% vs 1.2%), myalgia (5.4% vs 0%), and nausea (3.6% vs 1.2%).
Rates of treatment discontinuation were 9% in the plitidepsin arm, 6.5% in the dexamethasone arm, and 13.5% among patients who crossed over from the dexamethasone arm to the plitidepsin arm. Patients were allowed to cross over if they progressed after at least 8 weeks of treatment.
Based on these data, the CHMP was of the opinion that the benefits of plitidepsin did not outweigh its risks, so the committee recommended refusal of marketing authorization. After re-examination, the CHMP remained of the same opinion.
The European Commission (EC) has the final say on the marketing authorization application for plitidepsin. Though it is not required to do so, the EC typically follows the CHMP’s advice. The EC makes its decision within 67 days of the CHMP’s opinion.
PharmaMar has not announced whether it plans to continue developing plitidepsin for MM patients if the EC refuses to authorize the drug, and the company did not respond to a request for comment.
About plitidepsin
Plitidepsin is an investigational anticancer agent of marine origin, originally obtained from the ascidian Aplidium albicans. The drug specifically binds to eEF1A2 and targets the non-canonical role of this protein, resulting in cancer cell death via apoptosis.
Plitidepsin is currently in clinical development for hematologic malignancies.
In a phase 1b trial (NCT02100657), researchers are evaluating plitidepsin in combination with bortezomib and dexamethasone for patients with relapsed/refractory MM.
In a phase 2 trial (NCT03117361), researchers are investigating plitidepsin in combination with bortezomib and dexamethasone for patients with MM that is refractory to both lenalidomide and bortezomib.
Plitidepsin has received orphan drug designation in the European Union and the US.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of marketing authorization for plitidepsin (Aplidin).
PharmaMar is seeking approval for plitidepsin to treat adults with multiple myeloma (MM) who have received at least 3 prior treatments, including bortezomib and either lenalidomide or thalidomide.
Plitidepsin is intended to be used in combination with dexamethasone.
This is the second time the CHMP has recommended against authorizing plitidepsin for this indication. The first time was last December.
At that time, PharmaMar asked the CHMP to re-examine its opinion, and the CHMP obliged. The committee confirmed its negative opinion of plitidepsin last week.
The CHMP’s review
Upon its initial review, the CHMP was concerned that data from the main study of plitidepsin in MM—the phase 3 ADMYRE trial—did not demonstrate a sufficient benefit of plitidepsin plus dexamethasone, compared to dexamethasone alone, in MM patients who had received 3 to 6 prior therapies.
The CHMP noted that the data showed a modest increase in progression free-survival (PFS)—around 1 month—with plitidepsin. According to study investigators, the median PFS was 3.8 months in patients who received plitidepsin and 1.9 months in those who received dexamethasone alone. According to an independent review committee, the median PFS was 2.6 months and 1.7 months, respectively.
The CHMP also said improvement in overall survival (OS) was not sufficiently demonstrated in this trial. The median OS was 11.6 months in the plitidepsin arm and 6.4 months in the dexamethasone arm.
Finally, the CHMP noted that severe adverse events were reported more frequently in patients who received plitidepsin. The most common grade 3/4 treatment-related adverse events (in the plitidepsin and dexamethasone arms, respectively) were fatigue (10.8% vs 1.2%), myalgia (5.4% vs 0%), and nausea (3.6% vs 1.2%).
Rates of treatment discontinuation were 9% in the plitidepsin arm, 6.5% in the dexamethasone arm, and 13.5% among patients who crossed over from the dexamethasone arm to the plitidepsin arm. Patients were allowed to cross over if they progressed after at least 8 weeks of treatment.
Based on these data, the CHMP was of the opinion that the benefits of plitidepsin did not outweigh its risks, so the committee recommended refusal of marketing authorization. After re-examination, the CHMP remained of the same opinion.
The European Commission (EC) has the final say on the marketing authorization application for plitidepsin. Though it is not required to do so, the EC typically follows the CHMP’s advice. The EC makes its decision within 67 days of the CHMP’s opinion.
PharmaMar has not announced whether it plans to continue developing plitidepsin for MM patients if the EC refuses to authorize the drug, and the company did not respond to a request for comment.
About plitidepsin
Plitidepsin is an investigational anticancer agent of marine origin, originally obtained from the ascidian Aplidium albicans. The drug specifically binds to eEF1A2 and targets the non-canonical role of this protein, resulting in cancer cell death via apoptosis.
Plitidepsin is currently in clinical development for hematologic malignancies.
In a phase 1b trial (NCT02100657), researchers are evaluating plitidepsin in combination with bortezomib and dexamethasone for patients with relapsed/refractory MM.
