User login
Outcomes Associated With a Multidisciplinary Pain Oversight Committee to Facilitate Appropriate Management of Chronic Opioid Therapy
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
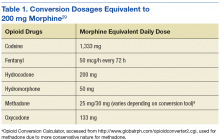
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
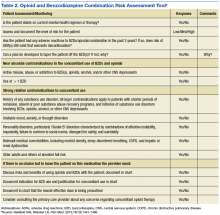
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
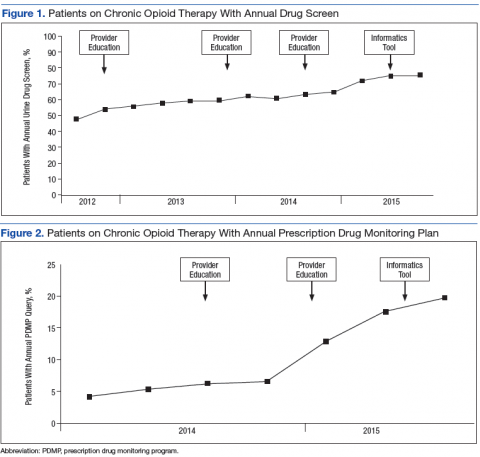
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
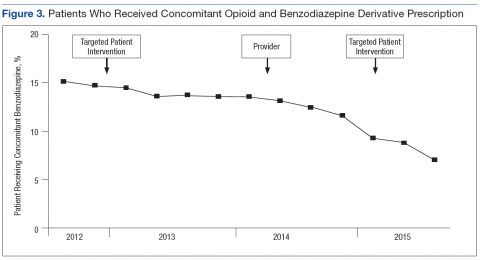
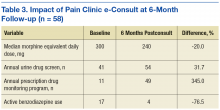
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
Who’s at greatest risk for delirium tremens
Ms. J, 42, was admitted to the OB-GYN service for a routine vaginal hysterectomy to treat dysfunctional uterine bleeding. In the presurgical history, she described having a few drinks daily. Shortly after a successful uncomplicated procedure, the patient became tremulous and was given several doses of lorazepam.
Two days after surgery, the patient became delirious. She complained of tactile and visual hallucinations, her level of consciousness waxed and waned, and she showed significant autonomic instability. A psychiatry consult was ordered. The consult team recommended IV fluids, IV diazepam, and haloperidol, supplemented with a multivitamin and 100 mg/d of thiamine. When the patient’s delirium resolved within 4 days, a more detailed discussion revealed a history of alcohol abuse and withdrawal seizures.
It is not uncommon for a patient to develop acute alcohol withdrawal and delirium tremens (DTs) while recovering from routine surgery. Delirium tremens remains a medical emergency, even though advances have reduced its associated mortality rates (Box).1-7
Psychiatrists who know the risk factors for DTs—also termed alcohol withdrawal delirium—can identify and protect patients who are susceptible to this life-threatening complication. We describe the clinical features of DTs, potential predisposing factors, theories behind its mechanisms, and strategies for preventing and managing DTs in patients experiencing alcohol withdrawal.
Clinical features
Disorientation and confusion are the hallmark features of DTs. Other clinical manifestations include vivid hallucinations, extreme tremulousness, autonomic hyperactivity, sweating, tachycardia, and agitation. Men experiencing DTs seem to demonstrate a greater degree of autonomic hyperactivity than women.8
Symptoms usually arise in the alcoholic patient between the third and fifth days of abstinence but have been known to occur several weeks after a patient’s last drink. Symptoms usually resolve within a few days9 but have been known to resolve within hours in some patients and to persist for several months in others.10
Differential diagnosis. Clinicians often fail to differentiate alcohol hallucinosis from DTs. Alcohol hallucinosis—which occurs in 3 to 10% of patients with severe alcohol withdrawal11 —manifests as auditory, visual, or tactile hallucinations with a clear sensorium. Patients experiencing DTs also may experience hallucinations but with confusion, disorientation, and severe autonomic hyperactivity. Unlike DTs, alcohol hallucinosis is not fatal.9
DTs also should be differentiated from:
- other causes of delirium, such as medication or infection. If the cause is identified and removed, the delirium should gradually resolve.
- Wernicke’s encephalopathy—caused by glucose exposure in the thiamine-deficient alcoholic—which is characterized by confusion, ophthalmoplegia, and ataxia.
Completing a thorough history and physical exam, talking to family members, and reviewing past medical charts are often the best ways to differentiate DTs from other conditions.
What causes DTs?
Vitamin deficiencies were initially thought to cause alcohol withdrawal.3 More recent evidence points toward multiple neuroadaptive changes in the brain associated with chronic alcohol exposure.12 Although numerous neurotransmitter systems may play a role in alcohol withdrawal, recent research has focused on glutamate13 and gamma-aminobutyric acid (GABA).14
Approximately 1.5 to 2 million Americans seek treatment for alcohol abuse or dependence each year.1 As many as 71% of these patients manifest symptoms of alcohol withdrawal.2 Of those individuals who experience alcohol withdrawal, delirium tremens (DTs) may occur in up to 5%.3-5 Utilizing these percentages, it can be estimated that as many as 50,000 to 70,000 individuals develop DTs each year in the United States alone.
