User login
Imatinib Mesylate–Induced Lichenoid Drug Eruption
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
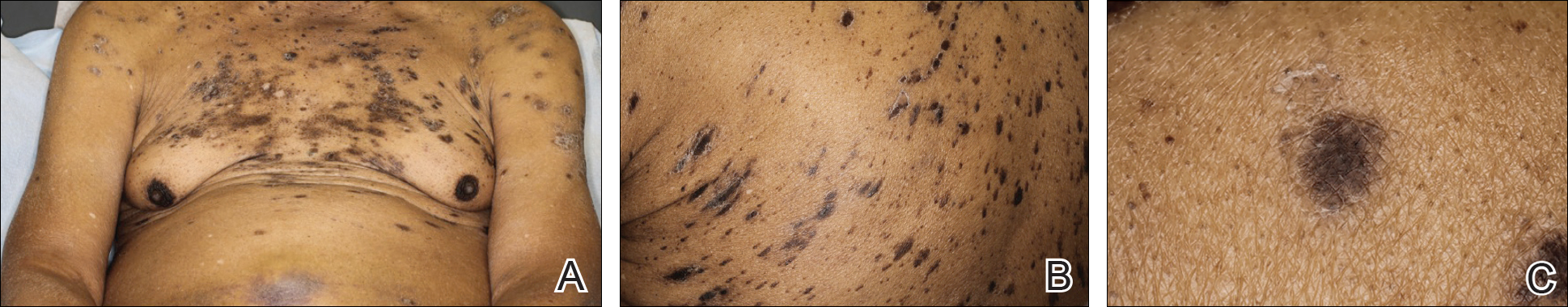
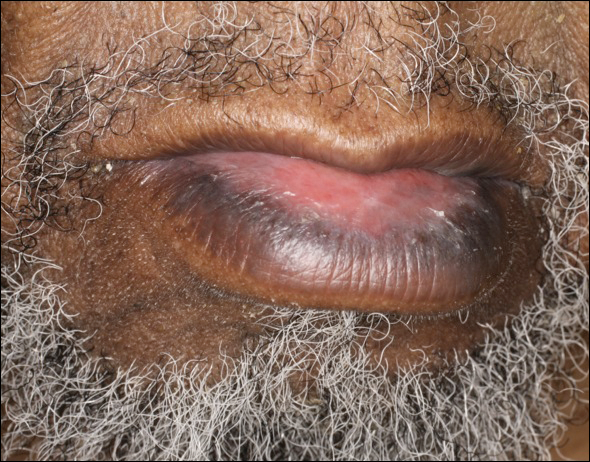
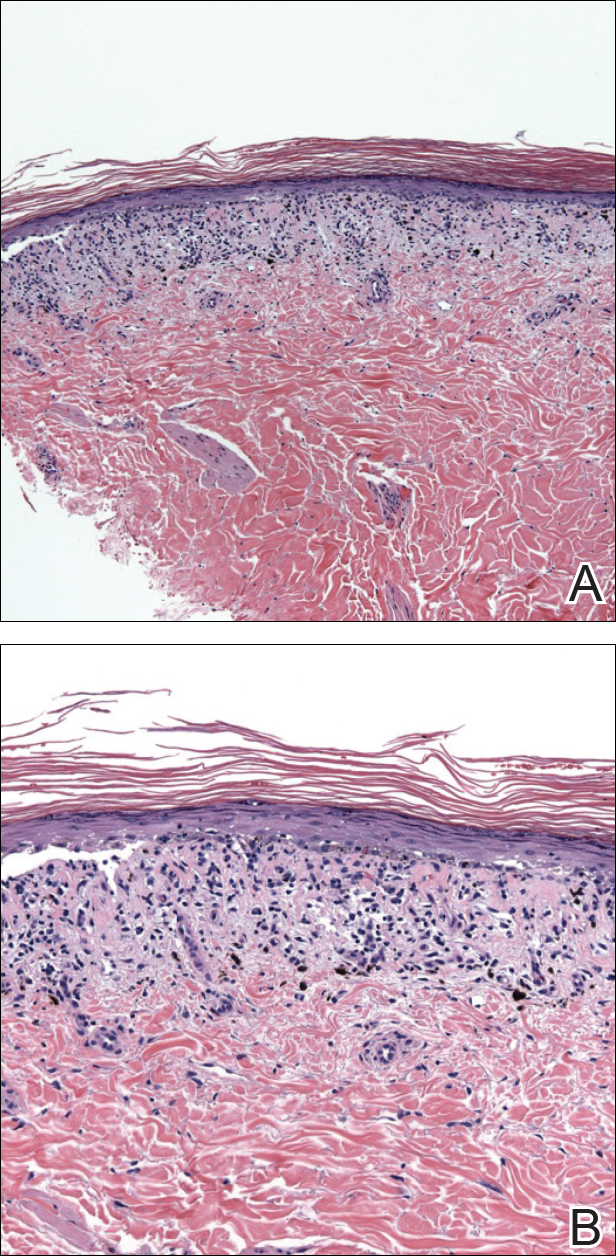
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.
Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.



Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.



Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
Practice Points
- Imatinib mesylate can cause cutaneous adverse reactions including dry skin, alopecia, facial edema, photosensitivity rash, and lichenoid drug eruption (LDE).
- Topical corticosteroids, oral acitretin, and oral steroids may be reasonable treatment options for imatinib-induced LDE if discontinuing imatinib is not possible in a symptomatic patient.
Rupioid Psoriasis and Other Skin Diseases With Rupioid Manifestations
Case Report
A 28-year-old man presented to the dermatology department with cone-shaped, oyster shell–like skin lesions on the scalp, trunk, arms, and legs of 1 month’s duration. He denied any fever, pruritus, pain, joint stiffness, or arthralgia. His family history was remarkable for psoriasis in his paternal grandfather and uncle.
A few years prior to the eruption, the patient developed a rash in the bilateral inguinal area but did not seek medical attention. One month prior to presentation, the rash began to spread to the scalp, trunk, arms, and legs. He was treated in the emergency department with a 5-day course of oral prednisone without any noticeable improvement. At the time of presentation to the dermatology clinic, he was found to have multiple well-demarcated erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crusts (Figure 1). Rapid plasma reagin testing was negative. A 4-mm punch biopsy specimen from the right upper arm demonstrated thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (Figure 2). In the stratum corneum, there was seroexudate with numerous red blood cells between the parakeratosis.
 |
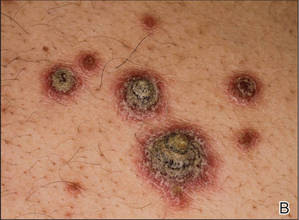 |
| Figure 1. Multiple well-demarcated erythematous plaques with hyperkeratotic crust on the back (A). Closer view of erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crust (B). |
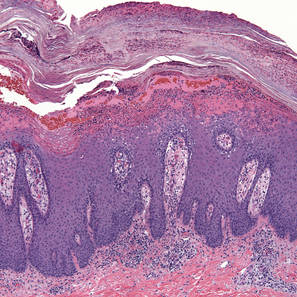 |
| Figure 2. Thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (H&E, original magnification ×100). |
The patient was diagnosed with rupioid psoriasis. The lesions dramatically improved with methotrexate 10 mg weekly and topical steroids. Two months following diagnosis the patient presented with persistent hyperkeratotic lesions on the back, as he had difficulty reaching the lesions to apply topical medications; intralesional steroid injections were added. This regimen resulted in near-complete resolution maintained at his most recent follow-up 2 years following diagnosis in our clinic.
Comment
Rupia is based on the Greek word rhupos, which means dirt or filth. The term rupioid has been used to describe well-demarcated, cone-shaped plaques with thick, dark, lamellate, and adherent crusts on the skin somewhat resembling oyster or limpet shells. Histologically, a serosanguineous exudate along with thick skin helps to impart a “dirty” appearance to rupioid lesions. Rupioid manifestations have been clinically observed in a variety of diseases, including rupioid psoriasis,1-3 reactive arthritis,4 disseminated histoplasmosis,5 keratotic scabies,6 secondary syphilis,7 and photosensitive skin lesions in association with aminoaciduria.8 To diagnose the underlying infectious or inflammatory diseases beneath the thick crusts, skin biopsy and a blood test for syphilis may be necessary.