In a phase 2 trial (NCT03117361), researchers are investigating plitidepsin in combination with bortezomib and dexamethasone for patients with MM that is refractory to both lenalidomide and bortezomib.
Plitidepsin has received orphan drug designation in the European Union and the US.
CHMP recommends approval for generic prasugrel
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization to a generic version of prasugrel called Prasugrel Mylan.
Mylan S.A.S. is seeking approval for this product to be co-administered with acetylsalicylic acid for the prevention of atherothrombotic events in adults with acute coronary syndrome (ie, unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction) undergoing primary or delayed percutaneous coronary intervention.
The CHMP’s opinion on Prasugrel Mylan will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
If approved, Prasugrel Mylan will be available as 5 mg and 10 mg film-coated tablets.
Prasugrel is an inhibitor of platelet activation and aggregation that acts through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
Since platelets participate in the initiation and/or evolution of thrombotic complications of atherosclerotic disease, inhibition of platelet function can reduce the risk of cardiovascular events such as death, myocardial infarction, or stroke.
Prasugrel Mylan is a generic of Efient, which has been authorized in the European Union since 2009.
According to the CHMP, studies have demonstrated that Prasugrel Mylan is of “satisfactory quality” and bioequivalent to Efient.
In the TRITON–TIMI 38 study, treatment with prasugrel (Efient) was associated with significantly reduced rates of ischemic events, including stent thrombosis, when compared to treatment with clopidogrel in patients with moderate- to high-risk acute coronary syndromes with scheduled percutaneous coronary intervention.
However, prasugrel was also associated with an increased risk of major bleeding, including fatal bleeding. Still, there was no significant difference in mortality between the treatment groups.
These results were published in NEJM in 2007.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization to a generic version of prasugrel called Prasugrel Mylan.
Mylan S.A.S. is seeking approval for this product to be co-administered with acetylsalicylic acid for the prevention of atherothrombotic events in adults with acute coronary syndrome (ie, unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction) undergoing primary or delayed percutaneous coronary intervention.
The CHMP’s opinion on Prasugrel Mylan will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
If approved, Prasugrel Mylan will be available as 5 mg and 10 mg film-coated tablets.
Prasugrel is an inhibitor of platelet activation and aggregation that acts through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
Since platelets participate in the initiation and/or evolution of thrombotic complications of atherosclerotic disease, inhibition of platelet function can reduce the risk of cardiovascular events such as death, myocardial infarction, or stroke.
Prasugrel Mylan is a generic of Efient, which has been authorized in the European Union since 2009.
According to the CHMP, studies have demonstrated that Prasugrel Mylan is of “satisfactory quality” and bioequivalent to Efient.
In the TRITON–TIMI 38 study, treatment with prasugrel (Efient) was associated with significantly reduced rates of ischemic events, including stent thrombosis, when compared to treatment with clopidogrel in patients with moderate- to high-risk acute coronary syndromes with scheduled percutaneous coronary intervention.
However, prasugrel was also associated with an increased risk of major bleeding, including fatal bleeding. Still, there was no significant difference in mortality between the treatment groups.
These results were published in NEJM in 2007.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization to a generic version of prasugrel called Prasugrel Mylan.
Mylan S.A.S. is seeking approval for this product to be co-administered with acetylsalicylic acid for the prevention of atherothrombotic events in adults with acute coronary syndrome (ie, unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction) undergoing primary or delayed percutaneous coronary intervention.
The CHMP’s opinion on Prasugrel Mylan will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
If approved, Prasugrel Mylan will be available as 5 mg and 10 mg film-coated tablets.
Prasugrel is an inhibitor of platelet activation and aggregation that acts through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
Since platelets participate in the initiation and/or evolution of thrombotic complications of atherosclerotic disease, inhibition of platelet function can reduce the risk of cardiovascular events such as death, myocardial infarction, or stroke.
Prasugrel Mylan is a generic of Efient, which has been authorized in the European Union since 2009.
According to the CHMP, studies have demonstrated that Prasugrel Mylan is of “satisfactory quality” and bioequivalent to Efient.
In the TRITON–TIMI 38 study, treatment with prasugrel (Efient) was associated with significantly reduced rates of ischemic events, including stent thrombosis, when compared to treatment with clopidogrel in patients with moderate- to high-risk acute coronary syndromes with scheduled percutaneous coronary intervention.
However, prasugrel was also associated with an increased risk of major bleeding, including fatal bleeding. Still, there was no significant difference in mortality between the treatment groups.
These results were published in NEJM in 2007.
ICER assesses value of CAR T-cell therapies
The Institute for Clinical and Economic Review (ICER) has made policy recommendations intended to ensure affordability and access to chimeric antigen receptor (CAR) T-cell therapies.