Although the incidence of DTs can be assumed to be relatively low, the condition should be considered a medical emergency. Studies list mortality rates for DTs as high as 15% and as low as 2 to 3%.7,8
The brain seems to compensate for alcohol’s enhancement of GABA (inhibitory) neurons by up-regulating excitatory neurons (glutamate). Alcohol has been shown to have some effects on neurons.15 The implication is that withdrawing alcohol triggers an “excitatory state” until the brain can readjust the fine balance between excitation and inhibition, a process that takes weeks to months. Some changes may never reverse because of the neurotoxic effects of alcohol and alcohol withdrawal.
Repeated alcohol exposure and withdrawal may lead to neuroadaptive changes in the brain and to more severe withdrawal symptoms, such as DTs. Repeated alcohol withdrawal episodes can produce a kindling effect. As outlined by Becker, kindling occurs “when a weak electrical or chemical stimulus, which initially causes no overt behavioral responses, results in the appearance of behavioral effects, such as seizures, when it is administered repeatedly.”16 Thus, repeated alcohol withdrawal worsens future episodes and eventually leads to alcohol withdrawal seizures.
Whereas most of these theories apply to alcohol withdrawal, they are also compatible with the neuronal mechanisms that may underlie DTs. Alcohol withdrawal and DTs share the presence of a “hyperactive state.” Most likely, DTs is the progression to more severe or pronounced neuroadaptive changes seen in mild to moderate alcohol withdrawal. One could certainly imagine that the possible neurotoxic effects of alcohol, alcohol withdrawal, and repeated detoxifications could sensitize the CNS to the more severe symptoms seen in DTs. Infection and metabolic abnormalities may also enhance the progression. Unfortunately, why some but not all patients experiencing alcohol withdrawal progress to DTs is unknown.
Table 1
RISK FACTORS FOR DELIRIUM TREMENS
| Comorbid medical illness (with electrolyte, fluid abnormalities)* |
| History of delirium tremens* |
| Blood alcohol level >300 mg/dL on presentation* |
| Presentation after an alcohol withdrawal seizure* |
| Older age* |
| Longer history of alcohol dependence |
| Intense alcohol craving |
| Abnormal liver function |
| * Supported with studies and/or in the medical literature |
| Source: Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World 1998;22(1):5-12. |
Table 2
STRATEGIES TO PREVENT AND TREAT DELIRIUM TREMENS
| Assess risk for DTs with a thorough history, including collaborative family information and medical charts |
| Admit patients with a history of serious withdrawal symptoms or potential for inpatient detoxification (based on degree of tolerance) |
| Check complete lab values (chemistry panel, liver function tests including ALT and AST, complete blood count, blood alcohol level, GGT, and others if relevant), and correct any fluid, vitamin, or electrolyte abnormalities |
| Treat comorbid medical conditions |
| Differentiate DTs from alcohol hallucinosis, other causes of delirium, and Wernicke’s encephalopathy |
| Consider giving benzodiazepines along with low-dose neuroleptics, if appropriate, and monitor for disinhibition/ confusion and extrapyramidal symptoms, respectively |
| Place the patient in a low-stimulation environment with frequent monitoring |
Predisposing risk factors
Past withdrawal complicated by seizures or DTs is the single best predictor of future alcohol withdrawal symptoms.17 Also consider the following patients to be at elevated risk:
- any individual who presents with a blood alcohol level >300 mg/dl or after experiencing a withdrawal seizure9
- patients with comorbid medical conditions, such as electrolyte abnormalities, infection, or poorly treated cardiovascular or respiratory diseases
- older persons, who tend to be susceptible to delirium associated with hospitalization, medical illnesses such as urinary tract infections or pneumonia, or use of certain medications.18
Potential risk factors for developing DTs are summarized in Table 1.1 Although few studies have been done, clinicians can use these factors as a guide for aggressively preventing DTs in at-risk patients.
Managing and preventing DTs
Management. Drug therapy is considered crucial to quell withdrawal symptoms and reduce the risk of death.19 Patients usually are treated with one of several benzodiazepines (such as chlordiazepoxide, diazepam, oxazepam, or lorazepam) to decrease autonomic instability and reduce seizure risk during acute alcohol withdrawal. Although dosages of these medications are estimated based on drinking history, some general starting ranges are often used in clinical practice:
- chlordiazepoxide, 50 to 100 mg tid
- lorazepam, 1 to 2 mg every 4 hours
- oxazepam, 15 to 30 mg qid
- diazepam, 10 to 20 mg tid/qid.
Treating DTs often requires the use of IV benzodiazepines because of their quick onset of action and benefit for acutely agitated patients who have difficulty taking medications by mouth.