Rupioid psoriasis is a morphologic subtype of plaque psoriasis with hyperkeratotic lesions that resemble an oyster or limpet shell. Patients with thick plaque psoriasis are more likely to be male with a higher incidence of nail disease and psoriatic arthritis as well as a greater body surface area affected than patients with thin plaque psoriasis.1 Although most cases of rupioid psoriasis were associated with psoriatic arthritis,3 our patient showed no evidence of psoriatic arthritis or nail changes.
Reactive arthritis may have a similar appearance to rupioid psoriasis but may be distinguished by a geographic relief map configuration with coalescing, keratotic and desquamating lesions, as well as associated urethritis, arthritis, and conjunctivitis.4 A rupioid eruption was reported as a manifestation of disseminated histoplasmosis with dirty-appearing, heaped-up, crusted lesions present on the cheeks, nose, and forehead on clinical examination and several intracellular and extracellular oval structures on histologic examination with periodic acid–Schiff and Gomori methenamine-silver stain.5 Malignant or rupioid syphilis refers to the stage in which papulopustules of pustular syphilis undergo central necrosis due to endarteritis obliterans and intravascular thrombosis.7
In our case, the patient’s psoriasis could have flared after discontinuation of the prednisone that was administered by the emergency department physician. Most cases have been treated with combined systemic and topical therapy.9 For systemic treatment, cyclosporine, intramuscular or oral methotrexate, adalimumab, and ustekinumab3 have been used with remarkable improvement. Hyperkeratotic types of psoriasis are generally thought to be resistant to topical therapy because of poor penetration of applied agents; however, a case of rupioid psoriasis without arthritis was successfully treated with topical steroids without concomitant systemic medications.2
Conclusion
Rupioid psoriasis is a morphological subtype of plaque psoriasis with hyperkeratotic lesions that resemble a limpet shell. Rupioid skin manifestations may be seen in a variety of diseases including rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria. Diagnosis of rupioid psoriasis often requires additional testing such as skin biopsy, skin scraping, and blood tests, and it typically requires systemic therapy for treatment.
1. Christensen TE, Callis KP, Papenfuss J, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397-2403.
2. Feldman SR, Brown KL, Heald P. ‘Coral reef’ psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
3. Necas M, Vasku V. Ustekinumab in the treatment of severe rupioid psoriasis: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:23-27.
4. Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter’s disease in a child. Dermatologica. 1985;170:77-79.
5. Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Bhagwat PV, Tophakhane RS, Rathod RM, et al. Rupioid syphilis in an HIV patient. Indian J Dermatol Venereol. 2009;75:201-202.
8. Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
9. Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
Case Report
A 28-year-old man presented to the dermatology department with cone-shaped, oyster shell–like skin lesions on the scalp, trunk, arms, and legs of 1 month’s duration. He denied any fever, pruritus, pain, joint stiffness, or arthralgia. His family history was remarkable for psoriasis in his paternal grandfather and uncle.
A few years prior to the eruption, the patient developed a rash in the bilateral inguinal area but did not seek medical attention. One month prior to presentation, the rash began to spread to the scalp, trunk, arms, and legs. He was treated in the emergency department with a 5-day course of oral prednisone without any noticeable improvement. At the time of presentation to the dermatology clinic, he was found to have multiple well-demarcated erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crusts (Figure 1). Rapid plasma reagin testing was negative. A 4-mm punch biopsy specimen from the right upper arm demonstrated thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (Figure 2). In the stratum corneum, there was seroexudate with numerous red blood cells between the parakeratosis.
 |
 |
| Figure 1. Multiple well-demarcated erythematous plaques with hyperkeratotic crust on the back (A). Closer view of erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crust (B). |
 |
| Figure 2. Thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (H&E, original magnification ×100). |
The patient was diagnosed with rupioid psoriasis. The lesions dramatically improved with methotrexate 10 mg weekly and topical steroids. Two months following diagnosis the patient presented with persistent hyperkeratotic lesions on the back, as he had difficulty reaching the lesions to apply topical medications; intralesional steroid injections were added. This regimen resulted in near-complete resolution maintained at his most recent follow-up 2 years following diagnosis in our clinic.