ICER released a Final Evidence Report on tisagenlecleucel (Kymriah, Novartis) and axicabtagene ciloleucel (Yescarta, Kite Pharma/Gilead), 2 CAR T-cell therapies approved in the US to treat B-cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphoma (NHL), respectively.
The report says the pricing of these therapies aligns with patient benefit, but changes will be needed in future pricing, payment, and delivery mechanisms to ensure patient access without threatening health system affordability.
“Given the currently available evidence, these therapies appear to be effective options for those with B-ALL or NHL, though uncertainty in the evidence raised questions around the long-term value for money,” said Dan Ollendorf, PhD, ICER’s chief scientific officer.
Net health benefit
ICER’s report says tisagenlecleucel provides a net health benefit for children with B-ALL, and both tisagenlecleucel and axicabtagene ciloleucel provide a net health benefit for adults with certain types of NHL. (Novartis is seeking approval for tisagenlecleucel in NHL).
The evidence suggests there is at least a small net health benefit of the CAR T-cell therapies compared to other therapies. The benefit may be substantial, but uncertainties remain.
The data show complete remission (CR), disease-free survival (DFS), and overall survival (OS) rates are superior for NHL patients who receive axicabtagene ciloleucel, compared to patients who receive standard chemoimmunotherapy regimens.
Similarly, B-ALL patients treated with tisagenlecleucel have superior CR, DFS, and OS rates to patients treated with standard therapies. CR and OS rates are also superior in NHL patients treated with tisagenlecleucel, but DFS has not been reported in this population.
The report says there is insufficient evidence to distinguish between the 2 CAR T-cell therapies for the treatment of NHL.
Toxicity and uncertainty
The report highlights the fact that cytokine release syndrome, neurological symptoms, and B-cell aplasia have been observed in patients who receive CAR T-cell therapies. However, these sometimes severe adverse events are generally “manageable.”
In addition to toxicity, the report highlights sources of uncertainty. These include the fact that studies of tisagenlecleucel and axicabtagene ciloleucel are small, single-arm trials with short follow-up; comparisons with historical controls may be misleading; and improvements in the CAR T-cell manufacturing process may change outcomes.
Cost-effectiveness
The report states that the cost-effectiveness of each therapy fell below or within commonly cited thresholds of $50,000 to $150,000 per quality-adjusted life-year (QALY) over a lifetime.
For its analyses, ICER used the wholesale acquisition cost (WAC) plus an assumed hospital mark-up. The analyses were also based on the assumption that survival benefits observed in clinical trials would continue after the trials ended.
For tisagenlecleucel in pediatric B-ALL, the WAC is $475,000. The long-term cost-effectiveness compared to clofarabine is $45,871 per QALY gained.
For axicabtagene ciloleucel in adults with NHL, the WAC is $373,000. The long-term cost-effectiveness compared to salvage chemotherapy is $136,078 per QALY gained. The effectiveness assumptions for chemotherapy were based on an average of salvage chemotherapy regimens from the SCHOLAR-1 trial, and the cost assumptions were based on the cost of the R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin) regimen.
The report says tisagenlecleucel’s price would remain in alignment with value even if price premiums of 102% to 194% were applied.
Meanwhile, axicabtagene ciloleucel’s price could be increased by up to 11% and remain in alignment with the upper threshold ($150,000 per QALY gained) but would need to be discounted by 28% to align with the lower threshold ($100,000 per QALY gained).
Tisagenlecleucel, as a treatment for B-ALL, is not expected to cross the $915 million threshold for annual budget impact.
However, the short-term costs of axicabtagene ciloleucel for relapsed/refractory NHL could exceed the threshold. Only 38% of the estimated 5900 eligible patients could receive axicabtagene ciloleucel in a year before crossing the threshold.
Because of these findings, ICER issued an “Affordability and Access Alert” for axicabtagene ciloleucel.
This alert is intended to signal when the added costs associated with a new treatment may be difficult for the healthcare system to absorb over the short-term without displacing other needed services or contributing to unsustainable growth in healthcare insurance costs.
“Based on current evidence, both therapies appear to be priced in alignment with their clinical value, but there are potential short-term affordability concerns—for axicabtagene ciloleucel under its current indication and for both treatments should they receive future approvals for broader patient populations,” Dr Ollendorf said.
Panel voting results
ICER’s report was reviewed at a public meeting of the California Technology Assessment Forum on March 2.
Most of the panel said tisagenlecleucel provides intermediate long-term value for money when treating B-ALL. However, the significant uncertainty surrounding the long-term risks and benefits of the therapy precluded a high-value vote.