Prevention. Correcting fluid and electrolyte abnormalities may be critical in preventing DTs (Table 2). In one study of patients who died while experiencing DTs, only 25% received adequate fluid replacement, which can be as much as 6 liters per day.20 Ideally, comorbid conditions should be addressed early in presentation and before DTs develop.21
High-dose benzodiazepine therapy does not completely protect a patient from DTs or reduce its duration,22 but it may reduce mortality. A meta-analysis of prospective, placebocontrolled trials reported a risk reduction of 4.9 cases of DTs per 100 patients treated with benzodiazepines. Mortality also seems to have been reduced in patients with DTs who were treated with sedative hypnotics.9 Benzodiazepines may cause increased confusion and disinhibition, as is frequently seen when patients with dementia are treated with these agents.23
Neuroleptics such as haloperidol have been used to prevent and treat DTs, but studies of their ability to reduce mortality have produced inconsistent results. What’s more, neuroleptics can reduce the seizure threshold and produce extrapyramidal symptoms.23 Atypical antipsychotics may offer a safer alternative, although more studies are needed to evaluate whether they decrease the occurrence and severity of DTs.
In summary, a rational approach to preventing and treating DTs is to:
- manage comorbid medical illnesses, and correct fluid and electrolyte abnormalities
- place the patient in a safe, low-stimulation environment with frequent monitoring
- use benzodiazepines judiciously.
Related resources
- National Institute on Alcohol Abuse and Alcoholism.
- www.niaaa.nih.gov
- 1995;30(6):765-70 (a thorough review of benzodiazepine use in alcohol withdrawal).
Drug brand names
- Chlordiazepoxide • Librium
- Diazepam • Valium
- Haloperidol • Haldol
- Lorazepam • Ativan
- Oxazepam • Serax
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World 1998;22(1):5-12.
2. Saitz R, Redmond MS, Mayo-Smith MF, et al. Individualized treatment for alcohol withdrawal. Randomized, double-blind, controlled trial. JAMA 1994;272:519-23.
3. Victor M, Adams RD. The effect of alcoholism on the nervous system. Research Publication of the Association for Research on Nervous and Mental Disorders 1953;32:526-73.
4. Victor M, Braush C. The role of abstinence in the genesis of alcoholic epilepsy. Epilepsia 1967;8:1-20.
5. Sellars EM, Kalant H. Alcohol intoxication and withdrawal. N Engl J Med 1976;294:757-62.
6. Victor M. Treatment of alcoholic intoxication and the withdrawal syndrome. Psychosomat Med 1966;28:636-50.
7. Guthrie S. The treatment of alcohol withdrawal. Pharmacotherapy 1989;9:131-43.
8. Trevisan LA, Boutrous N, Petrakis I, Krystal JH. Complications of alcohol withdrawal. Alcohol World 1998;22(1):61-66.
9. Mayo-Smith MF. Management of alcohol intoxication, overdose, and withdrawal. In: Graham AW, Schultz TK (eds). Principles of addiction medicine (2nd ed). Chevy Chase, MD: American Society of Addiction Medicine, 1998;434-41.
10. Wolf KM, Shaughnessy AF, Middleton DB. Prolonged delirium tremens requiring massive doses of medication. J Am Board Fam Pract 1993;6:502-4.
11. Platz WE, Oberlaender FA, Seidel ML. The phenomenology of perceptual hallucinations in alcohol-induced delirium tremens. Psychopathology 1995;28:247-55.
12. Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World 1998;22(1):13-24.
13. Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol 1990;7(3):229-31.
14. Morrow AL, Montpied P, Lingford-Hughes A, Paul SM. Chronic ethanol and pentobarbital administration in the rat: effects on GABAA receptor function and expression in brain. Alcohol 1990;7(3):237-44.
15. Begleiter H Kissin B (eds) The pharmacology of alcohol and alcohol dependence. New York: Oxford University Press, 1996.
16. Becker HC. Kindling in alcohol withdrawal. Alcohol World 1998;22(1):25-33.
17. Gold M, Miller N. Management of withdrawal syndromes and relapse prevention in drug and alcohol dependence. Am Fam Phys 1998;58:139-46.
18. Kraemer K, Mayo-Smith MF, Calkins R. Pharmacological management of alcohol withdrawal: a meta analysis of evidence-based practical guidelines. JAMA 1997;278:144-51.
19. Shaw GK. Detoxification: the use of benzodiazepines. Alcohol Alcohol 1995;30(6):765-70.
20. Victor M. Diagnosis and treatment of alcohol withdrawal states. Pract Gastroenteritis 1983;7(5):6-15.
21. Myrick H, Anton R. Treatment of alcohol withdrawal. Alcohol Health Res World 1998;22(1):38-43.
22. Hersh D, Kranzler HR, Meyer RE. Persistent delirium following cessation of heavy alcohol consumption: diagnostic and treatment implications. Am J Psychiatry 1997;154(6):846-51.
23. Myrick H, Anton R. Clinical management of alcohol withdrawal. CNS Spectrums 2000;5(2):22-32.
Ms. J, 42, was admitted to the OB-GYN service for a routine vaginal hysterectomy to treat dysfunctional uterine bleeding. In the presurgical history, she described having a few drinks daily. Shortly after a successful uncomplicated procedure, the patient became tremulous and was given several doses of lorazepam.
Two days after surgery, the patient became delirious. She complained of tactile and visual hallucinations, her level of consciousness waxed and waned, and she showed significant autonomic instability. A psychiatry consult was ordered. The consult team recommended IV fluids, IV diazepam, and haloperidol, supplemented with a multivitamin and 100 mg/d of thiamine. When the patient’s delirium resolved within 4 days, a more detailed discussion revealed a history of alcohol abuse and withdrawal seizures.