Comment
Rupia is based on the Greek word rhupos, which means dirt or filth. The term rupioid has been used to describe well-demarcated, cone-shaped plaques with thick, dark, lamellate, and adherent crusts on the skin somewhat resembling oyster or limpet shells. Histologically, a serosanguineous exudate along with thick skin helps to impart a “dirty” appearance to rupioid lesions. Rupioid manifestations have been clinically observed in a variety of diseases, including rupioid psoriasis,1-3 reactive arthritis,4 disseminated histoplasmosis,5 keratotic scabies,6 secondary syphilis,7 and photosensitive skin lesions in association with aminoaciduria.8 To diagnose the underlying infectious or inflammatory diseases beneath the thick crusts, skin biopsy and a blood test for syphilis may be necessary.
Rupioid psoriasis is a morphologic subtype of plaque psoriasis with hyperkeratotic lesions that resemble an oyster or limpet shell. Patients with thick plaque psoriasis are more likely to be male with a higher incidence of nail disease and psoriatic arthritis as well as a greater body surface area affected than patients with thin plaque psoriasis.1 Although most cases of rupioid psoriasis were associated with psoriatic arthritis,3 our patient showed no evidence of psoriatic arthritis or nail changes.
Reactive arthritis may have a similar appearance to rupioid psoriasis but may be distinguished by a geographic relief map configuration with coalescing, keratotic and desquamating lesions, as well as associated urethritis, arthritis, and conjunctivitis.4 A rupioid eruption was reported as a manifestation of disseminated histoplasmosis with dirty-appearing, heaped-up, crusted lesions present on the cheeks, nose, and forehead on clinical examination and several intracellular and extracellular oval structures on histologic examination with periodic acid–Schiff and Gomori methenamine-silver stain.5 Malignant or rupioid syphilis refers to the stage in which papulopustules of pustular syphilis undergo central necrosis due to endarteritis obliterans and intravascular thrombosis.7
In our case, the patient’s psoriasis could have flared after discontinuation of the prednisone that was administered by the emergency department physician. Most cases have been treated with combined systemic and topical therapy.9 For systemic treatment, cyclosporine, intramuscular or oral methotrexate, adalimumab, and ustekinumab3 have been used with remarkable improvement. Hyperkeratotic types of psoriasis are generally thought to be resistant to topical therapy because of poor penetration of applied agents; however, a case of rupioid psoriasis without arthritis was successfully treated with topical steroids without concomitant systemic medications.2
Conclusion
Rupioid psoriasis is a morphological subtype of plaque psoriasis with hyperkeratotic lesions that resemble a limpet shell. Rupioid skin manifestations may be seen in a variety of diseases including rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria. Diagnosis of rupioid psoriasis often requires additional testing such as skin biopsy, skin scraping, and blood tests, and it typically requires systemic therapy for treatment.
Case Report
A 28-year-old man presented to the dermatology department with cone-shaped, oyster shell–like skin lesions on the scalp, trunk, arms, and legs of 1 month’s duration. He denied any fever, pruritus, pain, joint stiffness, or arthralgia. His family history was remarkable for psoriasis in his paternal grandfather and uncle.
A few years prior to the eruption, the patient developed a rash in the bilateral inguinal area but did not seek medical attention. One month prior to presentation, the rash began to spread to the scalp, trunk, arms, and legs. He was treated in the emergency department with a 5-day course of oral prednisone without any noticeable improvement. At the time of presentation to the dermatology clinic, he was found to have multiple well-demarcated erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crusts (Figure 1). Rapid plasma reagin testing was negative. A 4-mm punch biopsy specimen from the right upper arm demonstrated thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (Figure 2). In the stratum corneum, there was seroexudate with numerous red blood cells between the parakeratosis.
 |
 |
| Figure 1. Multiple well-demarcated erythematous plaques with hyperkeratotic crust on the back (A). Closer view of erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crust (B). |
 |
| Figure 2. Thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (H&E, original magnification ×100). |
The patient was diagnosed with rupioid psoriasis. The lesions dramatically improved with methotrexate 10 mg weekly and topical steroids. Two months following diagnosis the patient presented with persistent hyperkeratotic lesions on the back, as he had difficulty reaching the lesions to apply topical medications; intralesional steroid injections were added. This regimen resulted in near-complete resolution maintained at his most recent follow-up 2 years following diagnosis in our clinic.