After deliberating on the value of axicabtagene ciloleucel to treat NHL, the panel’s votes were split between low-value and intermediate-value, driven by similar concerns about long-term uncertainty.
Policy recommendations
Following the voting session, ICER convened a policy roundtable of experts, including physicians, patient advocates, manufacturer representatives, and payer representatives.
Based on the roundtable discussion, ICER developed recommendations for enhanced stakeholder communication, innovative payment models, generation of additional evidence, settings of care, and patient education.
“With many other potentially transformative therapies in the pipeline, stakeholders must collaborate now to develop payment and delivery systems that can ensure timely patient access, manage short-term affordability for expensive one-time treatments, and continue to reward the innovation that brings these new treatments to market,” Dr Ollendorf said.
Some of ICER’s recommendations include:
- When launching novel therapies approved with limited clinical evidence, such as CAR T-cell therapies, manufacturers and payers should consider using a lower launch price that could be increased if substantial clinical benefits are confirmed or using a higher initial price tied to a requirement for refunds or rebates if real-world evidence fails to confirm high expectations.
- Outcomes-based pricing arrangements must be linked to “meaningful clinical outcomes assessed with sufficient follow up.”
- Hospital mark-up for CAR T-cell therapies “should reflect the expected additional cost for care delivered in the hospital, rather than a percentage of the drug cost to avoid perverse incentives in choosing the treatment location.”
- Initially, CAR T-cell therapies should be delivered in “manufacturer-accredited centers to ensure the quality and appropriateness of care.” Later, “centers of excellence accredited by specialty societies” can administer these therapies, as long as providers have “sufficient expertise” to manage serious side effects.
- Centers should ensure that patients understand what to expect from CAR T-cell therapy, including long-term consequences.
- Because additional evidence on CAR T-cell therapies is needed, all patients who receive these therapies should enter into a registry with planned long-term follow-up.
- Studies should determine the optimal timing of CAR T-cell therapy in the sequence of treatments for B-ALL and NHL.
Additional recommendations and more details are available in ICER’s report.
About ICER
ICER is an independent, non-profit research institute that produces reports analyzing evidence on the effectiveness and value of drugs and other medical services.
ICER’s reports include evidence-based calculations of prices for new drugs that reflect the degree of improvement expected in long-term patient outcomes, while also highlighting price levels that might contribute to unaffordable short-term cost growth for the overall healthcare system.
ICER’s reports incorporate input from stakeholders and are the subject of public hearings through 3 core programs: the California Technology Assessment Forum, the Midwest Comparative Effectiveness Public Advisory Council, and the New England Comparative Effectiveness Public Advisory Council.
These independent panels review ICER’s reports at public meetings to deliberate on the evidence and develop recommendations for how patients, clinicians, insurers, and policymakers can improve the quality and value of healthcare.
The Institute for Clinical and Economic Review (ICER) has made policy recommendations intended to ensure affordability and access to chimeric antigen receptor (CAR) T-cell therapies.
ICER released a Final Evidence Report on tisagenlecleucel (Kymriah, Novartis) and axicabtagene ciloleucel (Yescarta, Kite Pharma/Gilead), 2 CAR T-cell therapies approved in the US to treat B-cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphoma (NHL), respectively.
The report says the pricing of these therapies aligns with patient benefit, but changes will be needed in future pricing, payment, and delivery mechanisms to ensure patient access without threatening health system affordability.
“Given the currently available evidence, these therapies appear to be effective options for those with B-ALL or NHL, though uncertainty in the evidence raised questions around the long-term value for money,” said Dan Ollendorf, PhD, ICER’s chief scientific officer.
Net health benefit
ICER’s report says tisagenlecleucel provides a net health benefit for children with B-ALL, and both tisagenlecleucel and axicabtagene ciloleucel provide a net health benefit for adults with certain types of NHL. (Novartis is seeking approval for tisagenlecleucel in NHL).
The evidence suggests there is at least a small net health benefit of the CAR T-cell therapies compared to other therapies. The benefit may be substantial, but uncertainties remain.
The data show complete remission (CR), disease-free survival (DFS), and overall survival (OS) rates are superior for NHL patients who receive axicabtagene ciloleucel, compared to patients who receive standard chemoimmunotherapy regimens.
Similarly, B-ALL patients treated with tisagenlecleucel have superior CR, DFS, and OS rates to patients treated with standard therapies. CR and OS rates are also superior in NHL patients treated with tisagenlecleucel, but DFS has not been reported in this population.
The report says there is insufficient evidence to distinguish between the 2 CAR T-cell therapies for the treatment of NHL.