It is not uncommon for a patient to develop acute alcohol withdrawal and delirium tremens (DTs) while recovering from routine surgery. Delirium tremens remains a medical emergency, even though advances have reduced its associated mortality rates (Box).1-7
Psychiatrists who know the risk factors for DTs—also termed alcohol withdrawal delirium—can identify and protect patients who are susceptible to this life-threatening complication. We describe the clinical features of DTs, potential predisposing factors, theories behind its mechanisms, and strategies for preventing and managing DTs in patients experiencing alcohol withdrawal.
Clinical features
Disorientation and confusion are the hallmark features of DTs. Other clinical manifestations include vivid hallucinations, extreme tremulousness, autonomic hyperactivity, sweating, tachycardia, and agitation. Men experiencing DTs seem to demonstrate a greater degree of autonomic hyperactivity than women.8
Symptoms usually arise in the alcoholic patient between the third and fifth days of abstinence but have been known to occur several weeks after a patient’s last drink. Symptoms usually resolve within a few days9 but have been known to resolve within hours in some patients and to persist for several months in others.10
Differential diagnosis. Clinicians often fail to differentiate alcohol hallucinosis from DTs. Alcohol hallucinosis—which occurs in 3 to 10% of patients with severe alcohol withdrawal11 —manifests as auditory, visual, or tactile hallucinations with a clear sensorium. Patients experiencing DTs also may experience hallucinations but with confusion, disorientation, and severe autonomic hyperactivity. Unlike DTs, alcohol hallucinosis is not fatal.9
DTs also should be differentiated from:
- other causes of delirium, such as medication or infection. If the cause is identified and removed, the delirium should gradually resolve.
- Wernicke’s encephalopathy—caused by glucose exposure in the thiamine-deficient alcoholic—which is characterized by confusion, ophthalmoplegia, and ataxia.
Completing a thorough history and physical exam, talking to family members, and reviewing past medical charts are often the best ways to differentiate DTs from other conditions.
What causes DTs?
Vitamin deficiencies were initially thought to cause alcohol withdrawal.3 More recent evidence points toward multiple neuroadaptive changes in the brain associated with chronic alcohol exposure.12 Although numerous neurotransmitter systems may play a role in alcohol withdrawal, recent research has focused on glutamate13 and gamma-aminobutyric acid (GABA).14
Approximately 1.5 to 2 million Americans seek treatment for alcohol abuse or dependence each year.1 As many as 71% of these patients manifest symptoms of alcohol withdrawal.2 Of those individuals who experience alcohol withdrawal, delirium tremens (DTs) may occur in up to 5%.3-5 Utilizing these percentages, it can be estimated that as many as 50,000 to 70,000 individuals develop DTs each year in the United States alone.
Although the incidence of DTs can be assumed to be relatively low, the condition should be considered a medical emergency. Studies list mortality rates for DTs as high as 15% and as low as 2 to 3%.7,8
The brain seems to compensate for alcohol’s enhancement of GABA (inhibitory) neurons by up-regulating excitatory neurons (glutamate). Alcohol has been shown to have some effects on neurons.15 The implication is that withdrawing alcohol triggers an “excitatory state” until the brain can readjust the fine balance between excitation and inhibition, a process that takes weeks to months. Some changes may never reverse because of the neurotoxic effects of alcohol and alcohol withdrawal.
Repeated alcohol exposure and withdrawal may lead to neuroadaptive changes in the brain and to more severe withdrawal symptoms, such as DTs. Repeated alcohol withdrawal episodes can produce a kindling effect. As outlined by Becker, kindling occurs “when a weak electrical or chemical stimulus, which initially causes no overt behavioral responses, results in the appearance of behavioral effects, such as seizures, when it is administered repeatedly.”16 Thus, repeated alcohol withdrawal worsens future episodes and eventually leads to alcohol withdrawal seizures.
Whereas most of these theories apply to alcohol withdrawal, they are also compatible with the neuronal mechanisms that may underlie DTs. Alcohol withdrawal and DTs share the presence of a “hyperactive state.” Most likely, DTs is the progression to more severe or pronounced neuroadaptive changes seen in mild to moderate alcohol withdrawal. One could certainly imagine that the possible neurotoxic effects of alcohol, alcohol withdrawal, and repeated detoxifications could sensitize the CNS to the more severe symptoms seen in DTs. Infection and metabolic abnormalities may also enhance the progression. Unfortunately, why some but not all patients experiencing alcohol withdrawal progress to DTs is unknown.