Comment
Rupia is based on the Greek word rhupos, which means dirt or filth. The term rupioid has been used to describe well-demarcated, cone-shaped plaques with thick, dark, lamellate, and adherent crusts on the skin somewhat resembling oyster or limpet shells. Histologically, a serosanguineous exudate along with thick skin helps to impart a “dirty” appearance to rupioid lesions. Rupioid manifestations have been clinically observed in a variety of diseases, including rupioid psoriasis,1-3 reactive arthritis,4 disseminated histoplasmosis,5 keratotic scabies,6 secondary syphilis,7 and photosensitive skin lesions in association with aminoaciduria.8 To diagnose the underlying infectious or inflammatory diseases beneath the thick crusts, skin biopsy and a blood test for syphilis may be necessary.
Rupioid psoriasis is a morphologic subtype of plaque psoriasis with hyperkeratotic lesions that resemble an oyster or limpet shell. Patients with thick plaque psoriasis are more likely to be male with a higher incidence of nail disease and psoriatic arthritis as well as a greater body surface area affected than patients with thin plaque psoriasis.1 Although most cases of rupioid psoriasis were associated with psoriatic arthritis,3 our patient showed no evidence of psoriatic arthritis or nail changes.
Reactive arthritis may have a similar appearance to rupioid psoriasis but may be distinguished by a geographic relief map configuration with coalescing, keratotic and desquamating lesions, as well as associated urethritis, arthritis, and conjunctivitis.4 A rupioid eruption was reported as a manifestation of disseminated histoplasmosis with dirty-appearing, heaped-up, crusted lesions present on the cheeks, nose, and forehead on clinical examination and several intracellular and extracellular oval structures on histologic examination with periodic acid–Schiff and Gomori methenamine-silver stain.5 Malignant or rupioid syphilis refers to the stage in which papulopustules of pustular syphilis undergo central necrosis due to endarteritis obliterans and intravascular thrombosis.7
In our case, the patient’s psoriasis could have flared after discontinuation of the prednisone that was administered by the emergency department physician. Most cases have been treated with combined systemic and topical therapy.9 For systemic treatment, cyclosporine, intramuscular or oral methotrexate, adalimumab, and ustekinumab3 have been used with remarkable improvement. Hyperkeratotic types of psoriasis are generally thought to be resistant to topical therapy because of poor penetration of applied agents; however, a case of rupioid psoriasis without arthritis was successfully treated with topical steroids without concomitant systemic medications.2
Conclusion
Rupioid psoriasis is a morphological subtype of plaque psoriasis with hyperkeratotic lesions that resemble a limpet shell. Rupioid skin manifestations may be seen in a variety of diseases including rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria. Diagnosis of rupioid psoriasis often requires additional testing such as skin biopsy, skin scraping, and blood tests, and it typically requires systemic therapy for treatment.
1. Christensen TE, Callis KP, Papenfuss J, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397-2403.
2. Feldman SR, Brown KL, Heald P. ‘Coral reef’ psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
3. Necas M, Vasku V. Ustekinumab in the treatment of severe rupioid psoriasis: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:23-27.
4. Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter’s disease in a child. Dermatologica. 1985;170:77-79.
5. Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Bhagwat PV, Tophakhane RS, Rathod RM, et al. Rupioid syphilis in an HIV patient. Indian J Dermatol Venereol. 2009;75:201-202.
8. Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
9. Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
1. Christensen TE, Callis KP, Papenfuss J, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397-2403.
2. Feldman SR, Brown KL, Heald P. ‘Coral reef’ psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
3. Necas M, Vasku V. Ustekinumab in the treatment of severe rupioid psoriasis: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:23-27.
4. Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter’s disease in a child. Dermatologica. 1985;170:77-79.
5. Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Bhagwat PV, Tophakhane RS, Rathod RM, et al. Rupioid syphilis in an HIV patient. Indian J Dermatol Venereol. 2009;75:201-202.
8. Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
9. Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
- Diseases with rupioid manifestations include rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria.
- Skin biopsy, skin scraping, and blood tests may be necessary to diagnose the underlying diseases beneath the thick crusts and to rule out other diagnoses within the differential.
- Treatment of rupioid psoriasis is no different than typical plaque psoriasis, except for the need for systemic therapy in most cases due to the thick scale.