Toxicity and uncertainty
The report highlights the fact that cytokine release syndrome, neurological symptoms, and B-cell aplasia have been observed in patients who receive CAR T-cell therapies. However, these sometimes severe adverse events are generally “manageable.”
In addition to toxicity, the report highlights sources of uncertainty. These include the fact that studies of tisagenlecleucel and axicabtagene ciloleucel are small, single-arm trials with short follow-up; comparisons with historical controls may be misleading; and improvements in the CAR T-cell manufacturing process may change outcomes.
Cost-effectiveness
The report states that the cost-effectiveness of each therapy fell below or within commonly cited thresholds of $50,000 to $150,000 per quality-adjusted life-year (QALY) over a lifetime.
For its analyses, ICER used the wholesale acquisition cost (WAC) plus an assumed hospital mark-up. The analyses were also based on the assumption that survival benefits observed in clinical trials would continue after the trials ended.
For tisagenlecleucel in pediatric B-ALL, the WAC is $475,000. The long-term cost-effectiveness compared to clofarabine is $45,871 per QALY gained.
For axicabtagene ciloleucel in adults with NHL, the WAC is $373,000. The long-term cost-effectiveness compared to salvage chemotherapy is $136,078 per QALY gained. The effectiveness assumptions for chemotherapy were based on an average of salvage chemotherapy regimens from the SCHOLAR-1 trial, and the cost assumptions were based on the cost of the R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin) regimen.
The report says tisagenlecleucel’s price would remain in alignment with value even if price premiums of 102% to 194% were applied.
Meanwhile, axicabtagene ciloleucel’s price could be increased by up to 11% and remain in alignment with the upper threshold ($150,000 per QALY gained) but would need to be discounted by 28% to align with the lower threshold ($100,000 per QALY gained).
Tisagenlecleucel, as a treatment for B-ALL, is not expected to cross the $915 million threshold for annual budget impact.
However, the short-term costs of axicabtagene ciloleucel for relapsed/refractory NHL could exceed the threshold. Only 38% of the estimated 5900 eligible patients could receive axicabtagene ciloleucel in a year before crossing the threshold.
Because of these findings, ICER issued an “Affordability and Access Alert” for axicabtagene ciloleucel.
This alert is intended to signal when the added costs associated with a new treatment may be difficult for the healthcare system to absorb over the short-term without displacing other needed services or contributing to unsustainable growth in healthcare insurance costs.
“Based on current evidence, both therapies appear to be priced in alignment with their clinical value, but there are potential short-term affordability concerns—for axicabtagene ciloleucel under its current indication and for both treatments should they receive future approvals for broader patient populations,” Dr Ollendorf said.
Panel voting results
ICER’s report was reviewed at a public meeting of the California Technology Assessment Forum on March 2.
Most of the panel said tisagenlecleucel provides intermediate long-term value for money when treating B-ALL. However, the significant uncertainty surrounding the long-term risks and benefits of the therapy precluded a high-value vote.
After deliberating on the value of axicabtagene ciloleucel to treat NHL, the panel’s votes were split between low-value and intermediate-value, driven by similar concerns about long-term uncertainty.
Policy recommendations
Following the voting session, ICER convened a policy roundtable of experts, including physicians, patient advocates, manufacturer representatives, and payer representatives.
Based on the roundtable discussion, ICER developed recommendations for enhanced stakeholder communication, innovative payment models, generation of additional evidence, settings of care, and patient education.
“With many other potentially transformative therapies in the pipeline, stakeholders must collaborate now to develop payment and delivery systems that can ensure timely patient access, manage short-term affordability for expensive one-time treatments, and continue to reward the innovation that brings these new treatments to market,” Dr Ollendorf said.
Some of ICER’s recommendations include:
- When launching novel therapies approved with limited clinical evidence, such as CAR T-cell therapies, manufacturers and payers should consider using a lower launch price that could be increased if substantial clinical benefits are confirmed or using a higher initial price tied to a requirement for refunds or rebates if real-world evidence fails to confirm high expectations.
- Outcomes-based pricing arrangements must be linked to “meaningful clinical outcomes assessed with sufficient follow up.”
- Hospital mark-up for CAR T-cell therapies “should reflect the expected additional cost for care delivered in the hospital, rather than a percentage of the drug cost to avoid perverse incentives in choosing the treatment location.”
- Initially, CAR T-cell therapies should be delivered in “manufacturer-accredited centers to ensure the quality and appropriateness of care.” Later, “centers of excellence accredited by specialty societies” can administer these therapies, as long as providers have “sufficient expertise” to manage serious side effects.
- Centers should ensure that patients understand what to expect from CAR T-cell therapy, including long-term consequences.