Table 1
RISK FACTORS FOR DELIRIUM TREMENS
| Comorbid medical illness (with electrolyte, fluid abnormalities)* |
| History of delirium tremens* |
| Blood alcohol level >300 mg/dL on presentation* |
| Presentation after an alcohol withdrawal seizure* |
| Older age* |
| Longer history of alcohol dependence |
| Intense alcohol craving |
| Abnormal liver function |
| * Supported with studies and/or in the medical literature |
| Source: Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World 1998;22(1):5-12. |
Table 2
STRATEGIES TO PREVENT AND TREAT DELIRIUM TREMENS
| Assess risk for DTs with a thorough history, including collaborative family information and medical charts |
| Admit patients with a history of serious withdrawal symptoms or potential for inpatient detoxification (based on degree of tolerance) |
| Check complete lab values (chemistry panel, liver function tests including ALT and AST, complete blood count, blood alcohol level, GGT, and others if relevant), and correct any fluid, vitamin, or electrolyte abnormalities |
| Treat comorbid medical conditions |
| Differentiate DTs from alcohol hallucinosis, other causes of delirium, and Wernicke’s encephalopathy |
| Consider giving benzodiazepines along with low-dose neuroleptics, if appropriate, and monitor for disinhibition/ confusion and extrapyramidal symptoms, respectively |
| Place the patient in a low-stimulation environment with frequent monitoring |
Predisposing risk factors
Past withdrawal complicated by seizures or DTs is the single best predictor of future alcohol withdrawal symptoms.17 Also consider the following patients to be at elevated risk:
- any individual who presents with a blood alcohol level >300 mg/dl or after experiencing a withdrawal seizure9
- patients with comorbid medical conditions, such as electrolyte abnormalities, infection, or poorly treated cardiovascular or respiratory diseases
- older persons, who tend to be susceptible to delirium associated with hospitalization, medical illnesses such as urinary tract infections or pneumonia, or use of certain medications.18
Potential risk factors for developing DTs are summarized in Table 1.1 Although few studies have been done, clinicians can use these factors as a guide for aggressively preventing DTs in at-risk patients.
Managing and preventing DTs
Management. Drug therapy is considered crucial to quell withdrawal symptoms and reduce the risk of death.19 Patients usually are treated with one of several benzodiazepines (such as chlordiazepoxide, diazepam, oxazepam, or lorazepam) to decrease autonomic instability and reduce seizure risk during acute alcohol withdrawal. Although dosages of these medications are estimated based on drinking history, some general starting ranges are often used in clinical practice:
- chlordiazepoxide, 50 to 100 mg tid
- lorazepam, 1 to 2 mg every 4 hours
- oxazepam, 15 to 30 mg qid
- diazepam, 10 to 20 mg tid/qid.
Treating DTs often requires the use of IV benzodiazepines because of their quick onset of action and benefit for acutely agitated patients who have difficulty taking medications by mouth.
Prevention. Correcting fluid and electrolyte abnormalities may be critical in preventing DTs (Table 2). In one study of patients who died while experiencing DTs, only 25% received adequate fluid replacement, which can be as much as 6 liters per day.20 Ideally, comorbid conditions should be addressed early in presentation and before DTs develop.21
High-dose benzodiazepine therapy does not completely protect a patient from DTs or reduce its duration,22 but it may reduce mortality. A meta-analysis of prospective, placebocontrolled trials reported a risk reduction of 4.9 cases of DTs per 100 patients treated with benzodiazepines. Mortality also seems to have been reduced in patients with DTs who were treated with sedative hypnotics.9 Benzodiazepines may cause increased confusion and disinhibition, as is frequently seen when patients with dementia are treated with these agents.23
Neuroleptics such as haloperidol have been used to prevent and treat DTs, but studies of their ability to reduce mortality have produced inconsistent results. What’s more, neuroleptics can reduce the seizure threshold and produce extrapyramidal symptoms.23 Atypical antipsychotics may offer a safer alternative, although more studies are needed to evaluate whether they decrease the occurrence and severity of DTs.
In summary, a rational approach to preventing and treating DTs is to:
- manage comorbid medical illnesses, and correct fluid and electrolyte abnormalities
- place the patient in a safe, low-stimulation environment with frequent monitoring
- use benzodiazepines judiciously.
Related resources
- National Institute on Alcohol Abuse and Alcoholism.
- www.niaaa.nih.gov
- 1995;30(6):765-70 (a thorough review of benzodiazepine use in alcohol withdrawal).
Drug brand names
- Chlordiazepoxide • Librium
- Diazepam • Valium
- Haloperidol • Haldol
- Lorazepam • Ativan
- Oxazepam • Serax
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Ms. J, 42, was admitted to the OB-GYN service for a routine vaginal hysterectomy to treat dysfunctional uterine bleeding. In the presurgical history, she described having a few drinks daily. Shortly after a successful uncomplicated procedure, the patient became tremulous and was given several doses of lorazepam.
Two days after surgery, the patient became delirious. She complained of tactile and visual hallucinations, her level of consciousness waxed and waned, and she showed significant autonomic instability. A psychiatry consult was ordered. The consult team recommended IV fluids, IV diazepam, and haloperidol, supplemented with a multivitamin and 100 mg/d of thiamine. When the patient’s delirium resolved within 4 days, a more detailed discussion revealed a history of alcohol abuse and withdrawal seizures.