- Because additional evidence on CAR T-cell therapies is needed, all patients who receive these therapies should enter into a registry with planned long-term follow-up.
- Studies should determine the optimal timing of CAR T-cell therapy in the sequence of treatments for B-ALL and NHL.
Additional recommendations and more details are available in ICER’s report.
About ICER
ICER is an independent, non-profit research institute that produces reports analyzing evidence on the effectiveness and value of drugs and other medical services.
ICER’s reports include evidence-based calculations of prices for new drugs that reflect the degree of improvement expected in long-term patient outcomes, while also highlighting price levels that might contribute to unaffordable short-term cost growth for the overall healthcare system.
ICER’s reports incorporate input from stakeholders and are the subject of public hearings through 3 core programs: the California Technology Assessment Forum, the Midwest Comparative Effectiveness Public Advisory Council, and the New England Comparative Effectiveness Public Advisory Council.
These independent panels review ICER’s reports at public meetings to deliberate on the evidence and develop recommendations for how patients, clinicians, insurers, and policymakers can improve the quality and value of healthcare.
The Institute for Clinical and Economic Review (ICER) has made policy recommendations intended to ensure affordability and access to chimeric antigen receptor (CAR) T-cell therapies.
ICER released a Final Evidence Report on tisagenlecleucel (Kymriah, Novartis) and axicabtagene ciloleucel (Yescarta, Kite Pharma/Gilead), 2 CAR T-cell therapies approved in the US to treat B-cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphoma (NHL), respectively.
The report says the pricing of these therapies aligns with patient benefit, but changes will be needed in future pricing, payment, and delivery mechanisms to ensure patient access without threatening health system affordability.
“Given the currently available evidence, these therapies appear to be effective options for those with B-ALL or NHL, though uncertainty in the evidence raised questions around the long-term value for money,” said Dan Ollendorf, PhD, ICER’s chief scientific officer.
Net health benefit
ICER’s report says tisagenlecleucel provides a net health benefit for children with B-ALL, and both tisagenlecleucel and axicabtagene ciloleucel provide a net health benefit for adults with certain types of NHL. (Novartis is seeking approval for tisagenlecleucel in NHL).
The evidence suggests there is at least a small net health benefit of the CAR T-cell therapies compared to other therapies. The benefit may be substantial, but uncertainties remain.
The data show complete remission (CR), disease-free survival (DFS), and overall survival (OS) rates are superior for NHL patients who receive axicabtagene ciloleucel, compared to patients who receive standard chemoimmunotherapy regimens.
Similarly, B-ALL patients treated with tisagenlecleucel have superior CR, DFS, and OS rates to patients treated with standard therapies. CR and OS rates are also superior in NHL patients treated with tisagenlecleucel, but DFS has not been reported in this population.
The report says there is insufficient evidence to distinguish between the 2 CAR T-cell therapies for the treatment of NHL.
Toxicity and uncertainty
The report highlights the fact that cytokine release syndrome, neurological symptoms, and B-cell aplasia have been observed in patients who receive CAR T-cell therapies. However, these sometimes severe adverse events are generally “manageable.”
In addition to toxicity, the report highlights sources of uncertainty. These include the fact that studies of tisagenlecleucel and axicabtagene ciloleucel are small, single-arm trials with short follow-up; comparisons with historical controls may be misleading; and improvements in the CAR T-cell manufacturing process may change outcomes.
Cost-effectiveness
The report states that the cost-effectiveness of each therapy fell below or within commonly cited thresholds of $50,000 to $150,000 per quality-adjusted life-year (QALY) over a lifetime.
For its analyses, ICER used the wholesale acquisition cost (WAC) plus an assumed hospital mark-up. The analyses were also based on the assumption that survival benefits observed in clinical trials would continue after the trials ended.
For tisagenlecleucel in pediatric B-ALL, the WAC is $475,000. The long-term cost-effectiveness compared to clofarabine is $45,871 per QALY gained.
For axicabtagene ciloleucel in adults with NHL, the WAC is $373,000. The long-term cost-effectiveness compared to salvage chemotherapy is $136,078 per QALY gained. The effectiveness assumptions for chemotherapy were based on an average of salvage chemotherapy regimens from the SCHOLAR-1 trial, and the cost assumptions were based on the cost of the R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin) regimen.
The report says tisagenlecleucel’s price would remain in alignment with value even if price premiums of 102% to 194% were applied.
Meanwhile, axicabtagene ciloleucel’s price could be increased by up to 11% and remain in alignment with the upper threshold ($150,000 per QALY gained) but would need to be discounted by 28% to align with the lower threshold ($100,000 per QALY gained).
Tisagenlecleucel, as a treatment for B-ALL, is not expected to cross the $915 million threshold for annual budget impact.