It is not uncommon for a patient to develop acute alcohol withdrawal and delirium tremens (DTs) while recovering from routine surgery. Delirium tremens remains a medical emergency, even though advances have reduced its associated mortality rates (Box).1-7
Psychiatrists who know the risk factors for DTs—also termed alcohol withdrawal delirium—can identify and protect patients who are susceptible to this life-threatening complication. We describe the clinical features of DTs, potential predisposing factors, theories behind its mechanisms, and strategies for preventing and managing DTs in patients experiencing alcohol withdrawal.
Clinical features
Disorientation and confusion are the hallmark features of DTs. Other clinical manifestations include vivid hallucinations, extreme tremulousness, autonomic hyperactivity, sweating, tachycardia, and agitation. Men experiencing DTs seem to demonstrate a greater degree of autonomic hyperactivity than women.8
Symptoms usually arise in the alcoholic patient between the third and fifth days of abstinence but have been known to occur several weeks after a patient’s last drink. Symptoms usually resolve within a few days9 but have been known to resolve within hours in some patients and to persist for several months in others.10
Differential diagnosis. Clinicians often fail to differentiate alcohol hallucinosis from DTs. Alcohol hallucinosis—which occurs in 3 to 10% of patients with severe alcohol withdrawal11 —manifests as auditory, visual, or tactile hallucinations with a clear sensorium. Patients experiencing DTs also may experience hallucinations but with confusion, disorientation, and severe autonomic hyperactivity. Unlike DTs, alcohol hallucinosis is not fatal.9
DTs also should be differentiated from:
- other causes of delirium, such as medication or infection. If the cause is identified and removed, the delirium should gradually resolve.
- Wernicke’s encephalopathy—caused by glucose exposure in the thiamine-deficient alcoholic—which is characterized by confusion, ophthalmoplegia, and ataxia.
Completing a thorough history and physical exam, talking to family members, and reviewing past medical charts are often the best ways to differentiate DTs from other conditions.
What causes DTs?
Vitamin deficiencies were initially thought to cause alcohol withdrawal.3 More recent evidence points toward multiple neuroadaptive changes in the brain associated with chronic alcohol exposure.12 Although numerous neurotransmitter systems may play a role in alcohol withdrawal, recent research has focused on glutamate13 and gamma-aminobutyric acid (GABA).14
Approximately 1.5 to 2 million Americans seek treatment for alcohol abuse or dependence each year.1 As many as 71% of these patients manifest symptoms of alcohol withdrawal.2 Of those individuals who experience alcohol withdrawal, delirium tremens (DTs) may occur in up to 5%.3-5 Utilizing these percentages, it can be estimated that as many as 50,000 to 70,000 individuals develop DTs each year in the United States alone.
Although the incidence of DTs can be assumed to be relatively low, the condition should be considered a medical emergency. Studies list mortality rates for DTs as high as 15% and as low as 2 to 3%.7,8
The brain seems to compensate for alcohol’s enhancement of GABA (inhibitory) neurons by up-regulating excitatory neurons (glutamate). Alcohol has been shown to have some effects on neurons.15 The implication is that withdrawing alcohol triggers an “excitatory state” until the brain can readjust the fine balance between excitation and inhibition, a process that takes weeks to months. Some changes may never reverse because of the neurotoxic effects of alcohol and alcohol withdrawal.
Repeated alcohol exposure and withdrawal may lead to neuroadaptive changes in the brain and to more severe withdrawal symptoms, such as DTs. Repeated alcohol withdrawal episodes can produce a kindling effect. As outlined by Becker, kindling occurs “when a weak electrical or chemical stimulus, which initially causes no overt behavioral responses, results in the appearance of behavioral effects, such as seizures, when it is administered repeatedly.”16 Thus, repeated alcohol withdrawal worsens future episodes and eventually leads to alcohol withdrawal seizures.
Whereas most of these theories apply to alcohol withdrawal, they are also compatible with the neuronal mechanisms that may underlie DTs. Alcohol withdrawal and DTs share the presence of a “hyperactive state.” Most likely, DTs is the progression to more severe or pronounced neuroadaptive changes seen in mild to moderate alcohol withdrawal. One could certainly imagine that the possible neurotoxic effects of alcohol, alcohol withdrawal, and repeated detoxifications could sensitize the CNS to the more severe symptoms seen in DTs. Infection and metabolic abnormalities may also enhance the progression. Unfortunately, why some but not all patients experiencing alcohol withdrawal progress to DTs is unknown.