However, the short-term costs of axicabtagene ciloleucel for relapsed/refractory NHL could exceed the threshold. Only 38% of the estimated 5900 eligible patients could receive axicabtagene ciloleucel in a year before crossing the threshold.
Because of these findings, ICER issued an “Affordability and Access Alert” for axicabtagene ciloleucel.
This alert is intended to signal when the added costs associated with a new treatment may be difficult for the healthcare system to absorb over the short-term without displacing other needed services or contributing to unsustainable growth in healthcare insurance costs.
“Based on current evidence, both therapies appear to be priced in alignment with their clinical value, but there are potential short-term affordability concerns—for axicabtagene ciloleucel under its current indication and for both treatments should they receive future approvals for broader patient populations,” Dr Ollendorf said.
Panel voting results
ICER’s report was reviewed at a public meeting of the California Technology Assessment Forum on March 2.
Most of the panel said tisagenlecleucel provides intermediate long-term value for money when treating B-ALL. However, the significant uncertainty surrounding the long-term risks and benefits of the therapy precluded a high-value vote.
After deliberating on the value of axicabtagene ciloleucel to treat NHL, the panel’s votes were split between low-value and intermediate-value, driven by similar concerns about long-term uncertainty.
Policy recommendations
Following the voting session, ICER convened a policy roundtable of experts, including physicians, patient advocates, manufacturer representatives, and payer representatives.
Based on the roundtable discussion, ICER developed recommendations for enhanced stakeholder communication, innovative payment models, generation of additional evidence, settings of care, and patient education.
“With many other potentially transformative therapies in the pipeline, stakeholders must collaborate now to develop payment and delivery systems that can ensure timely patient access, manage short-term affordability for expensive one-time treatments, and continue to reward the innovation that brings these new treatments to market,” Dr Ollendorf said.
Some of ICER’s recommendations include:
- When launching novel therapies approved with limited clinical evidence, such as CAR T-cell therapies, manufacturers and payers should consider using a lower launch price that could be increased if substantial clinical benefits are confirmed or using a higher initial price tied to a requirement for refunds or rebates if real-world evidence fails to confirm high expectations.
- Outcomes-based pricing arrangements must be linked to “meaningful clinical outcomes assessed with sufficient follow up.”
- Hospital mark-up for CAR T-cell therapies “should reflect the expected additional cost for care delivered in the hospital, rather than a percentage of the drug cost to avoid perverse incentives in choosing the treatment location.”
- Initially, CAR T-cell therapies should be delivered in “manufacturer-accredited centers to ensure the quality and appropriateness of care.” Later, “centers of excellence accredited by specialty societies” can administer these therapies, as long as providers have “sufficient expertise” to manage serious side effects.
- Centers should ensure that patients understand what to expect from CAR T-cell therapy, including long-term consequences.
- Because additional evidence on CAR T-cell therapies is needed, all patients who receive these therapies should enter into a registry with planned long-term follow-up.
- Studies should determine the optimal timing of CAR T-cell therapy in the sequence of treatments for B-ALL and NHL.
Additional recommendations and more details are available in ICER’s report.
About ICER
ICER is an independent, non-profit research institute that produces reports analyzing evidence on the effectiveness and value of drugs and other medical services.
ICER’s reports include evidence-based calculations of prices for new drugs that reflect the degree of improvement expected in long-term patient outcomes, while also highlighting price levels that might contribute to unaffordable short-term cost growth for the overall healthcare system.
ICER’s reports incorporate input from stakeholders and are the subject of public hearings through 3 core programs: the California Technology Assessment Forum, the Midwest Comparative Effectiveness Public Advisory Council, and the New England Comparative Effectiveness Public Advisory Council.
These independent panels review ICER’s reports at public meetings to deliberate on the evidence and develop recommendations for how patients, clinicians, insurers, and policymakers can improve the quality and value of healthcare.
Leukemia research pioneer dies at 92
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
Product approved for hemophilia patients in Japan
Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved the bispecific factor IXa- and factor X-directed antibody emicizumab (Hemlibra), according to Chugai Pharmaceutical Co., Ltd.
Emicizumab is now approved for use in Japan as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in patients with hemophilia A and factor VIII inhibitors.
There are a few conditions for this approval, including requirements for a risk management plan, early phase post-marketing vigilance, and post-marketing drug use surveillance.
A risk management plan is intended to assess measures for appropriate management of the risks associated with a drug, either at regular intervals or in response to the progress of post-marketing surveillance.