Table 1
RISK FACTORS FOR DELIRIUM TREMENS
| Comorbid medical illness (with electrolyte, fluid abnormalities)* |
| History of delirium tremens* |
| Blood alcohol level >300 mg/dL on presentation* |
| Presentation after an alcohol withdrawal seizure* |
| Older age* |
| Longer history of alcohol dependence |
| Intense alcohol craving |
| Abnormal liver function |
| * Supported with studies and/or in the medical literature |
| Source: Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World 1998;22(1):5-12. |
Table 2
STRATEGIES TO PREVENT AND TREAT DELIRIUM TREMENS
| Assess risk for DTs with a thorough history, including collaborative family information and medical charts |
| Admit patients with a history of serious withdrawal symptoms or potential for inpatient detoxification (based on degree of tolerance) |
| Check complete lab values (chemistry panel, liver function tests including ALT and AST, complete blood count, blood alcohol level, GGT, and others if relevant), and correct any fluid, vitamin, or electrolyte abnormalities |
| Treat comorbid medical conditions |
| Differentiate DTs from alcohol hallucinosis, other causes of delirium, and Wernicke’s encephalopathy |
| Consider giving benzodiazepines along with low-dose neuroleptics, if appropriate, and monitor for disinhibition/ confusion and extrapyramidal symptoms, respectively |
| Place the patient in a low-stimulation environment with frequent monitoring |
Predisposing risk factors
Past withdrawal complicated by seizures or DTs is the single best predictor of future alcohol withdrawal symptoms.17 Also consider the following patients to be at elevated risk:
- any individual who presents with a blood alcohol level >300 mg/dl or after experiencing a withdrawal seizure9
- patients with comorbid medical conditions, such as electrolyte abnormalities, infection, or poorly treated cardiovascular or respiratory diseases
- older persons, who tend to be susceptible to delirium associated with hospitalization, medical illnesses such as urinary tract infections or pneumonia, or use of certain medications.18
Potential risk factors for developing DTs are summarized in Table 1.1 Although few studies have been done, clinicians can use these factors as a guide for aggressively preventing DTs in at-risk patients.
Managing and preventing DTs
Management. Drug therapy is considered crucial to quell withdrawal symptoms and reduce the risk of death.19 Patients usually are treated with one of several benzodiazepines (such as chlordiazepoxide, diazepam, oxazepam, or lorazepam) to decrease autonomic instability and reduce seizure risk during acute alcohol withdrawal. Although dosages of these medications are estimated based on drinking history, some general starting ranges are often used in clinical practice:
- chlordiazepoxide, 50 to 100 mg tid
- lorazepam, 1 to 2 mg every 4 hours
- oxazepam, 15 to 30 mg qid
- diazepam, 10 to 20 mg tid/qid.
Treating DTs often requires the use of IV benzodiazepines because of their quick onset of action and benefit for acutely agitated patients who have difficulty taking medications by mouth.
Prevention. Correcting fluid and electrolyte abnormalities may be critical in preventing DTs (Table 2). In one study of patients who died while experiencing DTs, only 25% received adequate fluid replacement, which can be as much as 6 liters per day.20 Ideally, comorbid conditions should be addressed early in presentation and before DTs develop.21
High-dose benzodiazepine therapy does not completely protect a patient from DTs or reduce its duration,22 but it may reduce mortality. A meta-analysis of prospective, placebocontrolled trials reported a risk reduction of 4.9 cases of DTs per 100 patients treated with benzodiazepines. Mortality also seems to have been reduced in patients with DTs who were treated with sedative hypnotics.9 Benzodiazepines may cause increased confusion and disinhibition, as is frequently seen when patients with dementia are treated with these agents.23
Neuroleptics such as haloperidol have been used to prevent and treat DTs, but studies of their ability to reduce mortality have produced inconsistent results. What’s more, neuroleptics can reduce the seizure threshold and produce extrapyramidal symptoms.23 Atypical antipsychotics may offer a safer alternative, although more studies are needed to evaluate whether they decrease the occurrence and severity of DTs.
In summary, a rational approach to preventing and treating DTs is to:
- manage comorbid medical illnesses, and correct fluid and electrolyte abnormalities
- place the patient in a safe, low-stimulation environment with frequent monitoring
- use benzodiazepines judiciously.
Related resources
- National Institute on Alcohol Abuse and Alcoholism.
- www.niaaa.nih.gov
- 1995;30(6):765-70 (a thorough review of benzodiazepine use in alcohol withdrawal).
Drug brand names
- Chlordiazepoxide • Librium
- Diazepam • Valium
- Haloperidol • Haldol
- Lorazepam • Ativan
- Oxazepam • Serax
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World 1998;22(1):5-12.
2. Saitz R, Redmond MS, Mayo-Smith MF, et al. Individualized treatment for alcohol withdrawal. Randomized, double-blind, controlled trial. JAMA 1994;272:519-23.
3. Victor M, Adams RD. The effect of alcoholism on the nervous system. Research Publication of the Association for Research on Nervous and Mental Disorders 1953;32:526-73.
4. Victor M, Braush C. The role of abstinence in the genesis of alcoholic epilepsy. Epilepsia 1967;8:1-20.
5. Sellars EM, Kalant H. Alcohol intoxication and withdrawal. N Engl J Med 1976;294:757-62.
6. Victor M. Treatment of alcoholic intoxication and the withdrawal syndrome. Psychosomat Med 1966;28:636-50.
7. Guthrie S. The treatment of alcohol withdrawal. Pharmacotherapy 1989;9:131-43.
8. Trevisan LA, Boutrous N, Petrakis I, Krystal JH. Complications of alcohol withdrawal. Alcohol World 1998;22(1):61-66.
9. Mayo-Smith MF. Management of alcohol intoxication, overdose, and withdrawal. In: Graham AW, Schultz TK (eds). Principles of addiction medicine (2nd ed). Chevy Chase, MD: American Society of Addiction Medicine, 1998;434-41.
10. Wolf KM, Shaughnessy AF, Middleton DB. Prolonged delirium tremens requiring massive doses of medication. J Am Board Fam Pract 1993;6:502-4.
11. Platz WE, Oberlaender FA, Seidel ML. The phenomenology of perceptual hallucinations in alcohol-induced delirium tremens. Psychopathology 1995;28:247-55.
12. Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World 1998;22(1):13-24.
13. Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol 1990;7(3):229-31.
14. Morrow AL, Montpied P, Lingford-Hughes A, Paul SM. Chronic ethanol and pentobarbital administration in the rat: effects on GABAA receptor function and expression in brain. Alcohol 1990;7(3):237-44.
15. Begleiter H Kissin B (eds) The pharmacology of alcohol and alcohol dependence. New York: Oxford University Press, 1996.
16. Becker HC. Kindling in alcohol withdrawal. Alcohol World 1998;22(1):25-33.
17. Gold M, Miller N. Management of withdrawal syndromes and relapse prevention in drug and alcohol dependence. Am Fam Phys 1998;58:139-46.
18. Kraemer K, Mayo-Smith MF, Calkins R. Pharmacological management of alcohol withdrawal: a meta analysis of evidence-based practical guidelines. JAMA 1997;278:144-51.
19. Shaw GK. Detoxification: the use of benzodiazepines. Alcohol Alcohol 1995;30(6):765-70.
20. Victor M. Diagnosis and treatment of alcohol withdrawal states. Pract Gastroenteritis 1983;7(5):6-15.
21. Myrick H, Anton R. Treatment of alcohol withdrawal. Alcohol Health Res World 1998;22(1):38-43.
22. Hersh D, Kranzler HR, Meyer RE. Persistent delirium following cessation of heavy alcohol consumption: diagnostic and treatment implications. Am J Psychiatry 1997;154(6):846-51.
23. Myrick H, Anton R. Clinical management of alcohol withdrawal. CNS Spectrums 2000;5(2):22-32.
1. Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World 1998;22(1):5-12.
2. Saitz R, Redmond MS, Mayo-Smith MF, et al. Individualized treatment for alcohol withdrawal. Randomized, double-blind, controlled trial. JAMA 1994;272:519-23.
3. Victor M, Adams RD. The effect of alcoholism on the nervous system. Research Publication of the Association for Research on Nervous and Mental Disorders 1953;32:526-73.
4. Victor M, Braush C. The role of abstinence in the genesis of alcoholic epilepsy. Epilepsia 1967;8:1-20.
5. Sellars EM, Kalant H. Alcohol intoxication and withdrawal. N Engl J Med 1976;294:757-62.
6. Victor M. Treatment of alcoholic intoxication and the withdrawal syndrome. Psychosomat Med 1966;28:636-50.
7. Guthrie S. The treatment of alcohol withdrawal. Pharmacotherapy 1989;9:131-43.
8. Trevisan LA, Boutrous N, Petrakis I, Krystal JH. Complications of alcohol withdrawal. Alcohol World 1998;22(1):61-66.
9. Mayo-Smith MF. Management of alcohol intoxication, overdose, and withdrawal. In: Graham AW, Schultz TK (eds). Principles of addiction medicine (2nd ed). Chevy Chase, MD: American Society of Addiction Medicine, 1998;434-41.
10. Wolf KM, Shaughnessy AF, Middleton DB. Prolonged delirium tremens requiring massive doses of medication. J Am Board Fam Pract 1993;6:502-4.
11. Platz WE, Oberlaender FA, Seidel ML. The phenomenology of perceptual hallucinations in alcohol-induced delirium tremens. Psychopathology 1995;28:247-55.
12. Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World 1998;22(1):13-24.
13. Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol 1990;7(3):229-31.
14. Morrow AL, Montpied P, Lingford-Hughes A, Paul SM. Chronic ethanol and pentobarbital administration in the rat: effects on GABAA receptor function and expression in brain. Alcohol 1990;7(3):237-44.
15. Begleiter H Kissin B (eds) The pharmacology of alcohol and alcohol dependence. New York: Oxford University Press, 1996.
16. Becker HC. Kindling in alcohol withdrawal. Alcohol World 1998;22(1):25-33.
17. Gold M, Miller N. Management of withdrawal syndromes and relapse prevention in drug and alcohol dependence. Am Fam Phys 1998;58:139-46.
18. Kraemer K, Mayo-Smith MF, Calkins R. Pharmacological management of alcohol withdrawal: a meta analysis of evidence-based practical guidelines. JAMA 1997;278:144-51.
19. Shaw GK. Detoxification: the use of benzodiazepines. Alcohol Alcohol 1995;30(6):765-70.
20. Victor M. Diagnosis and treatment of alcohol withdrawal states. Pract Gastroenteritis 1983;7(5):6-15.
21. Myrick H, Anton R. Treatment of alcohol withdrawal. Alcohol Health Res World 1998;22(1):38-43.
22. Hersh D, Kranzler HR, Meyer RE. Persistent delirium following cessation of heavy alcohol consumption: diagnostic and treatment implications. Am J Psychiatry 1997;154(6):846-51.
23. Myrick H, Anton R. Clinical management of alcohol withdrawal. CNS Spectrums 2000;5(2):22-32.