According to Japan’s Pharmaceuticals and Medical Devices Agency, a risk management plan must consist of 3 elements:
- Safety specification—Important adverse drug reactions and missing information
- Pharmacovigilance activities—Information collection activities performed in the post-marketing period
- Risk-minimization activities—Safety measures taken to minimize risks, which consists of providing information to healthcare professionals and setting the terms of use for a drug.
For the early phase post-marketing vigilance requirement, Chugai must provide safety information to healthcare professionals and collect information on adverse reactions to emicizumab in the early post-marketing phase.
Post-marketing drug use surveillance for emicizumab will include all patients receiving the product and is scheduled to continue until data is collected from approximately 100 people. The goal is to understand background information on patients receiving emicizumab, as well as to collect safety and efficacy data on the product and take necessary measures for the appropriate use of emicizumab.
The data collected via this surveillance effort will be reviewed to determine whether new surveillance or further safety measures are needed. Results of the surveillance will be reported to the regulatory authorities, and the data will be presented at scientific meetings, according to Chugai.
Phase 3 studies
The MHLW’s approval of emicizumab is based on data from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting in December.
HAVEN 1
This study enrolled 109 patients (age 12 and older) with hemophilia A and factor VIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs). Three had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with factor VIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related.
Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved the bispecific factor IXa- and factor X-directed antibody emicizumab (Hemlibra), according to Chugai Pharmaceutical Co., Ltd.
Emicizumab is now approved for use in Japan as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in patients with hemophilia A and factor VIII inhibitors.
There are a few conditions for this approval, including requirements for a risk management plan, early phase post-marketing vigilance, and post-marketing drug use surveillance.
A risk management plan is intended to assess measures for appropriate management of the risks associated with a drug, either at regular intervals or in response to the progress of post-marketing surveillance.
According to Japan’s Pharmaceuticals and Medical Devices Agency, a risk management plan must consist of 3 elements:
- Safety specification—Important adverse drug reactions and missing information
- Pharmacovigilance activities—Information collection activities performed in the post-marketing period
- Risk-minimization activities—Safety measures taken to minimize risks, which consists of providing information to healthcare professionals and setting the terms of use for a drug.
For the early phase post-marketing vigilance requirement, Chugai must provide safety information to healthcare professionals and collect information on adverse reactions to emicizumab in the early post-marketing phase.
Post-marketing drug use surveillance for emicizumab will include all patients receiving the product and is scheduled to continue until data is collected from approximately 100 people. The goal is to understand background information on patients receiving emicizumab, as well as to collect safety and efficacy data on the product and take necessary measures for the appropriate use of emicizumab.
The data collected via this surveillance effort will be reviewed to determine whether new surveillance or further safety measures are needed. Results of the surveillance will be reported to the regulatory authorities, and the data will be presented at scientific meetings, according to Chugai.
Phase 3 studies
The MHLW’s approval of emicizumab is based on data from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting in December.
HAVEN 1
This study enrolled 109 patients (age 12 and older) with hemophilia A and factor VIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs). Three had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with factor VIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related.
Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved the bispecific factor IXa- and factor X-directed antibody emicizumab (Hemlibra), according to Chugai Pharmaceutical Co., Ltd.
Emicizumab is now approved for use in Japan as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in patients with hemophilia A and factor VIII inhibitors.
There are a few conditions for this approval, including requirements for a risk management plan, early phase post-marketing vigilance, and post-marketing drug use surveillance.
A risk management plan is intended to assess measures for appropriate management of the risks associated with a drug, either at regular intervals or in response to the progress of post-marketing surveillance.
According to Japan’s Pharmaceuticals and Medical Devices Agency, a risk management plan must consist of 3 elements:
- Safety specification—Important adverse drug reactions and missing information
- Pharmacovigilance activities—Information collection activities performed in the post-marketing period
- Risk-minimization activities—Safety measures taken to minimize risks, which consists of providing information to healthcare professionals and setting the terms of use for a drug.
For the early phase post-marketing vigilance requirement, Chugai must provide safety information to healthcare professionals and collect information on adverse reactions to emicizumab in the early post-marketing phase.
Post-marketing drug use surveillance for emicizumab will include all patients receiving the product and is scheduled to continue until data is collected from approximately 100 people. The goal is to understand background information on patients receiving emicizumab, as well as to collect safety and efficacy data on the product and take necessary measures for the appropriate use of emicizumab.
The data collected via this surveillance effort will be reviewed to determine whether new surveillance or further safety measures are needed. Results of the surveillance will be reported to the regulatory authorities, and the data will be presented at scientific meetings, according to Chugai.
Phase 3 studies
The MHLW’s approval of emicizumab is based on data from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting in December.
HAVEN 1
This study enrolled 109 patients (age 12 and older) with hemophilia A and factor VIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs). Three had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with factor VIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related.